Effects of Exogenous Application of Phenolic Acid on Soil Nutrient Availability, Enzyme Activities, and Microbial Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Site Description
2.2. Sampling and Analyses
2.3. Soil Microorganism Analysis
2.4. Data Analysis
3. Results
3.1. The Impact of Various Treatments on the Chemical Characteristics of the Soil
3.2. Impact of Various Treatments on Soil Extracellular Enzyme Activity
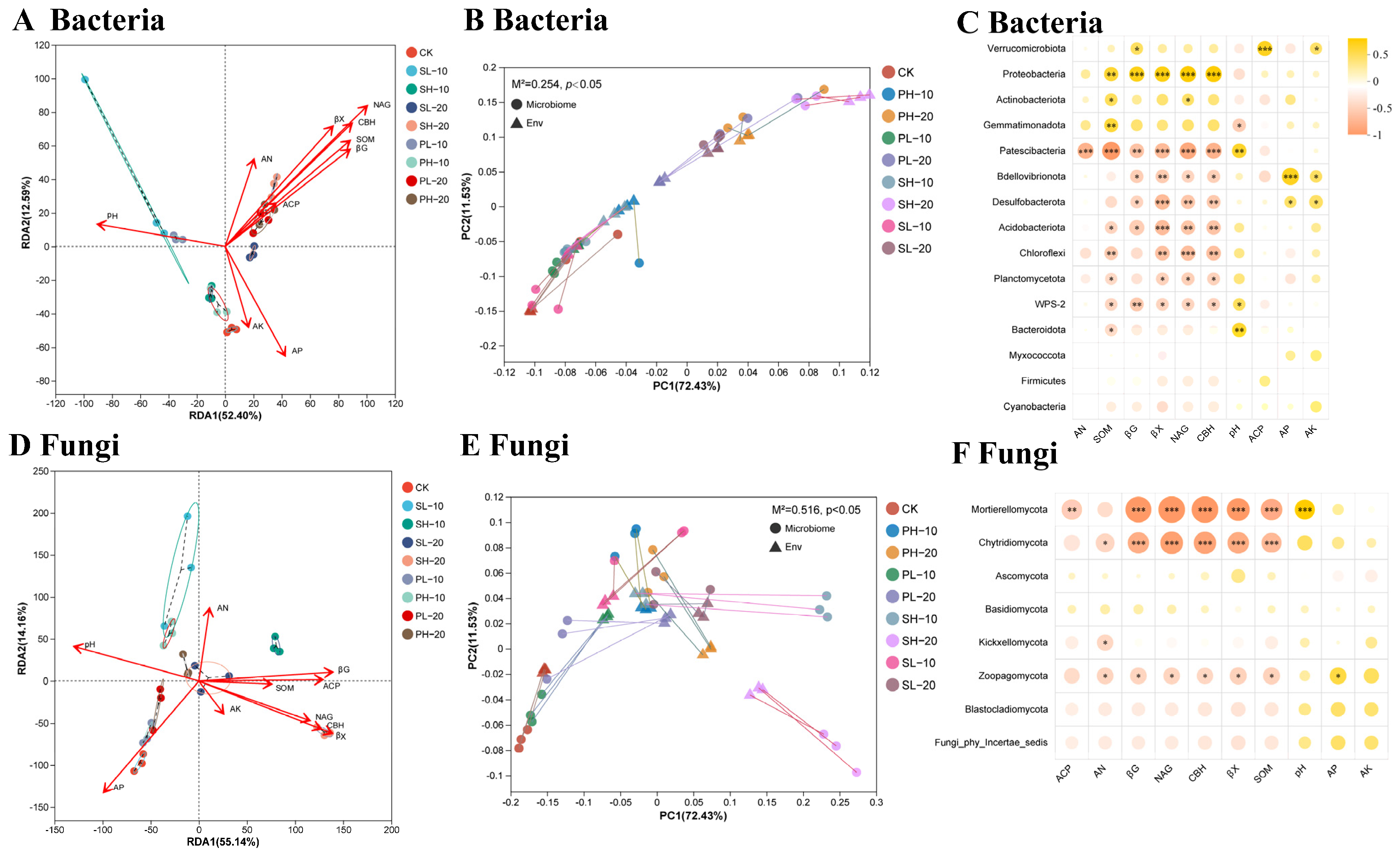
3.3. Effect of Different Treatments Onsoil Microorganisms
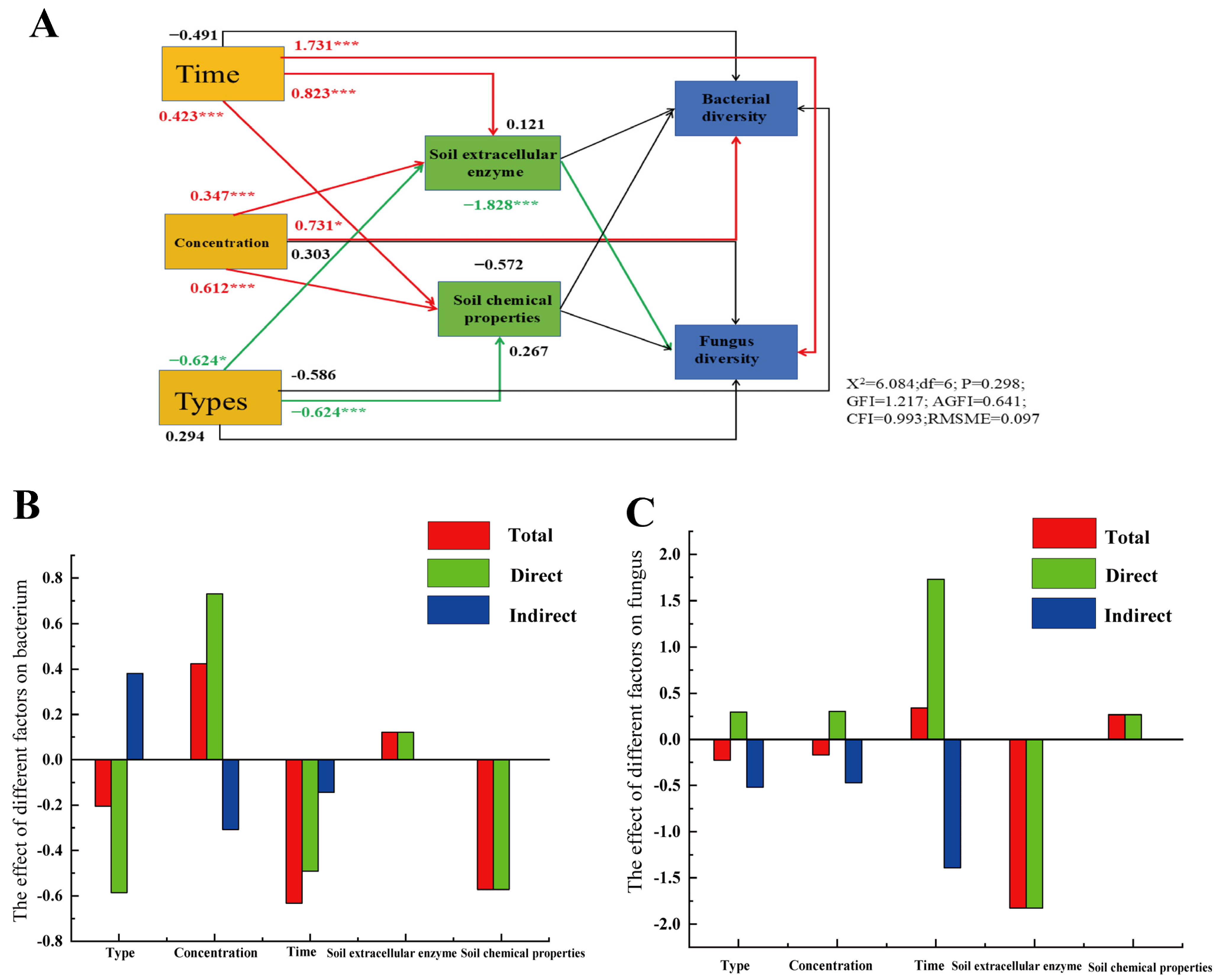
3.4. The Connection Between Soil Characteristics and Microorganisms Across Various Treatments
4. Discussion
4.1. Influence of Various Treatments on Chemical Characteristics of Soil
4.2. Effect of Different Treatments on Soil Microorganisms
4.3. The Relationship Between Soil Properties and Microbial Communities Under Various Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.L. Plant Nutrition, 2nd ed.; China Agriculture University Press: Beijing, China, 2003. [Google Scholar]
- Tian, L.; Hu, S.; Wang, X.; Guo, Y.; Huang, L.; Wang, L.; Li, W. Antagonism of Rhizosphere Streptomyces yangpuensis CM253 against the Pathogenic Fungi Causing Corm Rot in Saffron (Crocus sativus L.). Pathogens 2022, 11, 1195. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, N.; Scheu, S.; Jousset, A. Bacterial diversity stabilizes community productivity. PLoS ONE 2012, 7, e34517. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Dong, K.; Yang, Z.X.; Dong, Y.; Tang, L. Advance in Mechanism of Continuous Cropping Obstacle. Soils 2016, 48, 1068–1076. (In Chinese) [Google Scholar]
- Yuan, J.; Zhao, J.; Wen, T.; Li, R.; Pim, R.; Huang, Q.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 156. [Google Scholar] [CrossRef]
- Hu, L.F.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Meng, Y.; Li, B.; Daniele, M.; Noemie, C.; Thomas, S. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, X.F.; Yang, T.J.; Petri Friman, V.; Geisen, S.; Wei, Z.; Xu, Y.C.; Jousset, A.; Shen, Q.R. Chemical structure predicts the effect of plant-derived low-molecular weight compounds on soil microbiome structure and pathogen suppression. Funct. Ecol. 2020, 34, 2158–2169. [Google Scholar] [CrossRef]
- Zheng, G.D.; Shi, L.B.; Wu, H.Y.; Peng, D.L. Nematode communities in continuous tomato-cropping field soil infested by root-knot nematodes. Acta Agric. Scand. Sect. B-Soil Plant 2012, 62, 216–223. [Google Scholar] [CrossRef]
- Liu, J.J.; Yao, Q.; Li, Y.S.; Zhang, W.; Mi, G.; Chen, X.L.; Yu, Z.H.; Wang, G.H. Continuous cropping of soybean alters the bulk and rhizospheric soil fungal communities in a Mollisol of Northeast, PR China. Land Degrad. Dev. 2019, 30, 1725–1738. [Google Scholar] [CrossRef]
- Sanguin, H.; Sarniguet, A.; Gazengel, K.; Moënne-Loccoz, Y.; Grundmann, G.L. Rhizosphere bacterial communities associated with disease suppressiveness stages of take-all decline in wheat monoculture. New Phytol. 2010, 184, 694–707. [Google Scholar] [CrossRef]
- Bai, Y.X.; Yang, C.C.; Shi, P.Y.; Jia, M.; Yang, H.W.; Xu, Z.L.; Wang, G. Correlation analysis of main environmental factors and phenolic acids in continuous tobacco cropping soils using Mantel Test. Chin. J. Eco-Agric. 2019, 27, 369–379. (In Chinese) [Google Scholar]
- Zhang, Y.; Zhang, W.Q.; Han, L.L.; Shi, X.; Hikichi, Y.; Ohnishi, K. Involvement of a PadR regulator PrhP on virulence of Ralstonia solanacearum by controlling detoxification of phenolic acids and type III secretion system. Mol. Plant Pathol. 2019, 20, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wu, F.Z.; Zhou, X.G. Different toxic effects of ferulic and p-hydroxybenzoic acids on cucumber seedling growth were related to their different influences on rhizosphere microbial composition. Biol. Fert. Soils 2020, 56, 125–136. [Google Scholar] [CrossRef]
- Wang, X.B.; Luo, Y.M.; Liu, W.X.; Li, Z.G. Identification of peanut root exudates and their allelopathic effects. Chin. J. Ecol. 2011, 30, 2803–2808. (In Chinese) [Google Scholar]
- Li, X.G.; De Boer, W.; Zhang, Y.N.; Ding, C.F.; Zhang, T.L.; Wang, X.X. Suppression of soil-borne Fusarium pathogens of peanut by intercropping with the medicinal herb Atractylodes lancea. Soil Biol. Biochem. 2018, 116, 120–130. [Google Scholar] [CrossRef]
- You, C.; Yang, T.J.; Zhou, X.G.; Wang, X.F.; Xu, Y.C.; Shen, Q.R.; Wei, Z. Research Advances on Mechanisms and Preventions of Soil-borne Diseases Exacerbated by Root Exudates in Continuous Cropping Systems. Acta Pedol. Sin. 2024, 61, 1201–1211. (In Chinese) [Google Scholar]
- Liu, Y.X.; Li, X.; Cai, K.; Lu, N.; Shi, J. Identification of benzoic acid and 3-phenylpropanoic acid in tobacco root exudates and their role in the growth of rhizosphere microorganisms. Appl. Soil Ecol. 2015, 93, 78–87. [Google Scholar] [CrossRef]
- Zhang, S.S.; Yang, X.M.; Huang, Q.W.; Xu, Y.C.; Shen, Q.R. Effect of application of amino acid fertilizer on biological properties of cucumber plants and soil microorganisms under continuous mono-cropping. Acta Pedol. Sin. 2007, 44, 689–694. (In Chinese) [Google Scholar]
- Asaduzzaman, M.; Asao, T. Autotoxicity in beans and their allelochemicals. Sci. Hortic-Amst. 2012, 134, 26–31. [Google Scholar] [CrossRef]
- Zhou, X.G.; Wu, F.Z. Effects of amendments of ferulic acid on soil microbial communities in the rhizosphere of cucumber (Cucumis sativus L.). Eur. J. Soil Biol. 2012, 50, 191–197. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agriculture Chemistry Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Xia, H.; Shen, J.; Riaz, M.; Zu, C.L.; Ye, F.; Yan, Y.F.; Liu, B.; Jiang, C.Q. Soil microbiological assessment on diversified annual cropping systems in China. J. Environ. Manag. 2024, 371, 123284. [Google Scholar] [CrossRef] [PubMed]
- Schnecker, J.; Wild, B.; Takriti, M.; Alves, R.J.E.; Gentsch, N.; Gittel, A.; Hofer, A.; Klaus, K.; Knoltsch, A.; Lashchinskiy, N.; et al. Microbial community composition shapes enzyme patterns in topsoil and subsoil horizons along a latitudinal transect in Western Siberia. Soil Biol. Biochem. 2015, 83, 106–115. [Google Scholar] [CrossRef] [PubMed]
- He, Y.S. Soil problems and countermeasure in facility agriculture in China. Soils 2004, 36, 235–242. (In Chinese) [Google Scholar]
- Yan, S.Y. Characteristics of soil nutrient and enzymes activity in Pinus elliottii forests at different ages. J. For. Environ. 2020, 40, 24–29. (In Chinese) [Google Scholar]
- Li, R.R.; Zheng, M.H.; Cui, X.Y.; Wang, Y.; Xu, C.C. Screening of Lactic acid bacteria and its effects on Fermentation characteristics of Alfalfa Silage. Chin. J. Grassl. 2021, 43, 97–104. (In Chinese) [Google Scholar]
- Xia, H.; Jiang, C.Q.; Riaz, M.; Yu, F.; Dong, Q.; Yan, Y.F.; Zu, C.L.; Zhou, C.Y.; Wang, J.T.; Shen, J. Impacts of continuous cropping on soil fertility, microbial communities, and crop growth under different tobacco varieties in a field study. Environ. Sci. Eur. 2025, 37, 5. [Google Scholar] [CrossRef]
- Emani, C.S.; Gallant, J.L.; Wiid, I.J.; Bake, B. The role of low molecular weight thiols in Mycobacterium tuberculosis. Tuberculosis 2019, 116, 44–55. [Google Scholar] [CrossRef]
- He, B.; Xue, G.; Zhang, X.Q.; Xu, X.J.; Yao, J.; Yang, T.Z. Analysis on Chemical Mechanism of Potassium Release Process from Soil as Influenced by Organic Acids. Soils 2015, 47, 74–79. (In Chinese) [Google Scholar]
- Mei, P.P.; Gui, L.G.; Wang, P.; Huang, J.C.; Long, H.Y.; Christie, P. Maize/faba bean intercropping with rhizobia inoculation enhances productivity and recovery of fertilizer p in a reclaimed desert soil. Field Crop Res. 2012, 130, 19–27. [Google Scholar] [CrossRef]
- Moore, J.A.M.; Jiang, J.; Patterson, C.M.; Mayes, M.A.; Classen, A.T. Interactions among roots, mycorrhizas and free-living microbial communities differentially impact soil carbon processes. J. Ecol. 2015, 103, 1442–1453. [Google Scholar] [CrossRef]
- Clarholm, M.; Skyllberg, U.; Rosling, A. Organic acid induced release of nutrients from metal-stabilized soil organic matter-the unbutton model. Soil Biol Biochem. 2015, 84, 168–176. [Google Scholar] [CrossRef]
- Maly, S.; Královec, J.; Hampel, D. Effects of long-term mineral fertilization on microbial biomass, microbial activity, and the presence of r- and k-strategists in soil. Biol. Fert. Soils 2009, 45, 753–760. [Google Scholar] [CrossRef]
- Kamilova, F.; Kravchenko, L.V.; Shaposhnikov, A.I.; Azarova, T.; Makarova, N.; Lugtenberg, B. Organic acids, sugars, and l-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol. Plant Microbe 2006, 19, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Li, K.; Sun, Y.N.; Zhang, L.H.; Hu, X.X.; Xie, H.G. Allelopathic Effects and Identification of Allelochemicals in Grape Root Exudates. Acta Hortic. Sin. 2010, 37, 861–868. (In Chinese) [Google Scholar]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Chang. 2015, 5, 588–595. [Google Scholar] [CrossRef]
- Qiu, H.; Zheng, X.; Ge, T.; Dorodnikov, M.; Chen, X.; Hu, Y. Weaker priming and mineralisation of low molecular weight organic substances in paddy than in upland soil. Eur. J. Soil Biol. 2017, 83, 9–17. [Google Scholar] [CrossRef]
- Yuan, Y.S.; Huang, Z.X.; Chen, L.J.; Hua, J.Y. Different Influences of Exudate Components on Microbial and Enzymatic Activities in a Subalpine Spruce Plantation. Chin. J. Soil Sci. 2022, 53, 1079–1087. (In Chinese) [Google Scholar]
- Paterson, E.; Gebbing, T.; Abel, C.; Sim, A.; Telfer, G. Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytol. 2007, 173, 600–610. [Google Scholar] [CrossRef]
- Bradford, M.A.; Keiser, A.D.; Davies, C.A.; Mersmann, C.A.; Strickland, M.S. Empirical evidence that soil carbon formation from plant inputs is positively related to microbial growth. Biogeochemistry 2013, 113, 271–281. [Google Scholar] [CrossRef]
- Tian, R.; Ning, D.; He, Z.; Zhang, P.; Zhou, J. Small and mighty: Adaptation of superphylum patescibacteria to groundwater environment drives their genome simplicity. Microbiome 2020, 8, 51. [Google Scholar] [CrossRef]
- Dong, H. Succession of microbial communities in waste soils of an iron mine in eastern China. Microorganisms 2021, 9, 2463. [Google Scholar] [CrossRef] [PubMed]
- Liaw, S.J.; Lai, H.C.; Wang, W.B. Modulation of swarming and virulence by fatty acids through the RsbA protein in Proteus mirabilis. Infect. Immun. 2004, 72, 6836–6845. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.L.; Zhang, N.; Sun, B.; Liang, Y.T. Community Structure of Burkholderiales and Its Diversity in Typical Maize Rhizosphere Soil. Acta Pedol. Sin. 2020, 57, 975–985. (In Chinese) [Google Scholar]
- Song, Z.H.; Tian, P. A review of soil microorganism functions in soil improvement and remediation. Pratacultural Sci. 2024, 41, 2622–2636. (In Chinese) [Google Scholar]
- Li, L.L.; Li, T.L.; Zhang, E.P.; Zhang, W.B.; Xi, L.M.; Liu, W.E. Experimental study on degradation of four phenolic acids in soil. Chin. J. Soil Sci. 2010, 41, 1460–1465. (In Chinese) [Google Scholar]
- Fernandez, C.D.; Baath, E. Growth response of the bacterial community to pH in soils differing in pH. Fems Microbiol. Ecol. 2010, 7, 149–156. [Google Scholar]
- Wang, T.J.; Yang, H.M.; Gao, L.J.; Zhang, Y.; Hu, Z.Y.; Xu, C.K. Atmospheric sulfur deposition on farmland in East China. Pedosphere 2005, 15, 120–128. [Google Scholar]
- Xia, H.; Muhammad, R.; Saba, B.; Yan, L.; Li, Y.X.; Wang, X.L.; Jiang, C.C. Assessing the impact of biochar on microbes in acidic soils: Alleviating the toxicity of aluminum and acidity. J. Environ. Manag. 2023, 345, 118796. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhang, T.L.; Dai, C.C. Advance in Mechanism and Countermeasures of Peanut Succession Monocropping Obstacles. Soils 2010, 42, 505–512. (In Chinese) [Google Scholar]
- Li, C.G.; Li, X.M.; Kong, W.D.; Wu, Y.; Wang, J. Effect of monoculture soybean on soil microbial community in the Northeast China. Plant Soil 2010, 330, 423–433. [Google Scholar] [CrossRef]
- Li, X.G.; Ding, C.F.; Hua, K.; Zhang, T.L.; Zhang, Y.N.; Zhao, L.; Yang, Y.R.; Liu, J.D.; Wang, X.X. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 2014, 78, 149–159. [Google Scholar] [CrossRef]
- Li, X.G.; Zhang, T.L.; Wang, X.X. Advances in Mechanism of Peanut Continuous Cropping Obstacle. Soils 2015, 47, 266–271. (In Chinese) [Google Scholar]
- Kong, C.H.; Hu, F. Plant Allelopathy and Its Application; China Agriculture Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Zhang, H.S.; Wu, X.; Li, G.; Pei, Q. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol. Fert. Soils 2011, 47, 543–554. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Xu, Y.Y.; Hong, B.; Duan, S.L.; Zhang, H.Y.; Zou, J.L. Analysis of bacterial community diversity in rhizosphere soil of continuous cropping Andrographis paniculata based on high-throughput sequencing. J. South China Agric. Univ. 2021, 42, 55–63. (In Chinese) [Google Scholar]




| Treatment | AN (mg/kg) | AP (mg/kg) | AK (mg/kg) | SOM (%) | pH |
|---|---|---|---|---|---|
| CK | 51.36 ± 1.20 e | 63.04 ± 1.38 a | 180.06 ± 1.91 a | 1.08 ± 0.02 g | 6.48 ± 0.12 b |
| SL−10 | 62.11 ± 0.91 d | 30.19 ± 1.84 d | 169.11 ± 1.88 c | 1.13 ± 0.04 fg | 6.60 ± 0.13 a |
| SM−10 | 62.73 ± 2.14 cd | 30.83 ± 4.00 d | 170.21 ± 3.76 bc | 1.20 ± 0.01 cde | 6.36 ± 0.07 ab |
| SH−10 | 61.82 ± 0.67 d | 31.37 ± 2.09 d | 172.53 ± 4.84 bc | 1.21 ± 0.02 cd | 6.23 ± 0.09 cd |
| SL−20 | 62.61 ± 2.75 cd | 31.51 ± 5.71 d | 171.37 ± 3.09 bc | 1.19 ± 0.02 cde | 6.43 ± 0.09 ab |
| SM−20 | 62.58 ± 1.24 cd | 34.92 ± 4.03 cd | 173.53 ± 2.05 bc | 1.23 ± 0.05 c | 6.38 ± 0.02 ab |
| SH−20 | 62.64 ± 1.94 cd | 39.27 ± 5.31 c | 174.60 ± 3.43 abc | 1.29 ± 0.02 ab | 6.16 ± 0.02 d |
| PL−10 | 63.39 ± 0.59 cd | 45.94 ± 1.71 b | 170.21 ± 4.97 bc | 1.15 ± 0.02 ef | 6.41 ± 0.02 ab |
| PM−10 | 65.66 ± 1.63 bc | 46.96 ± 2.23 b | 171.48 ± 5.52 bc | 1.17 ± 0.02 def | 6.38 ± 0.05 ab |
| PH−10 | 69.54 ± 2.50 a | 47.25 ± 1.08 b | 172.84 ± 1.79 bc | 1.23 ± 0.02 bc | 6.35 ± 0.04 bc |
| PL−20 | 65.69 ± 1.42 bc | 46.57 ± 2.25 b | 172.81 ± 1.74 bc | 1.29 ± 0.02 ab | 6.36 ± 0.01 ab |
| PM−20 | 66.55 ± 0.78 b | 47.75 ± 1.18 b | 175.65 ± 1.90 abc | 1.31 ± 0.06 a | 6.33 ± 0.04 bc |
| PH−20 | 70.56 ± 1.73 a | 48.22 ± 3.46 b | 176.77 ± 5.03 ab | 1.33 ± 0.04 a | 6.31 ± 0.02 bc |
| Phenolic acid types | 64.67 ** | 167.56 ** | 0.37 | 12.15 ** | 0.08 |
| Concentration | 8.03 ** | 2.50 | 8.87 ** | 16.86 ** | 28.48 ** |
| Time | 2.58 | 5.77 ** | 0.38 | 77.78 ** | 7.86 ** |
| P × C | 9.37 ** | 0.66 | 2.87 | 1.19 | 14.23 ** |
| P × T | 0.84 | 2.79 | 1.60 | 10.55 ** | 0.44 |
| C × T | 0.29 | 0.85 | 3.54 ** | 0.14 | 1.99 |
| P × C × T | 0.17 | 0.69 | 3.14 | 1.47 | 1.98 |
| Treatment | N | P | C | Chemometric Characteristics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NAG | ACP | CBH | βG | βX | C/N | C/P | N/P | Vector Length | Vector Angle | |
| CK | 50.08 ± 1.31 g | 57.94 ± 2.62 e | 10.19 ± 0.60 d | 30.20 ± 1.18 f | 2.63 ± 1.03 d | 0.86 ± 0.03 e | 0.74 ± 0.05 g | 0.87 ± 0.03 d | 0.63 ± 0.02 e | 42.68 ± 0.66 g |
| SL−10 | 88.51 ± 1.89 f | 69.47 ± 1.10 d | 12.36 ± 1.05 d | 107.72 ± 14.65 e | 7.47 ± 1.06 cd | 1.44 ± 0.21 c | 1.84 ± 0.26 e | 1.27 ± 0.01 c | 0.87 ± 0.05 c | 47.65 ± 0.29 de |
| SH−10 | 96.56 ± 1.91 ef | 84.08 ± 2.23 c | 13.33 ± 1.49 d | 190.60 ± 24.51 d | 9.95 ± 2.48 cd | 2.22 ± 0.25 a | 2.54 ± 0.26 d | 1.15 ± 0.05 cd | 0.99 ± 0.03 a | 46.19 ± 0.44 f |
| SL−20 | 202.58 ± 1.20 c | 94.82 ± 17.48 | 40.71 ± 11.23 c | 301.90 ± 9.17 b | 19.63 ± 4.21 bc | 1.77 ± 0.06 b | 4.15 ± 0.38 b | 2.35 ± 0.26 b | 1.03 ± 0.01 a | 51.57 ± 0.70 c |
| SH−20 | 411.91 ± 28.75 a | 112.00 ± 1.10 a | 150.23 ± 3.31 a | 350.04 ± 31.28 a | 79.60 ± 19.73 a | 1.42 ± 0.22 c | 5.18 ± 0.44 a | 3.68 ± 0.29 a | 1.02 ± 0.03 a | 55.14 ± 1.46 b |
| PL−10 | 107.43 ± 1.76 de | 85.14 ± 1.11 c | 16.94 ± 2.11 d | 79.81 ± 5.25 e | 9.60 ± 2.81 cd | 0.99 ± 0.06 de | 1.25 ± 0.07 f | 1.26 ± 0.01 c | 0.75 ± 0.02 d | 48.15 ± 0.19 d |
| PH−10 | 117.50 ± 5.90 d | 98.45 ± 4.56 b | 26.01 ± 4.95 cd | 177.78 ± 11.42 d | 8.97 ± 1.42 cd | 1.81 ± 0.16 b | 2.16 ± 0.04 de | 1.20 ± 0.09 cd | 0.94 ± 0.02 b | 46.72 ± 0.80 ef |
| PL−20 | 215.24 ± 5.15 c | 63.24 ± 5.83 de | 36.83 ± 1.76 c | 162.23 ± 12.75 d | 23.15 ± 2.35 b | 1.03 ± 0.09 de | 3.52 ± 0.15 c | 3.43 ± 0.38 a | 0.93 ± 0.01 b | 56.90 ± 1.23 a |
| PH−20 | 292.98 ± 4.33 b | 80.02 ± 2.01 c | 80.47 ± 27.30 b | 263.13 ± 35.01 c | 20.83 ± 4.29 bc | 1.24 ± 0.05 cd | 4.56 ± 0.22 b | 3.66 ± 0.15 a | 0.99 ± 0.01 a | 55.94 ± 0.35 ab |
| Phenolic acid types | 14.40 ** | 13.25 ** | 10.39 ** | 61.73 ** | 19.60 ** | 47.42 ** | 27.15 ** | 11.02 ** | 58.48 ** | 29.64 ** |
| Concentration | 304.33 ** | 98.79 ** | 87.07 ** | 99.28 ** | 23.70 ** | 32.29 ** | 74.64 ** | 17.38 ** | 80.42 ** | 0.05 |
| Time | 1659.67 ** | 0.51 | 187.67 ** | 238.35 ** | 76.94 ** | 15.25 ** | 510.02 ** | 622.56 ** | 103.82 ** | 550.08 ** |
| P × C | 54.86 ** | 1.88 | 10.91 ** | 3.63 | 28.63 ** | 5.74 * | 0.26 | 9.83 ** | 11.91 ** | 11.72 ** |
| P × T | 69.78 ** | 148.73 ** | 27.01 ** | 29.44 ** | 21.29 ** | 0.04 | 0.41 | 9.68 ** | 1.72 | 15.06 ** |
| C × T | 236.34 ** | 3.96 | 66.96 ** | 0.69 | 20.84 ** | 45.94 ** | 1.11 | 28.34 ** | 40.31 ** | 17.54 ** |
| P × C × T | 58.34 ** | 1.01 | 17.89 ** | 1.01 | 23.45 ** | 3.94 | 0.22 | 12.18 ** | 0.04 | 12.01 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Liu, Y.; Jiang, C.; El-Desouki, Z.; Riaz, M.; Wang, C.; Zhang, X.; Ding, J.; Chen, Z.; Liu, H.; et al. Effects of Exogenous Application of Phenolic Acid on Soil Nutrient Availability, Enzyme Activities, and Microbial Communities. Agriculture 2025, 15, 1067. https://doi.org/10.3390/agriculture15101067
Zhou Y, Liu Y, Jiang C, El-Desouki Z, Riaz M, Wang C, Zhang X, Ding J, Chen Z, Liu H, et al. Effects of Exogenous Application of Phenolic Acid on Soil Nutrient Availability, Enzyme Activities, and Microbial Communities. Agriculture. 2025; 15(10):1067. https://doi.org/10.3390/agriculture15101067
Chicago/Turabian StyleZhou, Yi, Yihang Liu, Chaoqiang Jiang, Zeinab El-Desouki, Muhammad Riaz, Chenlu Wang, Xueping Zhang, Jiayi Ding, Zhenghao Chen, Huaiwei Liu, and et al. 2025. "Effects of Exogenous Application of Phenolic Acid on Soil Nutrient Availability, Enzyme Activities, and Microbial Communities" Agriculture 15, no. 10: 1067. https://doi.org/10.3390/agriculture15101067
APA StyleZhou, Y., Liu, Y., Jiang, C., El-Desouki, Z., Riaz, M., Wang, C., Zhang, X., Ding, J., Chen, Z., Liu, H., Shen, J., & Xia, H. (2025). Effects of Exogenous Application of Phenolic Acid on Soil Nutrient Availability, Enzyme Activities, and Microbial Communities. Agriculture, 15(10), 1067. https://doi.org/10.3390/agriculture15101067







