Analysis of Sublethal and Lethal Effects of Chlorantraniliprole on Loxostege sticticalis Based on Age-Stage, Two-Sex Life Table
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect and Insecticide
2.2. Bioassays
2.3. The Life-Table Study in Loxostege sticticalis
2.4. Data Analysis
3. Results and Discussion
3.1. Toxicity of Chlorantraniliprole on Loxostege sticticalis Larvae
3.2. Sublethal and Lethal Effects of Chlorantraniliprole on Loxostege sticticalis Based on the Life Table
3.2.1. Sublethal and Lethal Doses of Chlorantraniliprole Affect the Development Duration of Loxostege sticticalis
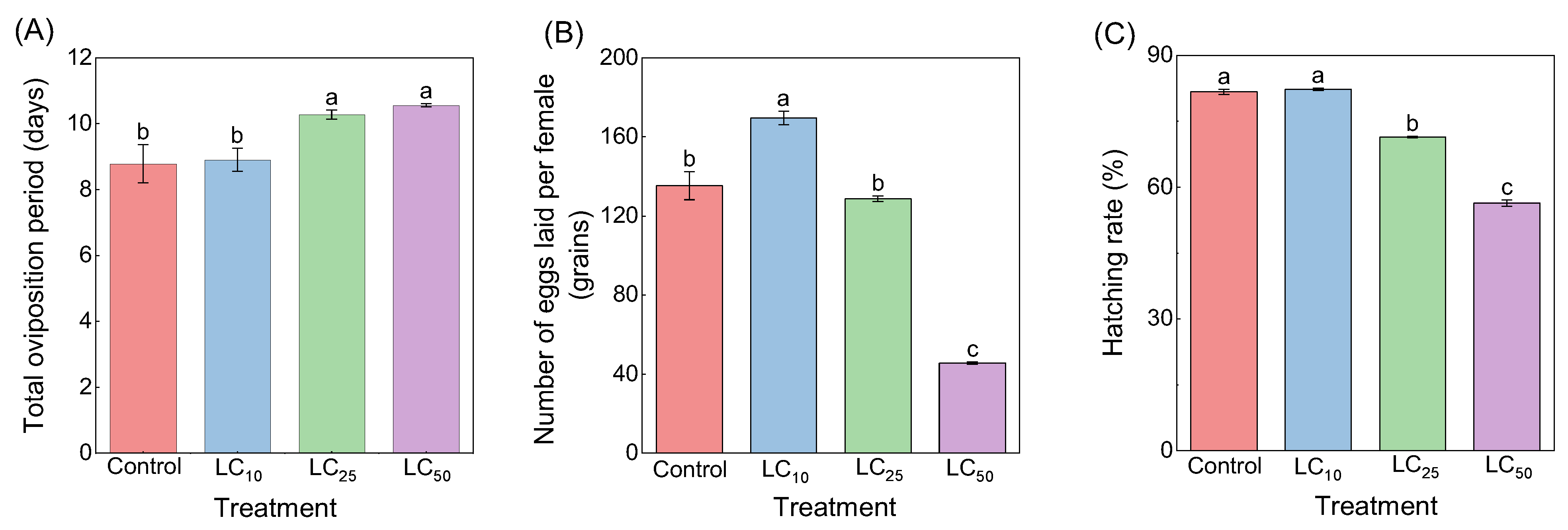
3.2.2. Sublethal and Lethal Doses of Chlorantraniliprole Affect the Reproduction of Loxostege sticticalis
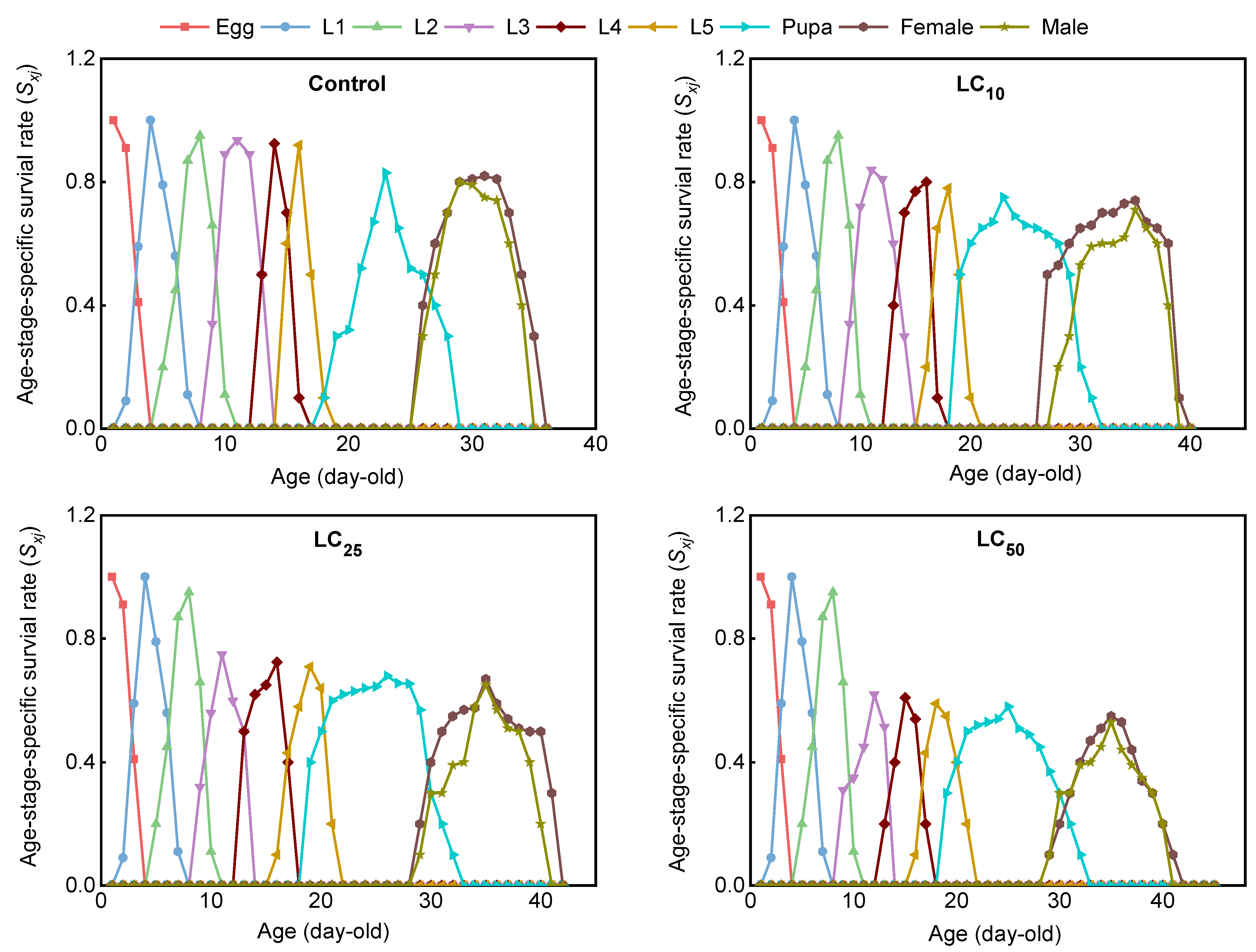
3.2.3. The Effect of Chlorantraniliprole on the Survival and Fecundity of Loxostege sticticalis Based on the Age-Stage, Two-Sex Life Table
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, L.; Tang, Y.; Zhang, L.; Jiang, X. Green manure crops as food source: Impact on the performance of the migratory beet webworm, Loxostege sticticalis (Lepidoptera: Pyralidae). Insects 2023, 14 Pt 8, 693. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Jiang, X.; Yin, J.; Chen, J.; Ding, T.; Tan, X. The adaptability of beet webworm (Loxostege sticticalis) to soybeans and other different host plants. Agronomy 2024, 14, 2595. [Google Scholar] [CrossRef]
- Huang, S.Z.; Zhang, L.; Xie, D.J.; Tang, J.H.; Jiang, Y.Y.; Mijidsuren, B.; Baasan, M.; Luo, L.Z.; Jiang, X.F. Transboundary migration of Loxostege sticticalis (Lepidoptera: Crambidae) among China, Russia and Mongolia. Pest Manag. Sci. 2024, 80, 4650–4664. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, H.; Tang, J.; Wu, M.; He, S.; Wan, H.; Li, J.; Ma, K. Chlorantraniliprole resistance in Spodoptera frugiperda: Resistance monitoring, resistance risk, and resistance mechanisms. J. Agric. Food Chem. 2024, 72, 16651–16660. [Google Scholar] [CrossRef]
- Calvin, W.; Palumbo, J.C. Chlorantraniliprole resistance associated with Diamondback moth (Lepidoptera: Plutellidae) outbreaks in Arizona brassica crops. J. Econ. Entomol. 2024, 117, 2608–2617. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, X.; He, X.; Guo, J.; Fu, Q.; Xu, H.; Lu, Z. Sublethal effects of chlorantraniliprole on growth, biochemical and molecular parameters in two chironomids, Chironomus kiiensis and Chironomus javanus. Ecotoxicol. Environ. Saf. 2023, 253, 114658. [Google Scholar] [CrossRef]
- Bartling, M.T.; Brandt, A.; Hollert, H.; Vilcinskas, A. Current insights into sublethal effects of pesticides on insects. Int. J. Mol. Sci. 2024, 25, 6007. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, N.; Yang, X.; Miao, L.; Jiang, H.; Ji, C.; Xu, B.; Qian, K.; Wang, J. Sublethal effects of chlorantraniliprole on molting hormone levels and mRNA expressions of three halloween genes in the rice stem borer, Chilo suppressalis. Chemosphere 2020, 238, 124676. [Google Scholar] [CrossRef]
- Kong, F.; Song, Y.; Zhang, Q.; Wang, Z.; Liu, Y. Sublethal effects of chlorantraniliprole on Spodoptera litura (Lepidoptera: Noctuidae) moth: Implication for attract-and-kill strategy. Toxics 2021, 9, 20. [Google Scholar] [CrossRef]
- Liu, X.G.; Wang, Q.G.; Liu, X.M.; Li, X.; Du, M.F.; Tian, C.H.; Zhang, Y.H.; An, S.H. Chronic sublethal exposure to chlorantraniliprole inhibits growth and development by disrupting the sugar and fatty acid metabolism in Spodoptera frugiperda. Pestic. Biochem. Physiol. 2025, 208, 106302. [Google Scholar] [CrossRef]
- Batool, N.; Abubakar, M.; Noureldeen, A.; Naqqash, M.N.; Alghamdi, A.; Dhafar, Z.M.; Baakdah, F.; Mozūratis, R. Toxicity and sublethal effect of chlorantraniliprole on multiple generations of Aedes aegypti L. (Diptera: Culicidae). Insects 2024, 15, 851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, C.; Wu, L.; Chen, W. Transgenerational sublethal effects of chlorantraniliprole and emamectin benzoate on the development and reproduction of Spodoptera frugiperda. Insects 2023, 14, 537. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, Z.R.; Afzal, A.; Idrees, A.; Zia, K.; Qadir, Z.A.; Ali, S.; Haq, I.U.; Ghramh, H.A.; Niaz, Y.; Tahir, M.B.; et al. Lethal, sub-Lethal and trans-generational effects of chlorantraniliprole on biological parameters, demographic traits, and fitness costs of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 881. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tian, J.; Ullah, F.; Desneux, N.; Guo, J.; Wang, S.; Xu, H.; Lu, Z. Sublethal and transgenerational effects of lufenuron on biological characteristics and expression of reproductive related genes in the fall armyworm, Spodoptera frugiperda. Pestic. Biochem. Physiol. 2023, 196, 105593. [Google Scholar] [CrossRef]
- Zhang, D.W.; Dai, C.C.; Ali, A.; Liu, Y.Q.; Pan, Y.; Desneux, N.; Lu, Y.H. Lethal and sublethal effects of chlorantraniliprole on the migratory moths Agrotis ipsilon and A. segetum: New perspectives for pest management strategies. Pest Manag. Sci. 2022, 78, 4105–4113. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, F.; Zheng, J.; Sun, Z.; Liu, X.; Ma, S.; Chen, K.; Ju, X.; Wang, Q. Effects of polyamide microplastics with different particle sizes on the development of silkworm Bombyx mori (Lepidoptera: Bombycidae) and its progeny: A study based on the age-stage, two-sex life table. Bull. Entomol. Res. 2025, 28, 1–11. [Google Scholar] [CrossRef]
- Campoy, A.; Lutsyk, M.; Pérez-Bañón, C.; Rojo, S. Age-stage two-sex life table analysis of Eristalinus aeneus (Diptera, Syrphidae) reared with two different larval media. Bull. Entomol. Res. 2022, 112, 13–20. [Google Scholar] [CrossRef]
- NY/T 1154.6-2006; Pesticides Guidelines for Laboratory Bioactivity Tests Part 6: The Immersion Test for Insecticide Activity. Ministry of Agriculture, Pesticide Testing Center: Beijing, China, 2006.
- Cheng, Y.; Hu, M.; Kang, A.; Xiao, Y.; Luo, L.; Jiang, X. The sex ratio indicates the conclusion and onset of population cycles in the beet webworm Loxostege sticticalis L. (Lepidoptera: Pyralidae). Insects 2023, 14, 781. [Google Scholar] [CrossRef]
- Chi, H.; You, M.S.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Zhang, Y.L.; Quandahor, P.; Gou, Y.P.; Li, C.C.; Zhang, K.X.; Liu, C.Z. Oviposition preference and age-stage, two-sex life table analysis of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different maize varieties. Insects 2023, 14, 413. [Google Scholar] [CrossRef]
- Lutz, A.L.; Bertolaccini, I.; Scotta, R.R.; Curis, M.C.; Favaro, M.A.; Fernandez, L.N.; Sánchez, D.E. Lethal and sublethal effects of chlorantraniliprole on Spodoptera cosmioides (Lepidoptera: Noctuidae). Pest Manag. Sci. 2018, 74, 2817–2821. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Zhang, S.; Shen, F.; Liu, M.; Ren, C.; Gao, X. Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (lepidoptera: Plutellidae). Pest Manag. Sci. 2012, 68, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.A.M.; Fouad, E.A.; Ibrahim, E.; Erdei, A.L.; Kárpáti, Z.; Fónagy, A. The comparative toxicity, biochemical and physiological impacts of chlorantraniliprole and indoxacarb on Mamestra brassicae (Lepidoptera: Noctuidae). Toxics 2023, 11, 212. [Google Scholar] [CrossRef]
- Zeng, X.; He, Y.; Wu, J.; Tang, Y.; Gu, J.; Ding, W.; Zhang, Y. Sublethal effects of cyantraniliprole and imidacloprid on feeding behavior and life table parameters of Myzus persicae (Hemiptera: Aphididae). J. Econ. Entomol. 2016, 109, 1595–1602. [Google Scholar] [CrossRef]
- Lv, S.; Guan, D.; Wei, J.; Ge, H.; Zhou, X.; Zheng, Y.; Qian, K.; Wang, J. Low concentrations of cyantraniliprole negatively affects the development of Spodoptera frugiperda by disruption of ecdysteroid biosynthesis and carbohydrate and lipid metabolism. Pestic. Biochem. Physiol. 2024, 200, 105827. [Google Scholar] [CrossRef]
- Yang, J.; Guan, D.; Wei, J.; Ge, H.; Cao, X.; Lv, S.; Zhou, X.; Zheng, Y.; Meng, X.; Wang, J.; et al. Mechanisms underlying the effects of low concentrations of chlorantraniliprole on development and reproduction of the fall armyworm, Spodoptera frugiperda. Pestic. Biochem. Physiol. 2023, 191, 105362. [Google Scholar] [CrossRef]
- Alimirzaee, S.; Khajehali, J.; Van Leeuwen, T. Hormetic effects of neonicotinoid insecticides on Rhizoglyphus robini (Acari: Acaridae). Pestic. Biochem. Physiol. 2023, 192, 105396. [Google Scholar] [CrossRef]
- Silva, W.R.; Pereira, R.C.; Mendonça, L.V.P.; Peçanha, L.S.; de Sales Abreu, L.M.; Abib, P.H.N.; Samuels, R.I.; Picanço, M.C.; Silva, G.A. Lethal and sublethal effects of insecticides used in the management of Plutella xylostella (Lepidoptera: Plutellidae) on the predator Cycloneda sanguinea L. (Coleoptera: Coccinellidae). Pest Manag. Sci. 2022, 78, 4397–4406. [Google Scholar] [CrossRef]
- Wu, H.M.; Feng, H.L.; Wang, G.D.; Zhang, L.L.; Zulu, L.; Liu, Y.H.; Zheng, Y.L.; Rao, Q. Sublethal effects of three insecticides on development and reproduction of Spodoptera frugiperda (Lepidoptera: Noctuidae). Agronomy 2022, 12, 1334. [Google Scholar] [CrossRef]
- Rix, R.R.; Cutler, G.C. Low doses of a neonicotinoid stimulate reproduction in a beneficial predatory insect. J. Econ. Entomol. 2020, 113, 2179–2186. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Z.; Li, M.; Mao, T.; Wang, H.; Li, F.; Sun, H.; Dai, M.; Ye, W.; Li, B. The mechanism of sublethal chlorantraniliprole exposure causing silkworm pupation metamorphosis defects. Pest Manag. Sci. 2020, 76, 2838–2845. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, N.; Shang, J.; Guo, L.; Zhu, X.; Zhang, K.; Niu, R.; Li, D.; Gao, X.; Wang, L.; Ji, J.; et al. Life table analysis and RNA-Seq reveal hormesis and transgenerational effects of deltamethrin on Aphis gossypii. Pest Manag. Sci. 2025, 81, 477–489. [Google Scholar] [CrossRef]

| Insecticides | Slope ± SE | LC50 (95% CI) ×10−2 μg/L | LC25 (95% CI) ×10−2 μg/L | LC10 (95% CI) ×10−2 μg/L | χ2 | R2 |
|---|---|---|---|---|---|---|
| Chlorantraniliprole | 0.843 ± 0.154 | 8.183 (3.418–14.524) | 1.297 (2.283–3.172) | 0.247 (0.018–0.892) | 0.523 | 0.986 |
| Developmental Stage (Days) | Groups | |||
|---|---|---|---|---|
| Control | LC10 | LC25 | LC50 | |
| Egg | 2.978 ± 0.051 a | 3.000 ± 0.051 a | 3.000 ± 0.089 a | 2.978 ± 0.033 a |
| 1st instar larvae | 2.592 ± 0.087 a | 2.592 ± 0.087 a | 2.581 ± 0.100 a | 2.581 ± 0.100 a |
| 2nd instar larvae | 2.570 ± 0.029 a | 2.570 ± 0.029 a | 2.570 ± 0.029 a | 2.570 ± 0.029 a |
| 3rd instar larvae | 2.258 ± 0.025 d | 2.618 ± 0.038 c | 2.818 ± 0.037 b | 3.140 ± 0.032 a |
| 4th instar larvae | 2.257 ± 0.012 d | 2.756 ± 0.029 c | 2.861 ± 0.017 b | 3.190 ± 0.027 a |
| 5th instar larvae | 2.259 ± 0.025 c | 2.817 ± 0.037 b | 2.861 ± 0.058 b | 3.310 ± 0.013 a |
| Pupa | 10.676 ± 0.025 d | 12.178 ± 0.051 c | 13.056 ± 0.096 b | 15.067 ± 0.115 a |
| Female | 9.440 ± 0.577 b | 12.222 ± 0.763 a | 12.278 ± 1.892 a | 12.556 ± 1.528 a |
| Male | 8.778 ± 0.577 b | 11.222 ± 0.577 a | 11.722 ± 1.041 a | 12.333 ± 1.323 a |
| Egg-adult | 35.193 ± 0.333 d | 40.276 ± 0.056 c | 41.178 ± 0.043 b | 44.081 ± 0.028 a |
| Groups | Net Reproductive Rate R0 (Offspring) | Intrinsic Rate of Increase r (d−1) | Finite Rate of Increase λ (d−1) | Mean Generation Time T (d) |
|---|---|---|---|---|
| Control | 82.1950 ± 0.7211 b | 0.2532 ± 0.0015 b | 1.2881 ± 0.0032 b | 17.4135 ± 0.0970 b |
| LC10 | 94.6087 ± 1.0504 a | 0.2704 ± 0.0006 a | 1.3105 ± 0.0021 a | 16.8266 ± 0.0470 c |
| LC25 | 56.1377 ± 0.9320 c | 0.2439 ± 0.0002 c | 1.2762 ± 0.0037 c | 16.5138 ± 0.0400 d |
| LC50 | 18.6342 ± 0.4619 d | 0.1030 ± 0.0001 d | 1.1085 ± 0.0006 d | 28.3953 ± 0.0021 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Fan, Y.; Mao, L.; Zhu, L.; Liu, X.; Zhang, L. Analysis of Sublethal and Lethal Effects of Chlorantraniliprole on Loxostege sticticalis Based on Age-Stage, Two-Sex Life Table. Agriculture 2025, 15, 1065. https://doi.org/10.3390/agriculture15101065
Pan X, Fan Y, Mao L, Zhu L, Liu X, Zhang L. Analysis of Sublethal and Lethal Effects of Chlorantraniliprole on Loxostege sticticalis Based on Age-Stage, Two-Sex Life Table. Agriculture. 2025; 15(10):1065. https://doi.org/10.3390/agriculture15101065
Chicago/Turabian StylePan, Xiaoxue, Yongmei Fan, Liangang Mao, Lizhen Zhu, Xingang Liu, and Lan Zhang. 2025. "Analysis of Sublethal and Lethal Effects of Chlorantraniliprole on Loxostege sticticalis Based on Age-Stage, Two-Sex Life Table" Agriculture 15, no. 10: 1065. https://doi.org/10.3390/agriculture15101065
APA StylePan, X., Fan, Y., Mao, L., Zhu, L., Liu, X., & Zhang, L. (2025). Analysis of Sublethal and Lethal Effects of Chlorantraniliprole on Loxostege sticticalis Based on Age-Stage, Two-Sex Life Table. Agriculture, 15(10), 1065. https://doi.org/10.3390/agriculture15101065






