Effects of Biofertilizer on Yield and Quality of Crops and Properties of Soil Under Field Conditions in China: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
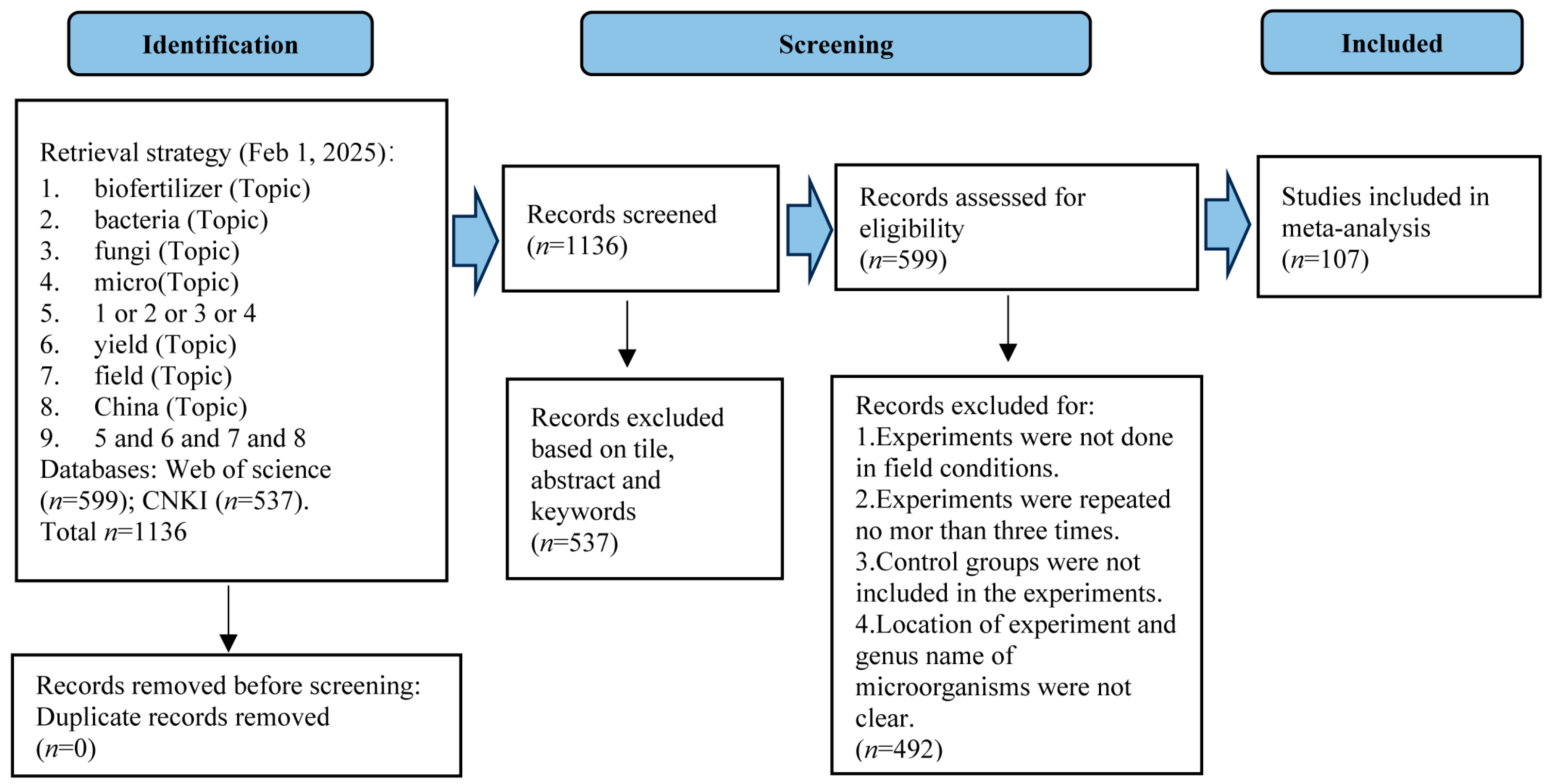
2.1. Data Collection
2.2. Effect Size Calculation and Its Normal Distribution Test
2.3. Heterogeneity and Publication Bias
2.4. Meta-Analysis
2.5. Statistics
3. Results
3.1. Normal Distribution and Heterogeneity of Biofertilizer Effect Size and Publication Bias Test
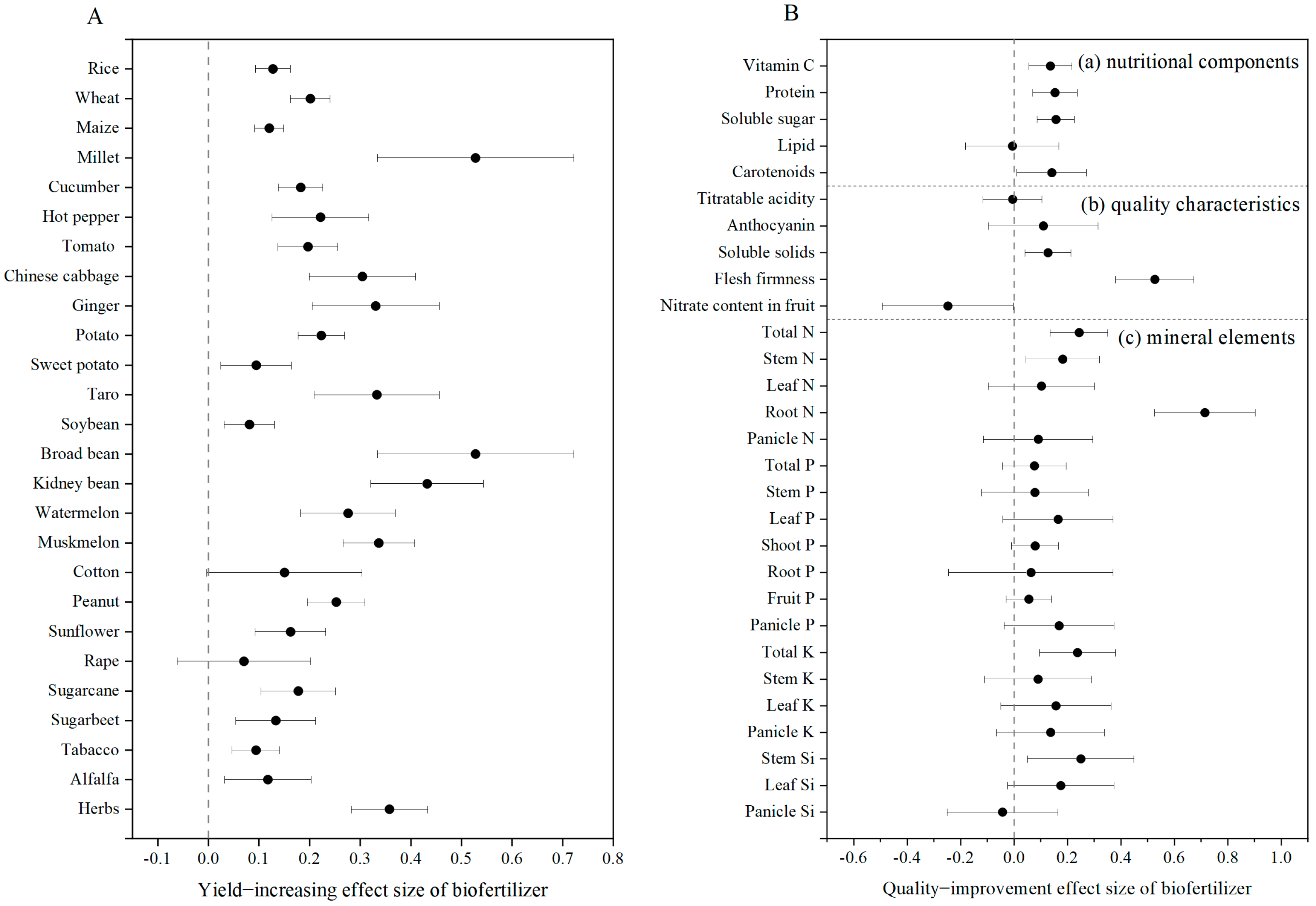
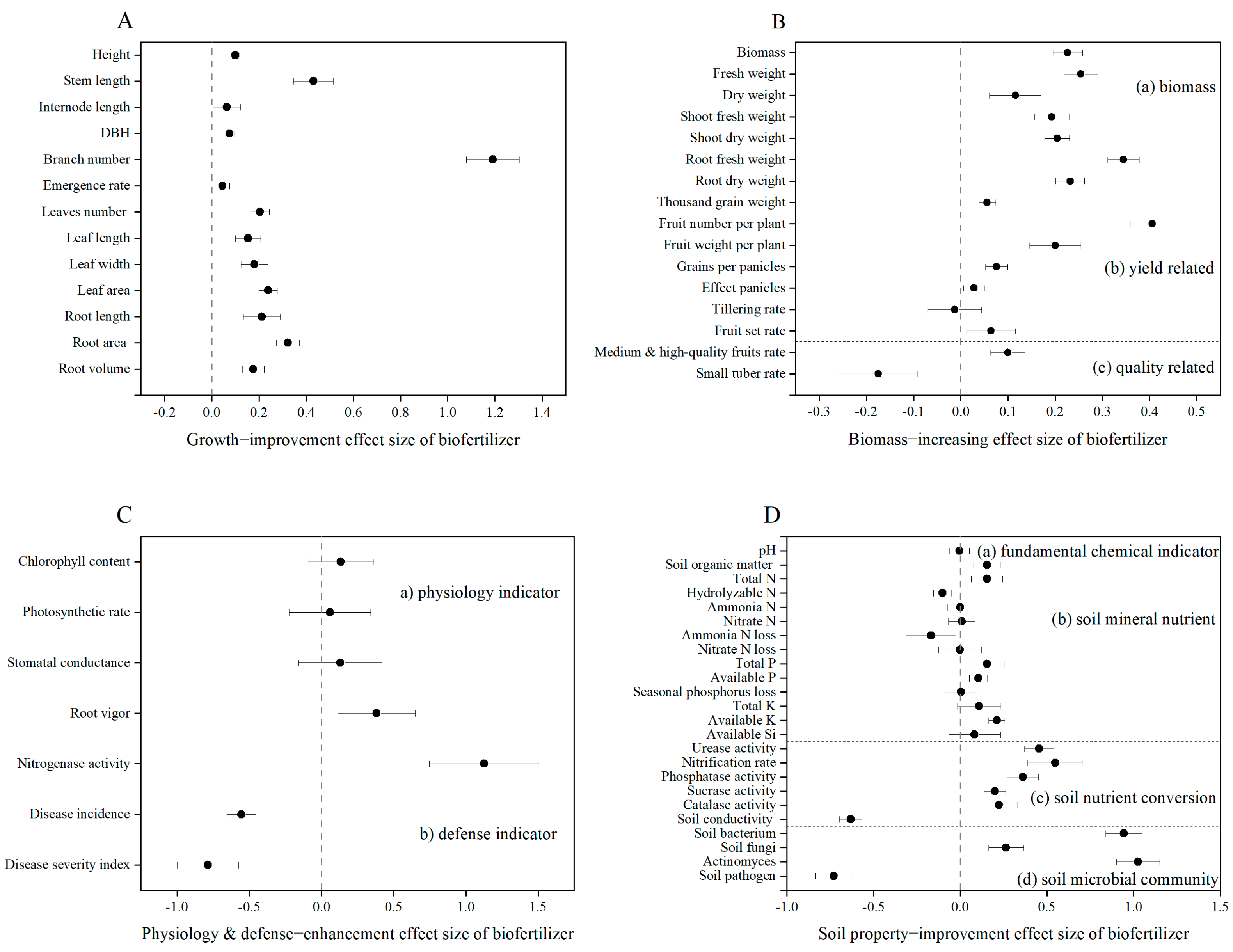
3.2. Biofertilizer Improves Yield and Quality of Crops Under Field Conditions in China
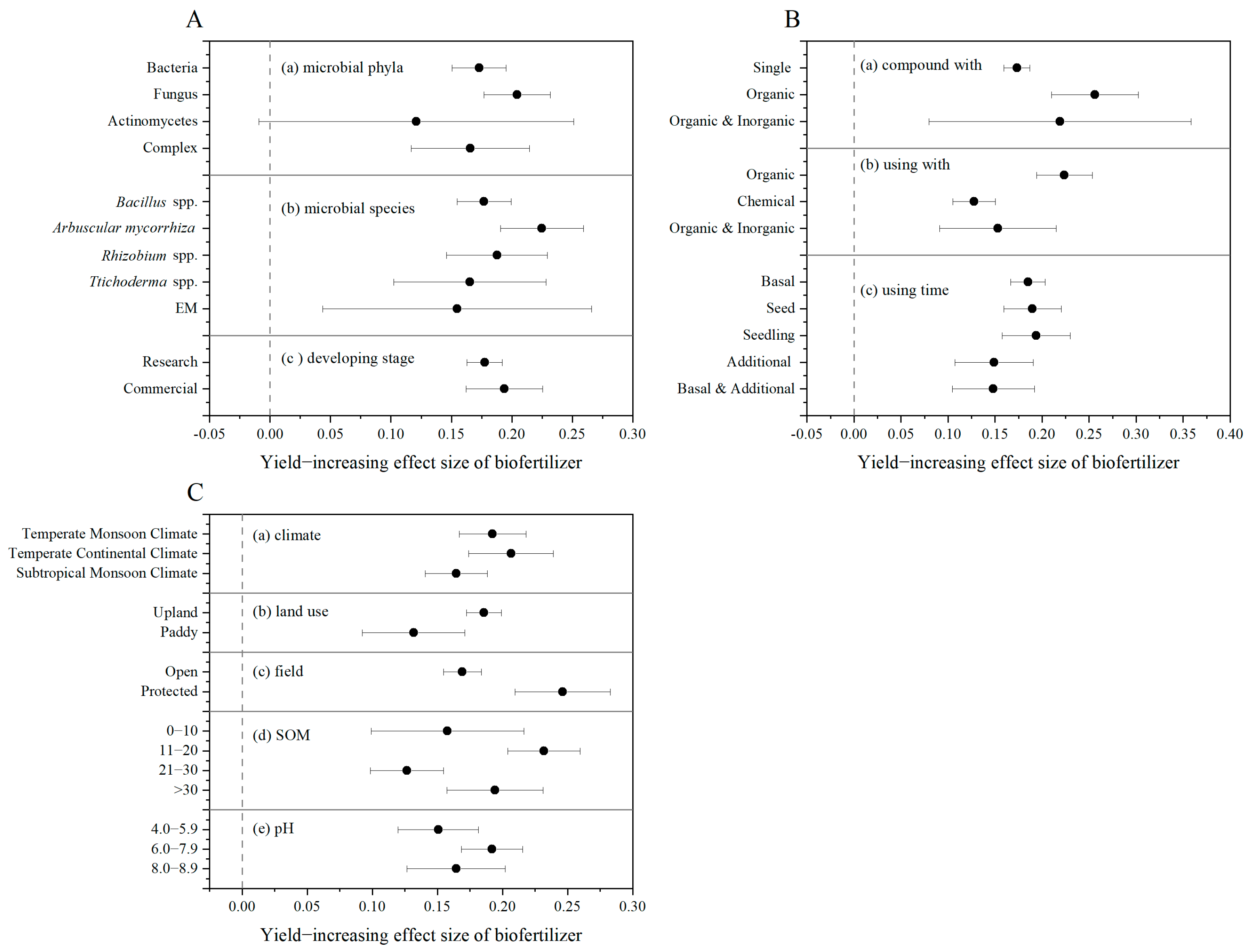
3.3. Impact Factors of Biofertilization on Crop Yield Under Field Conditions in China
3.4. Effects of Biofertilizer on the Growth and Defense of Crops
3.5. Effects of Biofertilizer on the Properties of Soils
3.6. Correlations of Crops’ Yield with Plant Growth, Soil Properties, and Soil-Borne Diseases
4. Discussion
4.1. Biofertilizer Is a Reliable Option for Sustainable Agriculture
4.2. Biofertilizers Enhance Crop Yield and Quality via Soil Microbiome-Mediated Nutrient and Growth Optimization
4.3. Determinants of Biofertilizer Efficacy: Specific Plant–Microbe Interactions and Management Practices
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNKI | China National Knowledge Infrastructure |
| DBH | diameter at breast height |
| EM | effective microorganisms |
| Nfs | fail-safe number |
| OLS | ordinary least squares |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SD | standard deviations |
| SE | standard errors |
| SOM | soil organic matter |
References
- United Nations, Department of Economic and Social Affairs. World Population Prospects 2024: Ten Key Messages, Population Division; United Nations: New York, NY, USA, 2024. [Google Scholar]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2023. Urbanization, Agrifood Systems Transformation and Healthy Diets Across the Rural–Urban Continuum; FAO: Rome, Italy, 2023. [Google Scholar]
- Le Page, M. Tackling the global food crisis. New Sci. 2022, 254, 18–20. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, M.; Zhang, W.; Chen, X.; Zhao, Z.; Yao, Z. Environmental impacts of crop production systems in subtropical plateau regions: Case study of Yunnan, China. Sci. Rep. 2024, 14, 30254. [Google Scholar] [CrossRef]
- Priyadarshini, P.; Choudhury, S.; Tilgam, J.; Bharati, A.; Sreeshma, N. Nitrogen fixing cereal: A rising hero towards meeting food security. Plant Physiol. Biochem. 2021, 167, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Remigi, P.; Zhu, J.; Young, J.P.W.; Masson-Boivin, C. Symbiosis within Symbiosis: Evolving Nitrogen-Fixing Legume Symbionts. Trends Microbiol. 2016, 24, 63–75. [Google Scholar] [CrossRef]
- Estrada-Bonilla, G.A.; Durrer, A.; Cardoso, E.J.B.N. Use of compost and phosphate-solubilizing bacteria affect sugarcane mineral nutrition, phosphorus availability, and the soil bacterial community. Appl. Soil Ecol. 2021, 157, 103760. [Google Scholar] [CrossRef]
- Khan, H.; Akbar, W.A.; Shah, Z.; Rahim, H.U.; Taj, A.; Alatalo, J.M. Coupling phosphate-solubilizing bacteria (PSB) with inorganic phosphorus fertilizer improves mungbean (Vigna radiata) phosphorus acquisition, nitrogen fixation, and yield in alkaline-calcareous soil. Heliyon 2022, 8, e09081. [Google Scholar] [CrossRef]
- Rawat, P.; Sharma, A.; Shankhdhar, D.; Shankhdhar, S.C. Improvement of phosphorus uptake, phosphorus use efficiency, and grain yield in upland rice (Oryza sativa L.) in response to phosphate-solubilizing bacteria blended with phosphorus fertilizer. Pedosphere 2022, 32, 752–763. [Google Scholar] [CrossRef]
- Gong, B.; Zhong, X.; Chen, X.; Li, S.; Hong, J.; Mao, X.; Liao, Z. Manipulation of composting oxygen supply to facilitate dissolved organic matter (DOM) accumulation which can enhance maize growth. Chemosphere 2021, 273, 129729. [Google Scholar] [CrossRef]
- Gigliotti, G.; Proietti, P.; Said-Pullicino, D.; Nasini, L.; Pezzolla, D.; Rosati, L.; Porceddu, P.R. Co-composting of olive husks with high moisture contents: Organic matter dynamics and compost quality. Int. Biodeterior. Biodegrad. 2012, 67, 8–14. [Google Scholar] [CrossRef]
- Eichmann, R.; Richards, L.; Schäfer, P. Hormones as go-betweens in plant microbiome assembly. Plant J. 2021, 105, 518–541. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Omae, N.; Tsuda, K. Inter-organismal phytohormone networks in plant-microbe interactions. Curr. Opin. Plant Biol. 2022, 68, 102258. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Nunes da Silva, M.; Pintado, M.E.; Sarmento, B.; Stamford, N.P.; Vasconcelos, M.W. A biofertilizer with diazotrophic bacteria and a filamentous fungus increases Pinus pinaster tolerance to the pinewood nematode (Bursaphelenchus xylophilus). Biol. Control. 2019, 132, 72–80. [Google Scholar] [CrossRef]
- Shen, Z.; Ruan, Y.; Wang, B.; Zhong, S.; Su, L.; Li, R.; Shen, Q. Effect of biofertilizer for suppressing Fusarium wilt disease of banana as well as enhancing microbial and chemical properties of soil under greenhouse trial. Appl. Soil Ecol. 2015, 93, 111–119. [Google Scholar] [CrossRef]
- Shen, Z.; Zhong, S.; Wang, Y.; Wang, B.; Mei, X.; Li, R.; Ruan, Y.; Shen, Q. Induced soil microbial suppression of banana fusarium wilt disease using compost and biofertilizers to improve yield and quality. Eur. J. Soil Biol. 2013, 57, 1–8. [Google Scholar] [CrossRef]
- Demir, Z. Effects of microbial bio-fertilizers on soil physicochemical properties under different soil water regimes in greenhouse grown eggplant (Solanum melongena L.). Commun. Soil Sci. Plant Anal. 2020, 51, 1888–1903. [Google Scholar] [CrossRef]
- Yang, L.Y.; Zhou, S.Y.; Lin, C.H.; Huang, X.R.; Neilson, R.; Yang, X.R. Effects of biofertilizer on soil microbial diversity and antibiotic resistance genes. Sci. Total Environ. 2022, 820, 153170. [Google Scholar] [CrossRef]
- Atieno, M.; Herrmann, L.; Nguyen, H.T.; Phan, H.T.; Nguyen, N.K.; Srean, P.; Than, M.M.; Zhiyong, R.; Tittabutr, P.; Shutsrirung, A.; et al. Assessment of biofertilizer use for sustainable agriculture in the Great Mekong Region. J. Environ. Manag. 2020, 275, 111300. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar]
- Luo, L.; Zhao, C.; Wang, E.; Raza, A.; Yin, C. Bacillus amyloliquefaciens as an excellent agent for biofertilizer and biocontrol in agriculture: An overview for its mechanisms. Microbiol. Res. 2022, 259, 127016. [Google Scholar] [CrossRef] [PubMed]
- Sakpirom, J.; Nunkaew, T.; Khan, E.; Kantachote, D. Optimization of carriers and packaging for effective biofertilizers to enhance Oryza sativa L. growth in paddy soil. Rhizosphere 2021, 19, 100383. [Google Scholar] [CrossRef]
- Ji, S.; Liu, Z.; Liu, B.; Wang, Y.; Wang, J. The effect of Trichoderma biofertilizer on the quality of flowering Chinese cabbage and the soil environment. Sci. Hortic. 2020, 262, 109069. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, L.; Yin, W. Soil Microorganisms: A New Dimension for Sustainable Agriculture and Environmental Development. J. Mirobiol. 2020, 40, 1–7. [Google Scholar]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving Crop Yield and Nutrient Use Efficiency via Biofertilization-A Global Meta-analysis. Front. Plant Sci. 2017, 8, 2204. [Google Scholar] [CrossRef]
- Du, Y.; Cui, B.; Zhang, Q.; Wang, Z.; Sun, J.; Niu, W. Effects of manure fertilizer on crop yield and soil properties in China: A meta-analysis. Catena 2020, 193, 104617. [Google Scholar] [CrossRef]
- Pei, B.; Zhang, Y.; Liu, T.; Cao, J.; Ji, H.; Hu, Z.; Wu, X.; Wang, F.; Lu, Y.; Chen, N.; et al. Effects of seaweed fertilizer application on crops’ yield and quality in field conditions in China—A meta-analysis. PLoS ONE 2024, 19, e0307517. [Google Scholar] [CrossRef]
- Cao, L.; Wang, Y.L.; Pu, J.W.; Song, T.J.; Liu, Y.T.; Wei, X.M.; Lin, Y.B. Effects of microbial agents on the active constituents and rhizosphere bacterial community of Gynostemma pentaphyllum. Acta Microbiol. Sin. 2024, 64, 2323–2336. [Google Scholar]
- Cao, Y.; Wen, H.X.; Deng, R.L.; Xiao, H.D. Effects of new type N2-fixing bacteria on growth and development of kidney bean. J. Foshan Univ. (Nat. Sci. Ed.) 2002, 20, 71–74. [Google Scholar]
- Chang, L.Y.; Wang, Z.H.; Li, F.M.; Gao, Z.Y.; Zhang, H.H.; Wang, Y.; Li, F.; Han, Y.L.; Jiang, Y. Screening Multi-functional Rhizobacteria from Maize Rhizosphere and Their Ehancing Effects on Winter Wheat-Summer Maize Rotation System. Biotechnol. Bull. 2024, 40, 231–242. [Google Scholar]
- Chen, L.; Li, K.; Shang, J.; Wu, Y.; Chen, T.; Wanyan, Y.; Wang, E.; Tian, C.; Chen, W.; Chen, W.; et al. Plant growth–promoting bacteria improve maize growth through reshaping the rhizobacterial community in low-nitrogen and low-phosphorus soil. Biol. Fertil. Soils 2021, 57, 1075–1088. [Google Scholar] [CrossRef]
- Chen, W.; Teng, Y.; Li, Z.; Liu, W.; Ren, W.; Luo, Y.; Christie, P. Mechanisms by which organic fertilizer and effective microbes mitigate peanut continuous cropping yield constraints in a red soil of south China. Appl. Soil Ecol. 2018, 128, 23–34. [Google Scholar] [CrossRef]
- Cheng, J.; Qiao, M.X.; Guo, Y.X.; Yang, Z.P.; Gao, Z.Q.; Liu, Y.T.; Lin, W. Effects of exogenous actinobacteria on growth of foxtail millet (Setaria italica) and culturable microorganisms in rhizosphere soil at Mature Stage. Soils 2022, 54, 978–985. [Google Scholar]
- Cheng, J.K.; Qian, C.X.; Yu, L.Y.; Wang, Q.; Qi, H.X.; Cheng, J.L. The effect of microbial agents on the growth and development of potatoes. Appl. Eng. Technol. 2022, 42, 15–17. [Google Scholar]
- Cheng, S.F.; Li, J.R.; Yao, T.Y.; Yu, J.X. Effect of increasing maize yields and saving nitrogen fertilizer by inoculation of Azospirillum brasilense UB37. Soils Fertil. 2002, 39, 37–40. [Google Scholar]
- Cheng, X.J.; Liu, K.M.; Xuan, M.G.; Shao, J.H.; Zhang, R.F. Screening and identification of plant growth-promoting rhizobacteria to enhance salt stress tolerance of crops and their effects in field experiment. J. Nanjing Agric. Univ. 2020, 43, 452–459. [Google Scholar]
- Cheng, Y.X.; Zhao, F.Q. Study on the Effect of Microbial Agents on the Yield of Celery. Agric. Technol. Equip. 2021, 37, 57–58. [Google Scholar]
- Dai, C.J.; Zhuo, Q. Study on the field control effect and yield of six Bacillus sp. microbial agents on melon wilt disease. Spec. Econ. Anim. Plant 2023, 26, 24–27. [Google Scholar]
- Dai, S.Y.; He, Y.J.; Shen, W.S.; Zhong, W.H.; Peng, Y. Mutagenesis of a phosphate dissolving bacterial strain by UV and its application to rice cultivation in red soil. Ecol. Environ. Sci. 2010, 19, 1646–1652. [Google Scholar]
- Deng, Y. Identification of Paenibacillus polymyxa as antagonist to Fusarium head blight of wheat and its field control efficacy. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2022, 51, 21–26. [Google Scholar]
- Deng, Z.P.; Tao, L.; Li, W.Q.; Wang, Y.; Cheng, S.F. Effects of microbial agents, biogas slurry and their combination on quality and yield of tomato, pepper and cucumber. J. Anhui Agric. Sci. 2011, 39, 9683–9686. [Google Scholar]
- Ding, C.; Shen, Q.; Zhang, R.; Chen, W. Evaluation of rhizosphere bacteria and derived bio-organic fertilizers as potential biocontrol agents against bacterial wilt (Ralstonia solanacearum) of potato. Plant Soil 2013, 366, 453–466. [Google Scholar] [CrossRef]
- Ding, X.S.; Zhu, H.Q.; Ding, Y.F.; Yao, Q.M.; Zhang, Q.Q.; Yao, C.X.; Zhou, X.G.; Yang, J.Z.; Ding, Y.M.; Cheng, J. The Field Application Effect of a Compound Microbial Agent on Sweet and Crisp Pea. Chin. Agric. Sci. Bull. 2022, 38, 15–19. [Google Scholar]
- Ding, Y.F.; Zhu, H.Q.; Yao, Q.M.; Zhang, H.Y.; Zhang, Q.Q.; Zhang, H.L.; Yan, J.; Yao, H.Y.; Ding, Y.M. Field application effect of compound microbial agent on tomato grown in the facility. Yunnan Agric. Sci. Technol. 2023, 52, 11–13+19. [Google Scholar]
- Dong, Y.; Yan, F.; Zhao, F.Y.; Hou, X.M.; Li, Q.Q.; Li, X.Y. Control effects of different microbial agents on Broomcorn Millet Smut. Heilongjiang Agric. Sci. 2023, 46, 18–21. [Google Scholar]
- Du, P.X.; Cheng, R.H.; Deng, Q.Q.; Lu, Q.; Liu, H.; Fan, C.G.; Li, S.X.; Hong, Y.B. Isolation, screening and application of peanut rhizobia. J. South. Agric. 2023, 54, 102–109. [Google Scholar]
- Fang, M.K.; Luo, X.C.; Wang, L.; Wang, Y.; Wu, M.Y.; Li, H.Z.; Yang, Y.Z. Effects of microbial liquid-soaked seeds on growth and yield of soybean, soil physicochemical properties and composition of phosphorus solubilizing bacteria community. Jiangsu Agric. Sci. 2024, 52, 190–198. [Google Scholar]
- Farmer, M.J.; Li, X.; Feng, G.; Zhao, B.; Chatagnier, O.; Gianinazzi, S.; Gianinazzi-Pearson, V.; van Tuinen, D. Molecular monitoring of field-inoculated AMF to evaluate persistence in sweet potato crops in China. Appl. Soil Ecol. 2007, 35, 599–609. [Google Scholar] [CrossRef]
- ten Berge, H.F.M.; Hijbeek, R.; van Loon, M.P.; Rurinda, J.; Tesfaye, K.; Zingore, S.; Craufurd, P.; van Heerwaarden, J.; Brentrup, F.; Schröder, J.J.; et al. Maize crop nutrient input requirements for food security in sub-Saharan Africa. Glob. Food Secur. 2019, 23, 9–21. [Google Scholar] [CrossRef]
- Gao, E.L. The effect of inoculating Bacillus subtilis on maize yield and growth. Appl. Eng. Technol. 2023, 43, 30–32. [Google Scholar]
- Gao, Q.; Yang, Z.X.; Liu, G.H.; Yang, L.X.; Tang, K.; Pu, N.B.; Li, L.; Liu, D.W. Effects of fungicides and rhizobia agents seed dressings on the rhizospheric microorganisms and yield of peanut. J. Peanut Sci. 2024, 53, 43–51. [Google Scholar]
- Ge, B.; Liu, B.; Nwet, T.T.; Zhao, W.; Shi, L.; Zhang, K. Bacillus methylotrophicus Strain NKG-1, Isolated from Changbai Mountain, China, Has Potential Applications as a Biofertilizer or Biocontrol Agent. PLoS ONE 2016, 11, e0166079. [Google Scholar] [CrossRef]
- Gong, X.G.; Hu, X.F.; Cheng, J.Q.; Yue, N.; Luo, Z.H.; Pan, W.Z. Effects of microbial agent application at different periods on growth and yield-quality of flue-cured tobacco. Soils Fertil. Sci. China 2014, 51, 106–110. [Google Scholar]
- Gong, Z.Y.; Si, T.R.; Sun, Y.F.; Wang, D.S.; Wang, B.; Li, R.; Sheng, Q.R. Continuous application of a bio-nursery substance to nursery seedlings improved greenhouse cucumber yield. Chin. J. Appl. Environ. Biol. 2018, 24, 967–971. [Google Scholar]
- Guo, F.T.; Feng, J.C.; Cheng, Z.B.; Zhao, J.W.; Li, F. Result Analysis for the Contrast Tests of Biotech Fertilizer (Bs Y1336 + Compound Organic Fertilizer) in the Rice Field. J. Agric. 2012, 16, 18–22+35. [Google Scholar]
- Guo, X.; Nanbiao, Z.; Zi, W.; Fangjun, D.; Zhonghao, Y.; Cui, X. Effects of the combination of Bacillus aryabhattai and calcium peroxide on soil silicon and potassium contents, the yield and quality of facility tomato. Arch. Agron. Soil Sci. 2024, 70, 1–12. [Google Scholar] [CrossRef]
- Guo, X.S.; Wei, J.H.; Sha, C.Y.; Gao, H.; Song, Z.Q.; Yin, J.; Ding, F.J. Effect of Optimizing Fertilization on Yield and Quality of Potato and Soil Nutrient Supply. Chin. Agric. Sci. Bull. 2024, 40, 84–90. [Google Scholar]
- Han, Z.S.; Zheng, M.N.; Liang, X.Z. Effect of Rhizobium Inoculation on Nitrogen Fixation Activity and Biomass of Medicago sativa L. Acta Agric. Boreali-Sin. 2016, 31, 214–219. [Google Scholar]
- He, G.Q.; Deng, Z.P.; Liu, Z.Z.; Xie, J.B.; Li, P.F.; Dong, R.J.; Pang, C.L.; Cheng, S.F. Effect of Azotobacter, slurry and their combination on economic characters and yield of maize. J. China Agric. Univ. 2011, 16, 24–29. [Google Scholar]
- He, G.Q.; Wang, L.; Deng, Z.P.; Li, H.M.; Gao, M.; Cheng, S.F. Effects of nitrogen-fixing, phosphate-solubilizing bacterial agents on wheat seed germination and wheat yield in the field. J. Anhui Agric. Sci. 2011, 39, 3875–3876+3888. [Google Scholar]
- Hu, M.; Xue, H.; Wade, A.J.; Gao, N.; Qiu, Z.; Long, Y.; Shen, W. Biofertilizer supplements allow nitrogen fertilizer reduction, maintain yields, and reduce nitrogen losses to air and water in China paddy fields. Agric. Ecosyst. Environ. 2024, 362, 108850. [Google Scholar] [CrossRef]
- Hu, X.; Roberts, D.P.; Xie, L.; Maul, J.E.; Yu, C.; Li, Y.; Jiang, M.; Liao, X.; Che, Z.; Liao, X. Formulations of Bacillus subtilis BY-2 suppress Sclerotinia sclerotiorum on oilseed rape in the field. Biol. Control 2014, 70, 54–64. [Google Scholar] [CrossRef]
- Hu, X.Y.; Zhou, S.; He, Y.G.; Liao, M.D.; Cheng, Y.M.; Cheng, Z.P. Growth Promoting Function and Application of Paecilomyces lilacinus in Tobacco Production. Southwest China J. Agric. Sci. 2018, 31, 973–979. [Google Scholar]
- Huang, Z.H.; Wu, G.L.; Zhang, T.; Liu, S.; Zhang, L.G.; Yuan, Y.; Guo, Y.N.; Wang, X.H. Quantitative analysis of the ability of Bacillus aryabhattai MB35-5 to dissolve silicon using incubation and field expriments. Soils Fertil. Sci. China 2021, 58, 140–143. [Google Scholar]
- Li, F.; Xu, L.J.; Xie, W.; Hao, Z.P.; Cheng, B.D. Effects of seedling mycorrhization on the growth and nutrient uptake of maize. J. Plant Nutr. Fertil. 2020, 26, 42–50. [Google Scholar]
- Li, G.Q.; Wang, N.; Li, Y.B.; Li, Y.L.; Wang, K.G.; Wang, R.; He, J.Y.; Liu, D.H.; Zhang, L.X.; Wang, Q.; et al. Effect Evaluation of Field Experiment of Two Paenibacillus sp. Agents in Wheat-Maize Rotation Area. J. Agric. Sci. Technol. 2020, 22, 147–152. [Google Scholar]
- Li, T.T.; Fu, Z.F.; Li, X. Effect of Inoculation of Arbuscular mycorrhizal Fungi and Phosphate-solubilizing Bacteria on Maize Growth and Phosphorus Uptake in Low Phosphorous Field. Chin. J. Soil Sci. 2017, 48, 922–929. [Google Scholar]
- Li, Y.B.; Li, Y.L.; Guan, G.H.; Cheng, S.F. Screening, Identification of Plant Growth Promoting Rhizobacteria and Its Effect on Reducing Fertilization While Increasing Efficiency in Wheat (Triticum aestivum). J. Agric. Biotechnol. 2020, 28, 1471–1476. [Google Scholar]
- Li, Y.L.; Wang, C.J.; Xu, B.M.; Zhang, X.G.; Wei, X.S.; Yang, S.H. Screening and Application of Suitable Symbiotic Combination Between Rhizobia and Soybean Cultivar Xudou 24. Soybean Sci. 2020, 39, 612–620. [Google Scholar]
- Liu, C.Y.; Cheng, M.F.; Jiang, H.M.; Zhao, F.K.; Fan, B.Q. Isolation of a high effective antagonistic bacterial strain YC16 against Sclerotinia sclerotiorum diseases in sunflower. Acta Microbiol. Sin. 2020, 60, 273–284. [Google Scholar]
- Liu, P.; Tian, Y.Z.; Zhong, Y.J.; Liao, H. Isolation and Application of Effective Rhizobium Strains in Peanut on Acidic Soils. Sci. Agric. Sin. 2019, 52, 3393–3403. [Google Scholar]
- Liu, Q.; Su, G.D.; Luo, X.Y.; Jiang, Y.P.; Cai, J.Z.; Yang, B.Y.; Yang, J.Q. The influence of applying mutant strain of Azospirllum braslense (CWV-22) on crop matter production and nitrogen circulation. J. Hunan Agric. Univ. (Nat. Sci.) 1991, 17, 419–426. [Google Scholar]
- Liu, R.J.; Li, M. Field Tests of Arbuscular mycorrhizal Fungi Applied as a Biological Fertilizer. J. Laiyang Agric. Coll. 2001, 18, 81–84. [Google Scholar]
- Liu, R.J.; Li, M.; Shi, Z.Y.; Han, Y.Z.; Li, X.L. Effect of Arbuscular mycorrhizal fungi on yield of peanut and sweet potato. Chin. J. Eco-Agric. 2003, 14, 42–43. [Google Scholar]
- Lu, L.W.; Lu, W.X.; Wang, X.; Wu, R.D.; Tan, Y.M.; Wei, Q.L.; Zhang, J.L.; Cheng, T.S. Influence of Arbuscular mycorrhizal fungi inoculation on sugarcane species “Liucheng 07-500” grown in field. Sugar Crops China 2016, 38, 5–7. [Google Scholar]
- Lu, Y.F.; Qiao, C.C.; Xu, H.; Gao, K.Y.; Li, R.; Sheng, Q.R. Development of Compound Microbial Liquid Fertilizer Containing Bacillus amyloliquefaciens SQR9 and Its Plant Growth Promotion Effect. Chin. J. Soil Sci. 2018, 49, 1150–1156. [Google Scholar]
- Luo, X.C.; Wang, Q.; Zhang, D.Y.; Huang, Y.H.; Kang, Z.C.; Shi, X.Y.; Zhu, J.Q.; Yang, Y.Z. Isolation of phosphate-solubilizing bacteria and its application in integrated rice-crawfish cultivation system. Jiangsu Agric. Sci. 2022, 50, 205–211. [Google Scholar]
- Lv, B.; Ding, L.; Guo, C.; Cheng, F.; Zhou, H.P.; Wang, X.S.; Dong, X.L.; Xiang, F.Y. Effects of compound microbial fertilizer on soil nutrients and rhizosphere bacterial community in cotton field. Crops 2024, 40, 209–215. [Google Scholar]
- Ma, Y.Q.; Wei, S.; Mao, Z.C.; Yang, Y.H.; Feng, D.X.; Xie, B.Y. Effects of bioorganic fertilizers with compound microbes on cucumber and root-knot nematode. Sci. Agric. Sin. 2016, 49, 2945–2954. [Google Scholar]
- Mei, P.P.; Wang, P.; Li, L.; Zhang, X.; Gu, L.G.; Huang, J.C. Construction of efficient nitrogen-fixing cropping pattern: Maize/faba bean intercrop with Rhizobium inoculation in reclaimed low-fertility soils. Chin. J. Eco-Agric. 2018, 26, 62–74. [Google Scholar]
- Qiao, C.C.; Wang, T.T.; Wang, R.F.; Liu, C.; Gao, Q.; Li, R.; Sheng, Q.R. Screening phosphate solubilizing bacterial strains from maize rhizosphere and research on their plant growth promotion effect. J. Nanjing Agric. Univ. 2017, 40, 664–670. [Google Scholar]
- Qiao, Z.W.; Hong, J.P.; Li, L.X.; Liu, C. Effect of phosphobacterias on nutrient, enzyme activities and phosphorus adsorption-desorption characteristics in a reclaimed soil. J. Soil Water Conserv. 2017, 31, 166–170+203. [Google Scholar]
- Shi, A.; Li, Q.; Huang, J.; Yuan, L. Influence of Arbuscular mycorrhizal Fungi on Growth, Mineral Nutrition and Chlorogenic Acid Content of Lonicera confusa Seedlings Under Field Conditions. Pedosphere 2013, 23, 333–339. [Google Scholar] [CrossRef]
- Shi, H.W.; Li, Y.B.; Li, P.F.; Wang, Z.M.; Cheng, S.F. Effect of nitrogen-fixing Paenibacillus spp. on wheat yield. J. China Agric. Univ. 2016, 21, 52–55. [Google Scholar]
- Shi, J.; Gao, Y.; Sun, Y.F.; Zhang, Z.H.; Liu, S.X.; Liu, Y.F. Inoculating Sinorhizobium meliloti SD101 Improving Nodule Number and Yield of Native Alfalfa. Chin. Agric. Sci. Bull. 2016, 32, 22–26. [Google Scholar]
- Song, F.Q.; Chen, J.; Chang, W.; Wang, C.X.; Kong, X.S.; Wang, J. The Impact of AM fungi on soybean growth with AM inoculum addition in Field. Chin. Agric. Sci. Bull. 2013, 29, 69–74. [Google Scholar]
- Sun, T.; Liu, Y.; Wu, S.; Zhang, J.; Qu, B.; Xu, J. Effects of background fertilization followed by co-application of two kinds of bacteria on soil nutrient content and rice yield in Northeast China. Int. J. Agric. Biol. Eng. 2020, 13, 154–162. [Google Scholar] [CrossRef]
- Tian, J. Application of rhizobium mixing technology for soybean production increase and fertilizer saving in Dengta Area. Mod. Agric. 2023, 53, 48–50. [Google Scholar]
- Tu, X.P.; Li, Z.X.; Song, Y. Effect of Effective Microorganisms on Rice Cultivation. J. Hubei Agric. Coll. 2000, 20, 298–300. [Google Scholar]
- Wang, C.; Cui, J.; Yang, L.; Zhao, C.; Wang, T.; Yan, L.; Liu, S. Phosphorus-Release Dynamics by Phosphate Solubilizing Actinomycetes and its Enhancement of Growth and Yields in Maize. Int. J. Agric. Biol. 2018, 20, 437–444. [Google Scholar] [CrossRef]
- Wang, H.L.; Liu, X.T.; Hou, X.N.; Si, H.L.; Yang, J.M.; Zhang, X.J. Effect of Antimicrobial Agents on Rice Yields. Ningxia J. Agric. For. Sci. Technol. 2020, 61, 18–20. [Google Scholar]
- Wang, H.Y.; Luo, J.J.; Wang, K.; Wang, R.N.; Wang, X.; Gao, G.R.; Fang, Y. Effects of different microbial agents on the disease prevention effect and yield of beetroot rot. Xinjiang Agric. Sci. 2024, 61, 448–454. [Google Scholar]
- Wang, H.Z.; Zhang, Z.W.; Jia, Q.H.; Zhang, L.Q.; Li, Z.Y.; Yang, L.Y. Infection potential and effects of VAM fungi on corn with seedling inoculation. Southwest China J. Agric. Sci. 2001, 1, 25–28. [Google Scholar]
- Wang, N.; Shi, Y.W.; Niu, X.X.; Yang, H.M.; Chu, M.; Zhan, F.Q.; Bao, H.F.; Yang, R.; Long, X.Q.; Ding, R.R. Inoculation technology and field application of cotton rhizosphere phosphorus solubilizing P. taiwanensis WJP-7. Xinjiang Agric. Sci. 2023, 60, 1263–1270. [Google Scholar]
- Wang, P.C.; Jin, G.H.; Zhang, C.Y.; Li, X. Biological control and growth promoting effect of potato common scab with different biocontrol agents and application method. Southwest China J. Agric. Sci. 2022, 35, 797–803. [Google Scholar]
- Wang, Q.L.; Huo, X.X.; Zhang, H.; Huang, Y.H.; Hao, Y.R.; Li, Z.; Zheng, Z.H.; Guo, K. Isolation, identification and field application of soybean rhizobia in saline-alkali land of the yellow river delta. Shandong Agric. Sci. 2023, 55, 143–151. [Google Scholar]
- Wang, X.; Cao, J.; Sun, R.; Liu, W.; Qi, L.; Song, P.; Yang, S. Improving dryland maize productivity and water efficiency with heterotrophic ammonia-oxidizing bacteria via nitrification and cytokinin activity. Crop J. 2024, 12, 880–887. [Google Scholar] [CrossRef]
- Wang, X.; Song, J.; Li, D.P.; Liu, Z.L.; Che, J.L.; Cheng, T.S. AMF and DSE: Effects on the Growth of Ginger in Field. Chin. Agric. Sci. Bull. 2021, 37, 62–67. [Google Scholar]
- Wang, X.B.; Qian, N.; Wang, X.L.; Cheng, L.; Zhang, H.M.; Wang, H.C.; Memon, S.P. Preparation and application of compound Bacillus sp. water dispersible granules with disease resistance and growth promoting Activity. Microbiology 2020, 47, 4349–4358. [Google Scholar]
- Wei, L.; Liang, Z.H.; Cheng, Y.R.; Cheng, Y.Q.; Wang, X.H.; Zhang, Y. Effects of Trichoderma harzianum TUV-13 on Growth of Houttuynia cordata and Southern Blight Disease Caused by Sclemtium roifsii. Chin. J. Biol. Control 2012, 28, 381–386. [Google Scholar]
- Wei, X.Y.; Lin, Y.; Cheng, T.; Tao, Z.X.; Zhao, H.Y.; Lin, S.; Lin, W.X. Effects of plant growth-promoting rhizobacteria on alleviating consecutive monoculture problem of Pseudostellaria heterophylla under field conditions. Oecologia 2018, 37, 399–408. [Google Scholar]
- Weng, C.Y.; Gao, Q.; Zhang, Y.; Li, R.; Sheng, Q.R. Development of Biological Matrix Produced by PGPR Strain LZ-8 and Analysis for Its Growth Promoting Effect. Soils 2016, 48, 414–417. [Google Scholar]
- Wu, F.; Li, Y.; Lu, Z.J.; Wang, M.; Sheng, Y.T. Field Control Effect of Six Antagonistic Bacteria against Bacterial Fruit Spot of Hami Melon. J. Seed Ind. Guide 2019, 39, 19–24. [Google Scholar]
- Wu, R.D.; Tan, C.L.; Li, T.H.; Lei, C.H.; Wei, J.F.; Yu, F.L.; Wang, X.; Tang, Y.M.; Cheng, T.S. Influence of inoculation of Arbuscular mycorrhizal Fungi on Sugarcane Variety Funong 41 Grown in Field. Sugarcane Canesugar 2015, 44, 20–23. [Google Scholar]
- Wu, R.D.; Wei, J.F.; Long, Y.Y.; Lei, C.H.; Tan, Y.M.; Li, T.H.; Cheng, T.S. Effect of Inoculation of Arbuscular mycorrhizal Fungi on Ratoon Sugarcane Grown in Field. Southwest China J. Agric. Sci. 2016, 29, 2648–2652. [Google Scholar]
- Wu, X.Q.; Zhao, Z.J.; Li, Z.; Hu, J.D.; Zhao, X.Y.; Wang, Y.L.; Huang, Y.J.; Li, J.S.; Yang, H.T. Impact of Trichoderma wettable powder application on winter wheat field growth. Shandong Sci. 2015, 28, 35–42. [Google Scholar]
- Xie, G.L.; Zhang, Z.H.; Wu, L.T.; Liu, J.W.; Xu, Q.J.; Fu, K.J.; Nie, Q.; Zhang, J.L.; Lin, C.; Cheng, W.H.; et al. Effect of biochar application combined with microbial agent on prevention and control of Plasmodiophora brassicae of Chinese cabbage. Southwest China J. Agric. Sci. 2023, 36, 105–111. [Google Scholar]
- Xie, Y.X.; Zeng, Q.B.; Yang, J.W.; Zhao, C.; Li, B.; Kang, X.; Cheng, W.M.; Feng, W.Q.; Cheng, Q.; Yu, X.M. Effects of growth-promoting rhizobacteria on quality and yield of flue-cured tobacco. Tob. Sci. Technol. 2017, 50, 14–21+30. [Google Scholar]
- Yan, F.F.; Kong, C.X.; Zhang, Y.J.; Mao, M.; Jian, L.J.; Wang, R. Biological Control of Tobacco Root-knot Nematode Disease by Penicillium purpurogenum K1. Chin. Agric. Sci. Bull. 2022, 38, 103–108. [Google Scholar]
- Yang, F.S.; Wang, Y.B.; Sun, C.; He, J.; Wang, S.J.; Ma, Y.K.; Fu, H.Y.; Liu, C.G. Highly Efficient Degradation Bacteria: Remediation Effect on Soil Polluted by Fomesafen. Chin. Agric. Sci. Bull. 2020, 36, 68–73. [Google Scholar]
- Yang, H.K.; Xu, C.T.; Liu, M.J.; Jiang, G.P. Effects of Different Microbial Agents Mixed with Tobacco Seedling-Nursing Substrates on Disease Resistance and Yield and Quality of Tobacco. Plant Dr. 2019, 32, 44–51. [Google Scholar]
- Yang, H.Y. The effect of combined application of microbial agents and chemical agents on the growth, occurrence of soft rot disease, and yield of konjac in Baoshan, Yunnan. Appl. Eng. Technol. 2023, 43, 28–30. [Google Scholar]
- Yang, Q.F.; Du, Y.H.; Zhao, W.J. Effects of different nitrogen treatment on application effect of Rhizobium arachis. J. Anhui Agric. Sci. 2013, 41, 8860–8861. [Google Scholar]
- Yang, T.; Hu, J.Y.; Lin, B.; Xiang, M.C. Field experiment of multi-microbial agents against root-knot nematode meloidogyne incognita on Lettuce. Chin. J. Biol. Control 2017, 33, 826–832. [Google Scholar]
- Yang, Z.G.; Ye, Y.J.; Chang, H.W.; Zuo, M.H.; Yu, X.X.; Hu, S.H. Effects of Microbial Fertilizer and Soil Amendment on the Growth, Quality and Yield of Dry Pepper. North. Hortic. 2020, 44, 1–7. [Google Scholar]
- Yang, Z.H.; Liu, Y.; Yang, Y.F.; Li, S.D.; Guo, R.J.; Lu, X.H.; Sun, M.H.; Luo, M. Control efficacy of Purpureocillium lilacinum and its compound formulations on root-knot nematode in tomato field. Plant Prot. 2024, 50, 118–125. [Google Scholar]
- Yin, J.; Yuan, L. Phytophthora disease control and growth promotion of pepper by Pythium oligandrum. Hortic. Plant J. 2017, 44, 2327–2337. [Google Scholar]
- Yu, Y.Y.; Xu, J.D.; Gao, M.Z.; Huang, T.X.; Zheng, Y.; Zhang, Y.Y.; Wang, Y.P.; Luo, Y.M.; Zhang, Y.; Hu, Y.H.; et al. Exploring plant growth promoting rhizobacteria potential for green agriculture system to optimize sweet potato productivity and soil sustainability in northern Jiangsu, China. Eur. J. Agron. 2023, 142, 126661. [Google Scholar] [CrossRef]
- Zeng, Q.B.; Zhang, Y.Y.; Cai, Y.; Ye, X.X.; Zhang, R.P.; Yang, J.W. Effect of Microbial Fertilizer on Agronomic and Economic Characters of Tobacco in Seedbed. J. Agric. 2017, 7, 52–56. [Google Scholar]
- Zeng, Z.H.; Sui, X.H.; Hu, Y.G.; Cheng, D.M.; Cheng, W.X.; Gao, R.L. Screening of highly-effective Sinorhizobium meliloti strains for Medicago sativa cultivars and their field inoculation. Acta Pratacult. Sin. 2004, 13, 95–100. [Google Scholar]
- Zhang, D.M.; Gao, Z.J.; Liu, D.; Gao, W.; Liu, M.X.; Liang, R.P.; Zhang, D.X. Field Efficacy Experiment of Microbial Agent on Potato. Chin. Agric. Sci. Bull. 2017, 33, 88–92. [Google Scholar]
- Zhang, J.H.; Cao, C.L.; Hao, J.L.; Bai, W.B.; Cao, J. Degradation characteristics of nicosulfuron degrading strain DT-4 and its mitigation effect on Sorghum phytotoxicity. Chin. J. Biol. Control 2022, 38, 1252–1260. [Google Scholar]
- Zhang, N.; Huang, Y.; Xu, X.; Zhang, B.; Deng, X.H.; Wang, D.S.; Tao, C.Y.; Wang, Q.Z.; Li, R.; Sheng, Q.R. Effects of seedlings colonized PGPR stains on bacterial wilt disease suppression and yield of tomato. Soils 2019, 51, 658–664. [Google Scholar]
- Zhang, S.; Wang, L.; Ma, F.; Zhang, X.; Fu, D. Arbuscular mycorrhiza improved phosphorus efficiency in paddy fields. Ecol. Eng. 2016, 95, 64–72. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Ma, F.; Zhang, X.; Li, Z.; Li, S.; Jiang, X. Can Arbuscular mycorrhiza and fertilizer management reduce phosphorus runoff from paddy fields? J. Environ. Sci. 2015, 33, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Ren, S.J.; Zhang, Y.; Li, Q.Z.; Wang, F.Q. The effect of increase soybean production by silicate bacterium inoculant. J. Heilongjiang Bayi Agric. Univ. 2000, 12, 36–39. [Google Scholar]
- Zhang, Y.; Wang, T.T.; Sun, Y.H.; Hu, G.M.; Li, R.; Yu, P.; Sheng, Q.R. Screening of Plant Growth-Promoting Rhizobacteria from Watermelon and Development of Bio-nursery Substrates. Acta Pedol. Sin. 2017, 54, 703–712. [Google Scholar]
- Zhao, F.L.; Xu, B.; Zhang, H.; Sun, S.R.; Wang, X.M. Effect of fast-growing Rhizobium japonicum on yield of soybean. J. Microbiol. 1989, 12, 30–34. [Google Scholar]
- Zhao, J.B.; Yang, X.H.; Zhang, Y.Y.; Bie, D.X.; You, S.F. Mycorrhizal colonization and yield potential of Brassica napus L. J. Southwest Univ. (Nat. Sci.) 2011, 33, 88–92. [Google Scholar]
- Zhao, W.S.; Guo, Q.G.; Zhang, X.Y.; Wang, B.B.; Su, Z.H.; Hu, Q.; Lu, X.Y.; Ma, P.; Li, S.Z. Development of Microbial Agent Bacillus amyloliquefaciens PHODG36 and Its Effect on Disease Control and Yield Increase of Potato. Chin. J. Biol. Control 2020, 36, 381–387. [Google Scholar]
- Zhou, S.X.; Zhang, L.X.; Lu, X.; Li, X.Y.; Li, Y. Biocontrol Effects of Ginseng Rust Rot by Two Strains of Streptomyces spp. J. Anhui Agric. Sci. 2012, 40, 803–804+806. [Google Scholar]
- Zhou, T.; Cheng, Y.X.; Zhou, Y.L.; Li, K.Q.; Wang, P.; Jiang, P.; Xu, K.M. Screening and preliminary application of high efficient soybean rhizobia strains in Sichuan province. J. Plant Nutr. Fertil. 2012, 18, 227–233. [Google Scholar]
- Zhu, S.R.; Tian, F.; Chao, J.; Cheng, Q.F.; Tian, M.C.; Dai, J.P.; Liu, J.Y.; Zhang, Z.H. Research on Application Effect of Photosynthetic Bacteria in Tobacco Planting. Hunan Agric. Sci. 2016, 46, 53–54+58. [Google Scholar]
- Zhu, Y.; Lv, G.; Chen, Y.; Gong, X.; Peng, Y.; Wang, Z.; Ren, A.; Xiong, Y. Inoculation of Arbuscular mycorrhizal fungi with plastic mulching in rainfed wheat: A promising farming strategy. Field Crops Res. 2017, 204, 229–241. [Google Scholar] [CrossRef]
- Li, S. Agroclimatic regionalization of China. J. Nat. Resour. 1987, 2, 71–83. [Google Scholar]
- Yan, F.; Zhao, H.; Wu, L.; Huang, Z.; Niu, Y.; Qi, B.; Zhang, L.; Fan, S.; Ding, Y.; Li, G.; et al. Basic Cognition of Melatonin Regulation of Plant Growth under Salt Stress: A Meta-Analysis. Antioxidants 2022, 11, 1610. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Huedo-Medina, T.B.; Sánchez-Meca, J.; Marín-Martínez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef]
- Qi, M.; Wang, Y.; Zhang, Y.; Feng, Y.; Liu, B. Potential neural mechanisms of acupuncture therapy on migraine: A systematic review and activation likelihood estimation meta-analysis update. Quant. Imaging Med. Surg. 2025, 15, 1653–1668. [Google Scholar] [CrossRef]
- Fan, W.; Zhao, R.; Liu, X.; Ge, L. Intelligent Robot Interventions for People with Dementia: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Med. Internet Res. 2025, 27, e59892. [Google Scholar] [CrossRef]
- Rosenberg, M.S. MetaWin 3: Open-source software for meta-analysis. Front. Bioinform. 2024, 4, 1305969. [Google Scholar] [CrossRef]
- Liu, F.; Xiao, X.; Qin, Y.; Yan, H.; Huang, J.; Wu, X.; Zhang, Y.; Zou, Z.; Doughty, R.B. Large spatial variation and stagnation of cropland gross primary production increases the challenges of sustainable grain production and food security in China. Sci. Total Environ. 2022, 811, 151408. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.T.; Kou, C.L.; Christie, P.; Dou, Z.X.; Zhang, F.S. Changes in the soil environment from excessive application of fertilizers and manures to two contrasting intensive cropping systems on the North China Plain. Environ. Pollut. 2007, 145, 497–506. [Google Scholar] [CrossRef]
- Ren, C.; Jin, S.; Wu, Y.; Zhang, B.; Kanter, D.; Wu, B.; Xi, X.; Zhang, X.; Chen, D.; Xu, J.; et al. Fertilizer overuse in Chinese smallholders due to lack of fixed inputs. J. Environ. Manag. 2021, 293, 112913. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, F.; Ahmad, Z.; Ul Hassan, M.; Wang, R.; Diao, X.; Li, X. Adaptation of Foxtail Millet (Setaria italica L.) to Abiotic Stresses: A Special Perspective of Responses to Nitrogen and Phosphate Limitations. Front. Plant Sci. 2020, 11, 187. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef]
- Serrano-Carreón, L.; Aranda-Ocampo, S.; Balderas-Ruíz, K.A.; Juárez, A.M.; Leyva, E.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Galindo, E. A case study of a profitable mid-tech greenhouse for the sustainable production of tomato, using a biofertilizer and a biofungicide. Electron. J. Biotechnol. 2022, 59, 13–24. [Google Scholar] [CrossRef]
- Kotopoulou, S.; Zampelas, A.; Magriplis, E. Dietary nitrate and nitrite and human health: A narrative review by intake source. Nutr. Rev. 2022, 80, 762–773. [Google Scholar] [CrossRef]
- Ali, S.M.S.; Soliman, H.; Abdallah, A.; Moharram, T.; Ahmed, S. Biofertilizers and their significance to environmental and sustainable agriculture. New Biotechnol. 2009, 25, S308. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Dragičević, V.; Simić, M.; Dolijanović, Ž.; Đorđević, S.; Stoiljković, M.; Dimkić, I.; Brankov, M. Combined effect of cover crops and bio-fertilizer on sustainable popcorn maize production. Front. Plant Sci. 2023, 14, 1250903. [Google Scholar] [CrossRef]
- Gong, M.; Wang, Y.; Bai, N.; Zhang, Q.; Kunkun, L.; Zhang, H. Co-inoculation of Potassium Solubilizing Bacteria and Rhizophagus irregularis Promotes the Growth and Potassium Accumulation of Robinia pseudoacacia L. Seedlings. Curr. Microbiol. 2025, 82, 142. [Google Scholar] [CrossRef]
- Sanadhya, S.; Jain, D.; Saheewala, H.; Sharma, D.; Chauhan, P.K.; Singh, G.; Upadhyay, S.K.; Mohanty, S.R. Efficacy of molecularly diversified phosphorus-solubilizing rhizobacterial isolates in phytostimulation, antimicrobial attributes and phosphorus-transporter genes mediated plant growth performance in maize (Zea mays L.). Plant Physiol. Biochem. 2025, 220, 109521. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Z.; Song, Z.; Wang, F.; Tian, D.; Mi, W.; Huang, X.; Wang, J.; Song, L.; Yang, Z.; et al. Vital roles of soil microbes in driving terrestrial nitrogen immobilization. Glob. Change Biol. 2021, 27, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, B.; Hao, Y.; Lu, M.; Ding, D.; Niu, S.; Xiang, H.; Huang, Z.; Li, J. Two-stage inoculation with lignocellulose-degrading microorganisms in composting: Enhanced humification efficiency and underlying mechanisms. Environ. Res. 2025, 271, 120906. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhang, Y.; Hao, Y.; Lu, M.; Xiang, H.; Ding, D.; Niu, S.; Li, K.; Li, J.; Huang, Z. Insights into nitrogen metabolism and humification process in aerobic composting facilitated by microbial inoculation. Environ. Res. 2025, 269, 120894. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, J.; Feng, Y.; Zhou, C.; Li, X.; Yan, X.; Ruan, R.; Cheng, P. Microalgae-based biofertilizers improve fertility and microbial community structures in the soil of potted tomato. Front. Plant Sci. 2024, 15, 1461945. [Google Scholar] [CrossRef]
- Nabati, J.; Nezami, A.; Yousefi, A.; Oskoueian, E.; Oskoueian, A.; Ahmadi-Lahijani, M.J. Biofertilizers containing plant growth promoting rhizobacteria enhance nutrient uptake and improve the growth and yield of chickpea plants in an arid environment. Sci. Rep. 2025, 15, 8331. [Google Scholar] [CrossRef]
- Ayed, F.; Aydi Ben Abdallah, R.; Ben Khedher, S.; Jabnoun-Khiareddine, H.; Daami-Remadi, M. Biocontrol of Agroathelia rolfsii associated with stem rot disease in tomato (Solanum lycopersicum L.) and growth promotion using compost-associated actinobacteria. Braz. J. Microbiol. 2025. [Google Scholar] [CrossRef]
- Batista, B.D.; Dourado, M.N.; Figueredo, E.F.; Hortencio, R.O.; Marques, J.P.R.; Piotto, F.A.; Bonatelli, M.L.; Settles, M.L.; Azevedo, J.L.; Quecine, M.C. The auxin-producing Bacillus thuringiensis RZ2MS9 promotes the growth and modifies the root architecture of tomato (Solanum lycopersicum cv. Micro-Tom). Arch. Microbiol. 2021, 203, 3869–3882. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen Biocontrol Using Plant Growth-Promoting Bacteria (PGPR): Role of Bacterial Diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef]
- Lopes, M.; Cardoso, A.F.; Dias-Filho, M.B.; Gurgel, E.S.C.; da Silva, G.B. Brazilian Amazonian microorganisms: A sustainable alternative for plant development. Aims Microbiol. 2025, 11, 150–166. [Google Scholar] [CrossRef]
- Manjunatha, B.S.; Nivetha, N.; Krishna, G.K.; Elangovan, A.; Pushkar, S.; Chandrashekar, N.; Aggarwal, C.; Asha, A.D.; Chinnusamy, V.; Raipuria, R.K.; et al. Plant growth-promoting rhizobacteria Shewanella putrefaciens and Cronobacter dublinensis enhance drought tolerance of pearl millet by modulating hormones and stress-responsive genes. Physiol. Plant. 2022, 174, e13676. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-Tolerant Plant Growth-Promoting Rhizobacteria Associated with Foxtail Millet in a Semi-arid Agroecosystem and Their Potential in Alleviating Drought Stress. Front. Microbiol. 2017, 8, 2580. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Lian, Z.; Lyu, X.; Yan, C.; Yan, S.; Gong, Z.; Li, S.; Ma, C. Nitrate Inhibits Nodule Nitrogen Fixation by Accumulating Ureide in Soybean Plants. Plants 2024, 13, 2045. [Google Scholar] [CrossRef] [PubMed]
- Paliya, S.; Mandpe, A.; Kumar, S.; Kumar, M.S. Enhanced nodulation and higher germination using sludge ash as a carrier for biofertilizer production. J. Environ. Manag. 2019, 250, 109523. [Google Scholar] [CrossRef]
- Debray, R.; Herbert, R.A.; Jaffe, A.L.; Crits-Christoph, A.; Power, M.E.; Koskella, B. Priority effects in microbiome assembly. Nat. Rev. Microbiol. 2022, 20, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Rensing, C.; Zhang, X. Early inoculation and bacterial community assembly in plants: A review. Microbiol. Res. 2025, 296, 128141. [Google Scholar] [CrossRef]
- Thakur, R.; Dhar, H.; Mathew, S.; Gulati, A. PGPR inoculants journey from lab to land: Challenges and limitations. Microbiol. Res. 2024, 289, 127910. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
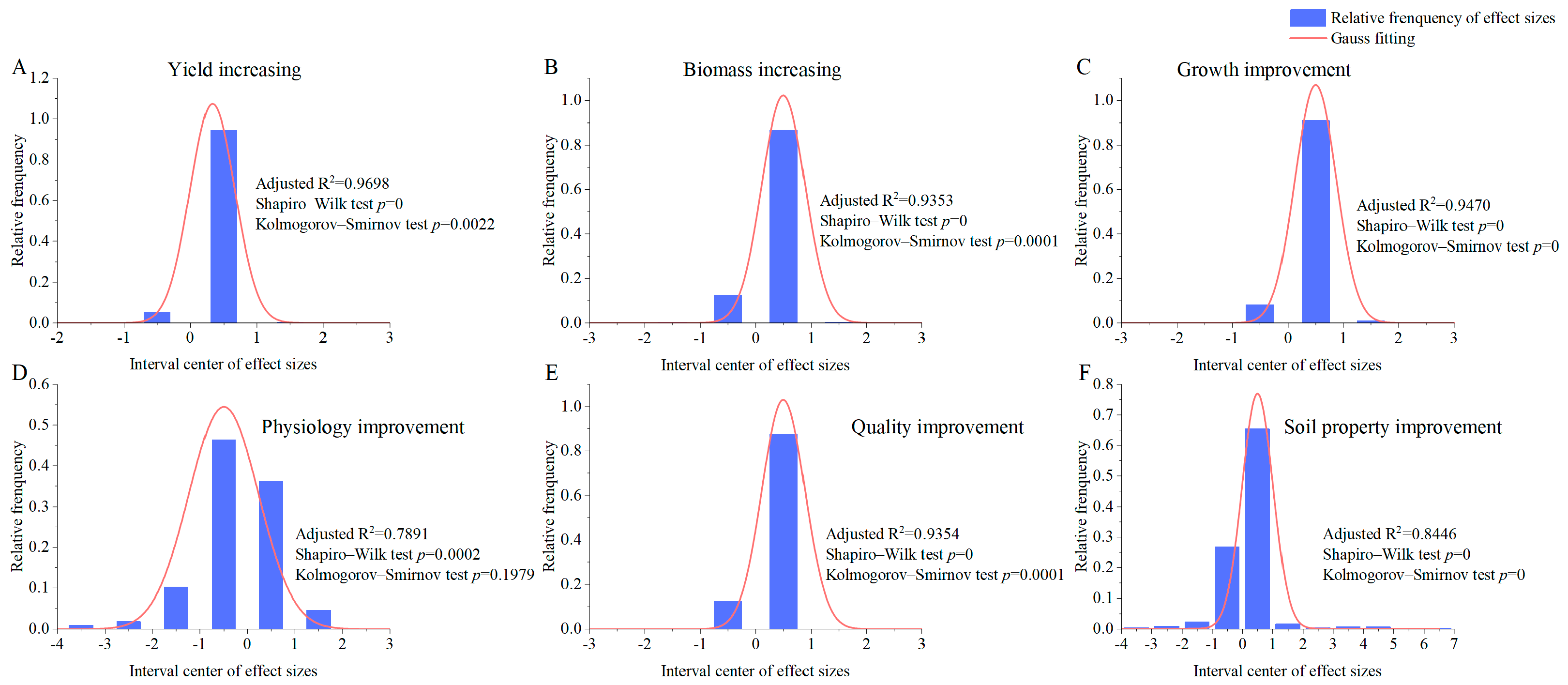

| Biofertilizer Effect | N | 5N + 10 | Rosenthal’s Nfs | p Value of Egger’s Regression |
|---|---|---|---|---|
| Yield increasing | 376 | 1890 | 68,727.17 | 0.1985 |
| Biomass increasing | 351 | 1765 | 55,749.10 | 0.8089 |
| Growth promotion | 341 | 1715 | 72,679.67 | 0.1014 |
| Physiology enhancement | 108 | 550 | 1676.32 | 0.1188 |
| Quality promotion | 235 | 1185 | 7015.59 | 0.0614 |
| Soil property improvement | 415 | 2085 | 3669.21 | 0.7107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, B.; Liu, T.; Xue, Z.; Cao, J.; Zhang, Y.; Yu, M.; Liu, E.; Xing, J.; Wang, F.; Ren, X.; et al. Effects of Biofertilizer on Yield and Quality of Crops and Properties of Soil Under Field Conditions in China: A Meta-Analysis. Agriculture 2025, 15, 1066. https://doi.org/10.3390/agriculture15101066
Pei B, Liu T, Xue Z, Cao J, Zhang Y, Yu M, Liu E, Xing J, Wang F, Ren X, et al. Effects of Biofertilizer on Yield and Quality of Crops and Properties of Soil Under Field Conditions in China: A Meta-Analysis. Agriculture. 2025; 15(10):1066. https://doi.org/10.3390/agriculture15101066
Chicago/Turabian StylePei, Baolei, Ting Liu, Ziyan Xue, Jian Cao, Yunpeng Zhang, Mulan Yu, Engang Liu, Jincheng Xing, Feibing Wang, Xuqin Ren, and et al. 2025. "Effects of Biofertilizer on Yield and Quality of Crops and Properties of Soil Under Field Conditions in China: A Meta-Analysis" Agriculture 15, no. 10: 1066. https://doi.org/10.3390/agriculture15101066
APA StylePei, B., Liu, T., Xue, Z., Cao, J., Zhang, Y., Yu, M., Liu, E., Xing, J., Wang, F., Ren, X., & Zhang, Z. (2025). Effects of Biofertilizer on Yield and Quality of Crops and Properties of Soil Under Field Conditions in China: A Meta-Analysis. Agriculture, 15(10), 1066. https://doi.org/10.3390/agriculture15101066








