Bacterial Endophytes and Their Contributions to Alleviating Drought and Salinity Stresses in Wheat: A Systematic Review of Physiological Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Analysis
2.2.1. Systematic Analysis
2.2.2. Descriptive Analysis
2.2.3. Statistical Analysis
3. Results and Discussion
3.1. Performance Analysis
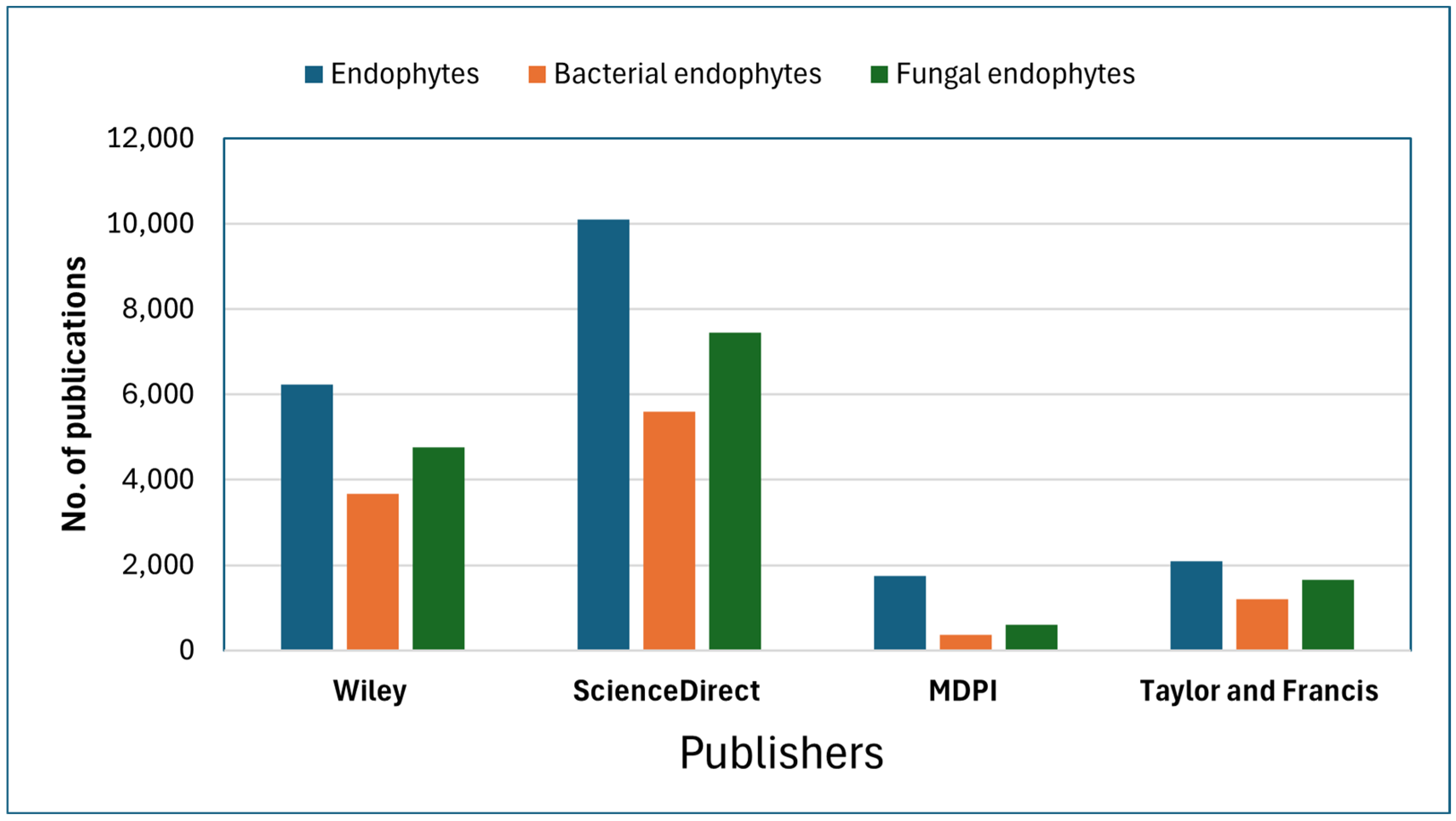
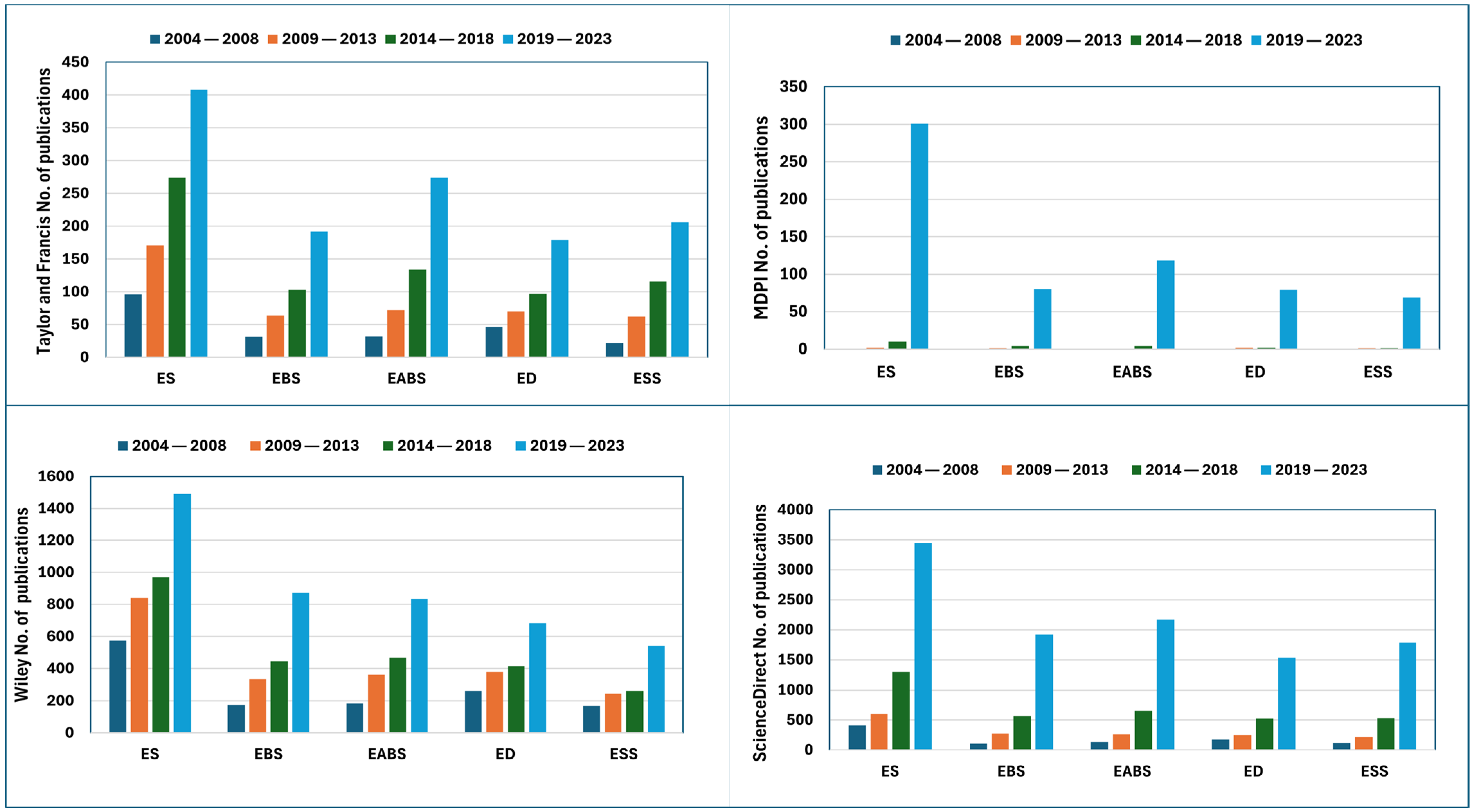
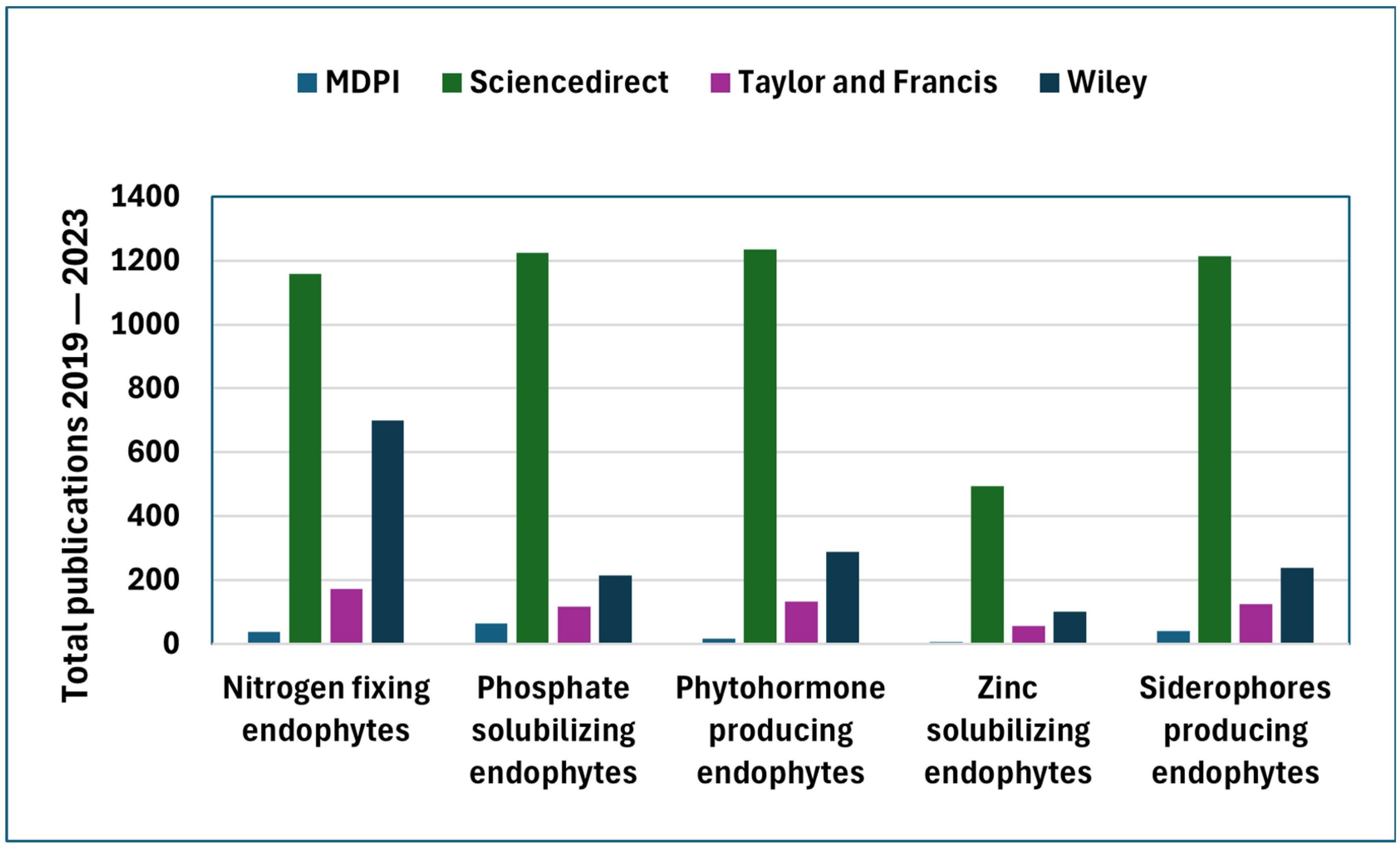
3.2. Dynamics of Endophyte–Host Interaction
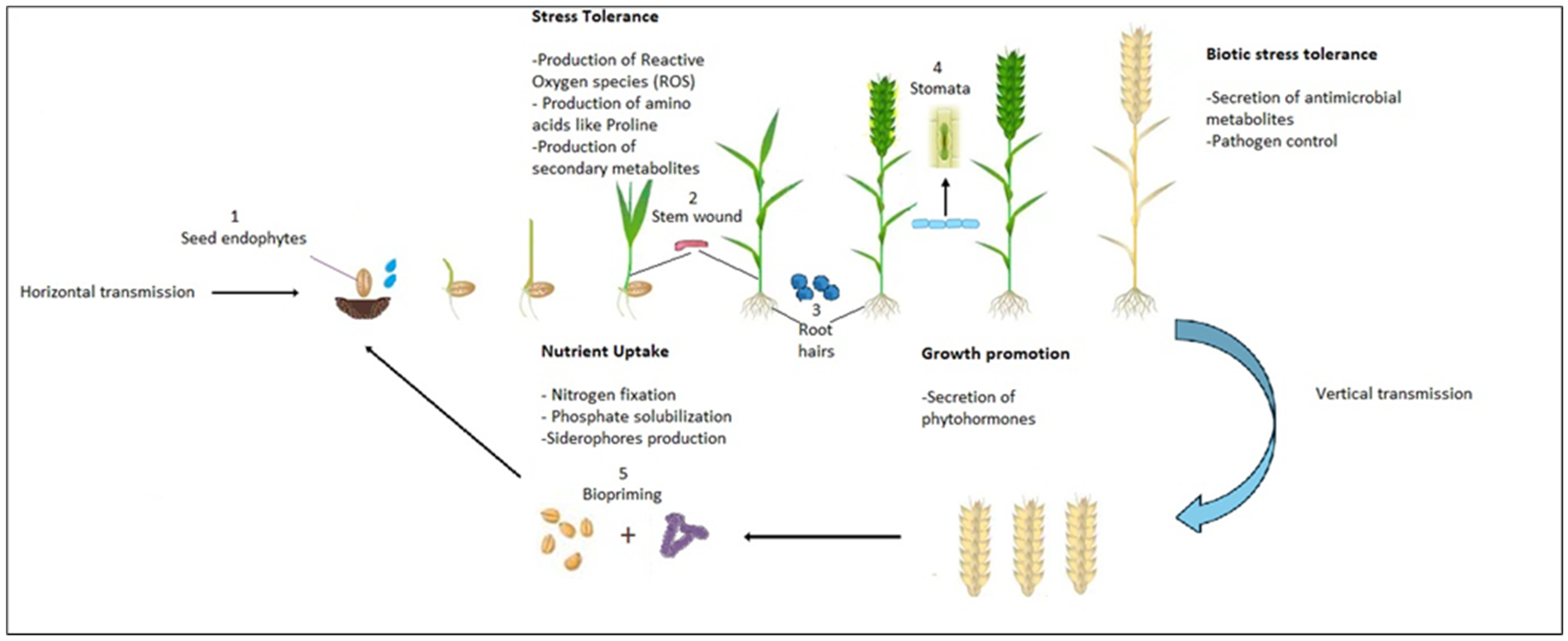
3.2.1. Bacterial Endophyte Entry Mechanisms in Plants
3.2.2. Reproduction and Transmission of Endophytes in Plant Parts
- (1)
- Colonization and Establishment:
- (2)
- Growth and Multiplication:
- (3)
- Biofilm Formation:
- (4)
- Dissemination:
- (5)
- Seed Transmission:
3.2.3. Mechanisms of Horizontal and Vertical Transmission of Bacterial Endophytes within Plants
Horizontal Transmission
Vertical Transmission
| Endophyte | Mechanism of Action | Wheat Genotype | Type of Abiotic Stress | References |
|---|---|---|---|---|
| Bacillus subtilis | Regulate proline production. Regulate malondialdehyde (MDA) production. | T. aestivum | Drought | [99] |
| Pantoea alhagi | Regulate the production of proline. Regulate malondialdehyde (MDA) production. Regulate the metabolism of chlorophyll pigment. | T. turgidum subsp. durum | Drought | [97] |
| Salicornia brachiate Bacillus spp. | The production of IAA. The production of siderophores. Produce lytic enzymes. Nitrogen fixation. Phosphate solubilization. | Triticum spp. | Salinity | [43] |
| Pseudomonas spp. | Phosphate solubilization. | T. aestivum | Salinity | [98] |
| Azotobacter chroococcum | Nitrogen fixation. | T. turgidum subsp. durum | Salinity | [103] |
| Kocuria rhizophila Cronobacter sakazakii | The production of (ACC) deaminase. | T. turgidum subsp. durum | Salinity | [124] |
| Bacillus spp. Proteobacteria Firmicutes Actinobacteria | The production of IAA. Phosphate solubilization. Zinc and potassium solubilization. The production of siderophores. | T. aestivum | Heat | [20] |
| Pseudomonas Bacillus spp. Stenotrophomonas Methylobacterium Arthrobacter Pantoea Achromobacter Acinetobacter Exiguobacterium Staphylococcus Enterobacter Providencia | The production of siderophores. The production of ammonia. The production of hydrogen cyanide. The production of (IAA), gibberellic acid (GA), and cytokinin. Nitrogen fixation. Phosphate solubilization. Zinc and potassium solubilization. | T. aestivum | Cold stress | [45] |
3.3. Analysis of Wheat Growth Response to Endophyte Inoculation under Drought and Salinity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Albdaiwi, R.N.; Khyami-Horani, H.; Ayad, J.Y. Plant growth promoting Rhizobacteria: An emerging method for the enhancement of wheat tolerance against salinity stress. Jordan J. Biol. Sci. 2019, 12, 525–534. [Google Scholar]
- Akman, Z. Comparison of high temperature tolerance in maize, rice and sorghum seeds by plant growth regulators. J. Anim. Vet. Adv. 2009, 8, 358–361. [Google Scholar]
- Huntingford, C.; Zelazowski, P.; Galbraith, D.; Mercado, L.M.; Sitch, S.; Fisher, R.; Lomas, M.; Walker, A.P.; Jones, C.D.; Booth, B.B. Simulated resilience of tropical rainforests to CO2 induced climate change. Nat. Geosci. 2013, 6, 268–273. [Google Scholar] [CrossRef]
- Ren, C.; Bilyeu, K.D.; Beuselinck, P. Composition, vigor, and proteome of mature soybean seeds developed under high temperature. Crop. Sci. 2009, 49, 1010–1022. [Google Scholar] [CrossRef]
- Savin, R.; Cossani, C.M.; Dahan, R.; Ayad, J.Y.; Albrizio, R.; Todorovic, M.; Karrou, M.; Slafer, G.A. Intensifying cereal management in dryland Mediterranean agriculture: Rainfed wheat and barley responses to nitrogen fertilization. Eur. J. Agron. 2022, 137, 126518. [Google Scholar] [CrossRef]
- Chai, Y.; Senay, S.; Horvath, D.; Pardey, P. Multi-peril pathogen risks to global wheat production: A probabilistic loss and investment assessment. Front. Plant Sci. 2022, 13, 1034600. [Google Scholar] [CrossRef]
- FAO. Climate Change and Food Security: Risks and Responses. 2015. Available online: www.fao.org/3/i5188e/I5188E.pdf (accessed on 1 January 2023).
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Jayakumar, A.; Krishna, A.; Nair, I.C.; Radhakrishnan, E.K. Drought-tolerant and plant growth-promoting endophytic Staphylococcus sp. Having synergistic effect with silicate supplementation. Ach. Microbiol. 2020, 1899–1906. [Google Scholar] [CrossRef]
- Ek-Ramos, M.J.; Gomez-Flores, R.; Orozco-Flores, A.A.; Rodríguez-Padilla, C.; González-Ochoa, G.; Tamez-Guerra, P. Bioactive products from plant-endophytic gram-positive bacteria. Front. Microbiol. 2019, 10, 463. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Chauhan, B.S. Genetic diversity, conservation, and utilization of plant genetic resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Abaid-Ullah, M.; Hassan, M.N.; Jamil, M.; Brader, G.; Shah, M.K.N.; Sessitsch, A.; Hafeez, F.Y. Plant growth promoting rhizobacteria: An alternate way to improve yield and quality of wheat (Triticum aestivum). Int. J. Agric. Biol. 2015, 17, 51–60. [Google Scholar]
- Prasanna, R.; Bidyarani, N.; Babu, S.; Hossain, F.; Shivay, Y.S.; Nain, L. Cyanobacterial inoculation elicits plant defense response and enhanced Zn mobilization in maize hybrids. Cogent Food Agric. 2015, 1, 998507. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Kaur, T.; Sheikh, I.; Yadav, A.N.; Kumar, V.; Suman, A.; Dhaliwal, H.S. Endophytic microbes from diverse wheat genotypes and their potential biotechnological applications in plant growth promotion and nutrient uptake. Proc. Natl. Acad. Sci. India Sect. B—Biol. Sci. 2020, 90, 969–979. [Google Scholar] [CrossRef]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front. Microbiol. 2018, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.; Kashyap, P.L.; Bhardwaj, A.K.; Kuppusamy, P.; Srivastava, A.K.; Tiwari, R.K. Bacterial endophyte mediated plant tolerance to salinity: Growth responses and mechanisms of action. World J. Microbiol. Biotech. 2020, 36, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, Y.; Ma, D.; Xu, W.; Zhou, J.; Yan, H.; Yang, L.; Yin, J. Screening, identification, and optimization of fermentation conditions of an antagonistic endophyte to wheat head blight. Agronomy 2019, 9, 476. [Google Scholar] [CrossRef]
- Ridout, M.E.; Schroeder, K.L.; Hunter, S.S.; Styer, J.; Newcombe, G. Priority effects of wheat seed endophytes on a rhizosphere symbiosis. Symbiosis 2019, 78, 19–31. [Google Scholar] [CrossRef]
- Aswini, K.; Suman, A.; Sharma, P.; Singh, P.K.; Gond, S.; Pathak, D. Seed endophytic bacterial profiling from wheat varieties of contrasting heat sensitivity. Front. Plant Sci. 2023, 14, 1101818. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Khan, M.S.; Aziz, S.; Ali, H.; Pecoraro, L. Molecular and biochemical characterization, antimicrobial activity, stress tolerance, and plant growth-promoting effect of endophytic bacteria isolated from wheat varieties. Microorganisms 2021, 10, 21. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, Y.J.; Yuan, B.; Xu, P.Y.; Xing, K.; Wang, J.; Jiang, J.H. Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil 2014, 374, 753–766. [Google Scholar] [CrossRef]
- Elmagzob, A.A.H.; Ibrahim, M.M.; Zhang, G.F. Seasonal diversity of endophytic bacteria associated with Cinnamomum camphora (L.) Presl. Diversity 2019, 11, 112. [Google Scholar] [CrossRef]
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial endophyte colonization and distribution within plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Fadiji, A.E.; Ayilara, M.S.; Igiehon, O.N.; Nwachukwu, B.C.; Babalola, O.O. Strategies to enhance the use of endophytes as bioinoculants in agriculture. Horticulturae 2022, 8, 498. [Google Scholar] [CrossRef]
- Min, J.; Ling, C.; Hai-Liang, X.; Cheng-Jian, Z.; Khalid, R.; Ting, H.; Lu-Ping, Q. A Friendly relationship between endophytic fungi and medicinal plants: A systematic review. Front. Microbiol. 2016, 7, 906. [Google Scholar]
- Ahn, E.; Kang, H. Introduction to systematic review and meta-analysis. Korean J. Anesthesiol. 2018, 71, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Othman, Y.A.; Al-Assaf, A.; Tadros, M.J.; Albalawneh, A. Heavy metals and microbes accumulation in soil and food crops irrigated with wastewater and the potential human health risk: A metadata analysis. Water 2021, 13, 3405. [Google Scholar] [CrossRef]
- Gao, F.; Li, H.; Mu, X.; Gao, H.; Zhang, Y.; Li, R.; Cao, K.; Ye, L. Effects of organic fertilizer application on tomato yield and quality: A meta-analysis. Appl. Sci. 2023, 13, 2184. [Google Scholar] [CrossRef]
- Ullah, R.; Asghar, I.; Griffiths, M.G. An integrated methodology for bibliometric analysis: A case study of internet of things in healthcare applications. Sensors 2023, 23, 67. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Marc Lim, W. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Snyder, H. Literature review as a research methodology: An overview and guidelines. J. Bus. Res. 2019, 104, 333–339. [Google Scholar] [CrossRef]
- Zaidi, A.; Khan, M.S.; Ahemad, M.; Oves, M. Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol. Immunol. Hung. 2009, 56, 263–284. [Google Scholar] [CrossRef]
- Al-Khoury, A.; Hussein, S.A.; Abdulwhab, M.; Aljuboori, Z.M.; Haddad, H.; Ali, M.A.; Abed, I.A.; Flayyih, H.H. Intellectual capital history and trends: A bibliometric analysis using scopus database. Sustainability 2022, 14, 11615. [Google Scholar] [CrossRef]
- Ankrah, J.; Monteiro, A.; Madureira, H. Bibliometric analysis of data sources and tools for shoreline change analysis and detection. Sustainability 2022, 14, 4895. [Google Scholar] [CrossRef]
- Watts, D.; Palombo, E.A.; Jaimes Castillo, A.; Zaferanloo, B. Endophytes in agriculture: Potential to improve yields and tolerances of agricultural crops. Microorganisms 2023, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D. Meta-Analysis of the Effects of Endophytes and Their Secondary Metabolites on Herbivory from Insects. Master’s Thesis, Graduate School of Clemson University, Clemson, SC, USA, May 2023. Available online: https://tigerprints.clemson.edu/all_theses/4032 (accessed on 1 March 2024).
- Sarsaiya, S.; Shi, J.; Chen, J. A comprehensive review on fungal endophytes and its dynamics on Orchidaceae plants: Current research, challenges, and future possibilities. Bioengineered 2019, 10, 316–334. [Google Scholar] [CrossRef]
- Battu, L.; Reddy, M.M.; Goud, B.S.; Ulaganathan, K.; Kandasamy, U. Genome inside genome: NGS based identification and assembly of endophytic Sphingopyxis granuli and Pseudomonas aeruginosa genomes from rice genomic reads. Genomics 2017, 109, 141–146. [Google Scholar] [CrossRef]
- Oita, S.; Carey, J.; Kline, I.; Ibáñez, A.; Yang, N.; Hom, E.F.Y.; Carbone, I.; U’Ren, J.M.; Arnold, A.E. Methodological approaches frame insights into endophyte richness and community composition. Microb. Ecol. 2021, 82, 21–34. [Google Scholar] [CrossRef]
- Cohen, A.C.; Travaglia, C.N.; Bottini, R.; Piccoli, P.N. Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 2009, 87, 462–487. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Thissera, B.; Bouket, A.; Saadaoui, N.; Silini, E.; Eshelli, M.; Alenezi, M.; Vallat, A.; Luptakova, A.; Yahiaoui, B.; et al. Durum wheat stress tolerance induced by endophyte pantoea agglomerans with genes contributing to plant functions and secondary metabolite arsenal. Int. J. Mol. Sci. 2019, 20, 3989. [Google Scholar] [CrossRef]
- Hadj Brahim, A.; Ben Ali, M.; Daoud, L.; Jlidi, M.; Akremi, I.; Hmani, H.; Feto, N.A.; Ben Ali, M. Biopriming of durum wheat seeds with endophytic diazotrophic bacteria enhances tolerance to fusarium head blight and salinity. Microorganisms 2022, 10, 970. [Google Scholar] [CrossRef] [PubMed]
- Thaweenut, N.; Hachisuka, Y.; Ando, S.; Yanagisawa, S.; Yoneyama, T. Two seasons’ study on nifH gene expression and nitrogen fixation by diazotrophic endophytes in sugarcane (Saccharum spp. Hybrids): Expression of nifH genes similar to those of rhizobia. Plant Soil 2010, 338, 435–449. [Google Scholar] [CrossRef]
- Verma, P.; Yadav, A.N.; Khannam, K.S.; Panjiar, N.; Kumar, S.; Saxena, A.K.; Suman, A. Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann. Microbiol. 2015, 65, 1885–1899. [Google Scholar] [CrossRef]
- Li, C.; Ng, C.K.-Y.; Fan, L.-M. MYB transcription factors, active players in abiotic stress signaling. Environ. Exp. Bot. 2015, 114, 80–91. [Google Scholar] [CrossRef]
- Mei, C.; Flinn, B.S. The use of beneficial microbial endophytes for plant biomass and stress tolerance improvement. Recent Pat. Biotechnol. 2010, 4, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh Kumar, R.; Singh, R.P.; Mishra, P. Endophytes as emphatic communication barriers of quorum sensing in Gram-positive and Gram-negative bacteria—A review. Environ. Sustain. 2019, 2, 455–468. [Google Scholar] [CrossRef]
- Hussain, A.; Zahir, Z.A.; Asghar, H.N.; Ahmad, M.; Jamil, M.; Naveed, M.; Zaman Akhtar, M.F.U. Zinc solubilizing bacteria for zinc biofortification in cereals: A step toward sustainable nutritional security. In Role of Rhizospheric Microbes in Soil: Volume 2: Nutrient Management and Crop Improvement; Springer: Berlin/Heidelberg, Germany, 2018; pp. 203–227. [Google Scholar]
- Upadhayay, V.K.; Singh, A.V.; Khan, A. Cross talk between zinc-solubilizing bacteria and plants: A short tale of bacterial-assisted zinc biofortification. Front. Soil Sci. 2022, 1, 788170. [Google Scholar] [CrossRef]
- Upadhayay, V.K.; Singh, A.V.; Pareek, N. An insight in decoding the multifarious and splendid role of microorganisms in crop biofortification. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2407–2418. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (pgpr): Emergence in agriculture. World J. Microbiol. Biotechn. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Bogino, P.C.; Oliva, M.D.L.M.; Sorroche, F.G. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef] [PubMed]
- Reinhold-Hurek, B.; Hurek, T. Living inside plants: Bacterial endophytes. Curr. Opin. Plant Biol. 2011, 14, 435–443. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Luo, Y.; Freitas, H. Inoculation of endophytic bacteria on host and non-host plants—Effects on plant growth and Ni uptake. J. Hazard. Mater. 2011, 195, 230–237. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Hardoim, C.C.; Van Overbeek, L.S.; Van Elsas, J.D. Dynamics of seed-borne rice endophytes on early plant growth stages. PLoS ONE 2012, 7, e30438. [Google Scholar] [CrossRef]
- Khan, Z.; Guelich, G.; Phan, H.; Redman, R.; Doty, S. Bacterial and yeast endophytes from poplar and willow promote growth in crop plants and grasses. ISRN Agron. 2012, 2012, 890280. [Google Scholar] [CrossRef]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef]
- Lennon, J.T.; Jones, S.E. Microbial seed banks: The ecological and evolutionary implications of dormancy. Nat. Rev. Microbiol. 2011, 9, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.B. Microbial dynamics and interactions in the spermosphere. Annu. Rev. Phytopathol. 2004, 42, 271–309. [Google Scholar] [CrossRef]
- Schiltz, S.; Gaillard, I.; Pawlicki-Jullian, N.; Thiombiano, B.; Mesnard, F.; Gontier, E. A review: What is the spermosphere and how can it be studied? J. Appl. Microbiol. 2015, 119, 1467–1481. [Google Scholar] [CrossRef]
- Roberts, D.P.; Baker, C.J.; McKenna, L.; Liu, S.; Buyer, J.S.; Kobayashi, D.Y. Influence of host seed on metabolic activity of Enterobacter cloacae in the spermosphere. Soil Biol. Biochem. 2009, 41, 754–761. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Rolli, E.; Marasco, R.; Vigani, G.; Ettoumi, B.; Mapelli, F.; Deangelis, M.L.; Gandolfi, C.; Casati, E.; Previtali, F.; Gerbino, R.; et al. Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ. Microbiol. 2015, 17, 316–331. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Lebeis, S.L.; Paredes, S.H.; Lundberg, D.S.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; Glavina del Rio, T.; Jones, C.D.; Tringe, S.G.; et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 2015, 349, 860–864. [Google Scholar] [CrossRef]
- Saleem, M.; Law, A.D.; Moe, L.A. Nicotiana roots recruit rare rhizosphere taxa as major root-inhabiting microbes. Microb. Ecol. 2016, 71, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Bodenhausen, N.; Horton, M.W.; Bergelson, J. Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLoS ONE 2013, 8, e56329. [Google Scholar] [CrossRef] [PubMed]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Burrows, S.M.; Elbert, W.; Lawrence, M.G.; Pöschl, U. Bacteria in the global atmosphere—Part 1: Review and synthesis of literature data for different ecosystems. Atmos. Chem. Phys. 2009, 9, 9263–9280. [Google Scholar] [CrossRef]
- Womack, A.M.; Bohannan, B.J.M.; Green, J.L. Biodiversity and biogeography of the atmosphere. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3645–3653. [Google Scholar] [CrossRef]
- Frank, A.C.; Saldierna Guzmán, J.P.; Shay, J.E. Transmission of bacterial endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Madmony, A.; Chernin, L.; Pleban, S.; Peleg, E.; Riov, J. Enterobacter cloacae, an obligatory endophyte of pollen grains of Mediterranean pines. Folia Microbiol. Praha 2005, 50, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Shade, A.; Jacques, M.-A.; Barret, M. Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr. Opin. Microbiol. 2017, 37, 15–22. [Google Scholar] [CrossRef]
- Nelson, E.B. The seed microbiome: Origins, interactions, and impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- Charkowski, A.O.; Sarreal, C.Z.; Mandrell, R.E. Wrinkled alfalfa seeds harbor more aerobic bacteria and are more difficult to sanitize than smooth seeds. J. Food Prot. 2001, 64, 1292–1298. [Google Scholar] [CrossRef]
- Cottyn, B.; Regalado, E.; Lanoot, B.; De Cleene, M.; Mew, T.; Swings, J. Bacterial populations associated with rice seed in the tropical environment. Phytopathology 2001, 91, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zuo, S.; Zou, Y.; Wang, J.; Song, W. Investigation on diversity and population succession dynamics of endophytic bacteria from seeds of maize (Zea mays L.; Nongda108) at different growth stages. Ann. Microbiol. 2013, 63, 71–79. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Raizada, M.N. Conservation and diversity of seed associated endophytes in Zea across boundaries of evolution, ethnography and ecology. PLoS ONE 2011, 6, e20396. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Rogel, M.A.; Ormeno-Orrillo, E.; Martínez-Romero, J.; Martínez-Romero, E. Phaseolus vulgaris seed-borne endophytic community with novel bacterial species such as Rhizobium endophyticum sp. nov. Syst. Appl. Microbiol. 2010, 33, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Zawoznik, M.S.; Vázquez, S.C.; Díaz Herrera, S.M.; Groppa, M.D. Search for endophytic diazotrophs in barley seeds. Braz. J. Microbiol. 2014, 45, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Fürnkranz, M.; Lukesch, B.; Müller, H.; Huss, H.; Grube, M.; Berg, G. Microbial diversity inside pumpkins: Microhabitat-specific communities display a high antagonistic potential against phytopathogens. Microb. Ecol. 2012, 63, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Puente, M.E.; Li, C.Y.; Bashan, Y. Rock-degrading endophytic bacteria in cacti. Environ. Exp. Bot. 2009, 66, 389–401. [Google Scholar] [CrossRef]
- Goggin, D.E.; Emery, R.J.N.; Kurepin, L.V.; Powles, S.B. A potential role for endogenous microflora in dormancy release, cytokinin metabolism and the response to fluridone in Lolium rigidum seeds. Ann. Bot. 2015, 115, 293–301. [Google Scholar] [CrossRef]
- Ferreira, A.; Quecine, M.C.; Lacava, P.T.; Oda, S.; Azevedo, J.L.; Araújo, W.L. Diversity of endophytic bacteria from Eucalyptus species seeds and colonization of seedlings by Pantoea agglomerans. FEMS Microbiol. Lett. 2008, 287, 8–14. [Google Scholar] [CrossRef]
- Clark, S.E. Organ formation at the vegetative shoot meristem. Plant Cell 1997, 9, 1067–1076. [Google Scholar] [CrossRef]
- Barret, M.; Briand, M.; Bonneau, S.; Préveaux, A.; Valière, S.; Bouchez, O.; Hunault, G.; Simoneau, P.; Jacques, M.-A. Emergence shapes the structure of the seed microbiota. Appl. Environ. Microbiol. 2015, 81, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Ambika Manirajan, B.; Ratering, S.; Rusch, V.; Schwiertz, A.; Geissler-Plaum, R.; Cardinale, M.; Schnell, S. Bacterial microbiota associated with flower pollen is influenced by pollination type, and shows a high degree of diversity and species-specificity. Environ. Microbiol. 2016, 18, 5161–5174. [Google Scholar] [CrossRef]
- Zasloff, M. Pollen has a microbiome: Implications for plant reproduction, insect pollination and human allergies: Pollen has a microbiome. Environ. Microbiol. 2017, 19, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xin, K.; Liu, H.; Cheng, J.; Shen, X.; Wang, Y.; Zhang, L. Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci. Rep. 2017, 7, 41564. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Lastochkina, O.; Yakupova, A.; Avtushenko, I.; Lastochkin, A.; Yuldashev, R. Effect of seed priming with endophytic Bacillus subtilis on some physio-biochemical parameters of two wheat varieties exposed to drought after selective herbicide application. Plants 2023, 12, 1724. [Google Scholar] [CrossRef] [PubMed]
- Belaouni, H.A.; Compant, S.; Antonielli, L.; Nikolic, B.; Zitouni, A.; Sessitsch, A. In-depth genome analysis of Bacillus sp. BH32, a salt stress-tolerant endophyte obtained from a halophyte in a semiarid region. Appl. Microbiol. Biotechnol. 2022, 106, 3113–3137. [Google Scholar] [CrossRef] [PubMed]
- Latef, A.A.H.A.; Omer, A.M.; Badawy, A.A. Strategy of salt tolerance and interactive impact of Azotobacter chroococcum and/or Alcaligenes faecalis inoculation on canola (Brassica napus l.) plants grown in saline soil. J. Plants. 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Blasco, B.; Martos, V. Combating salinity through natural plant extracts based biostimulants: A review. Front. Plant Sci. 2022, 13, 862034. [Google Scholar] [CrossRef] [PubMed]
- Silini, A.; Cherif-Silini, H.; Yahiaoui, B. Growing varieties durum wheat (Triticum durum) in response to the effect of osmolytes and inoculation by Azotobacter chroococcum under salt stress. Afr. J. Microbiol. Res. 2016, 10, 387–399. [Google Scholar]
- Kaur, M.; Karnwal, A. Screening of endophytic Bacteria from stress-tolerating plants for abiotic stress tolerance and plant growth-promoting properties: Identification of potential strains for bioremediation and crop enhancement. J. Agric. Food Res. 2023, 14, 100723. [Google Scholar] [CrossRef]
- AL-Shwaiman, H.; Shahid, M.; Elgorban, A.; Siddique, K.; Syed, A. Beijerinckia fluminensis BFC-33, a novel multi-stress-tolerant soil bacterium: Deciphering the stress amelioration, phytopathogenic inhibition and growth promotion in Triticum aestivum (L.). Chemosphere 2022, 295, 133843. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; Bhat, J.A.; Devoto, A. The influence of endophytes on rice fitness under environmental stresses. Plant Mol. Biol. 2002, 109, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Wadhwa, N. Screening and characterization of potential plant growth promoting endophytes of wheat (Triticum aestivum). Curr. App. Sci. Tech. 2023, 23, 19. [Google Scholar] [CrossRef]
- Jodder, J. miRNA-mediated regulation of auxin signaling pathway during plant development and stress responses. J. Bios. 2020, 45, 91. [Google Scholar] [CrossRef]
- Parveen, A.; Aslam, M.M.; Iqbal, R.; Ali, M.; Kamran, M.; Alwahibi, M.S.; Akram, M.; Elshikh, M.S. Effect of natural phytohormones on growth, nutritional status, and yield of mung bean (Vigna radiata L.) and N availability in sandy-loam soil of sub-tropics. Soil Syst. 2023, 7, 34. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotech. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Chowdappa, S.; Jagannath, S.; Konappa, N.; Udayashankar, A.C.; Jogaiah, S. Detection and characterization of antibacterial siderophores secreted by endophytic fungi from Cymbidium aloifolium. Biomolecules 2020, 10, 1412. [Google Scholar] [CrossRef]
- Yadav, A.N. Beneficial plant-microbe interactions for agricultural sustainability. J. Appl. Biol. Biotechnol. 2021, 9, i–iv. [Google Scholar] [CrossRef]
- Aznar, A.; Chen, N.W.; Thomine, S.; Dellagi, A. Immunity to plant pathogens and iron homeostasis. Plant Sci. 2015, 240, 90–97. [Google Scholar] [CrossRef]
- Nagata, T.; Oobo, T.; Aozasa, O. Efficacy of a bacterial siderophore, pyoverdine, to supply iron to Solanum lycopersicum plants. J. Biosci. Bioeng. 2013, 115, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Env. Sci. Pollut. Res. Int. 2016, 23, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Rajeev, K.; Saxena, A.K.; Arora, D.K. Diversity and phylogeny of plant growth-promoting bacilli from moderately acidic soil. J. Basic Microbiol. 2011, 51, 98–106. [Google Scholar] [CrossRef]
- Ghazy, N.; El-Nahrawy, S. Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Arch. Microbiol. 2021, 203, 1195–1209. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Glick, B.R. The role of plant growth- promoting bacteria in metal phytoremediation zhaoyu. Adv. Microbiol. Physiol. 2017, 71, 327–353. [Google Scholar]
- Leguizamo, M.A. Role of plant species in bioremediation of heavy metals from polluted areas and wastewaters. In Advanced Materials for Wastewater Treatment; Scrivener Publishing LLC: Beverly, MA, USA, 2017; pp. 223–261. [Google Scholar]
- Ustiatik, R.; Nuraini, Y.; Handayanto, E. Siderophore production of the Hg-resistant endophytic bacteria isolated from local grass in the Hg-contaminated soil. J. Ecol. Eng. 2021, 22, 129–138. [Google Scholar] [CrossRef]
- Meneely, K.M.; Lamb, A.L. Biochemical characterization of an FAD-dependent monooxygenase, the ornithine hydroxylase from Pseudomonas aeruginosa, suggests a novel reaction mechanism. Biochemistry 2007, 46, 11930–11937. [Google Scholar] [CrossRef] [PubMed]
- Van de Poel, B.; Van Der Straeten, D. 1-aminocyclopropane-1-carboxylic acid (ACC) in plants: More than just the precursor of ethylene! Front. Plant Sci. 2014, 5, 640. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, M.; Lopez-Calcagno, P.E.; Raines, C.A.; Furbank, R.T.; von Caemmerer, S. Overexpression of the Rieske FeS protein of the Cytochrome b6f complex increases C4 photosynthesis in Setaria viridis. Commun. Biol. 2019, 2, 314. [Google Scholar] [CrossRef]
- Afridi, M.S.; Amna; Sumaira; Mahmood, T.; Salam, A.; Mukhtar, T.; Mehmood, S.; Ali, J.; Khatoon, Z.; Bibi, M.; et al. Induction of tolerance to salinity in wheat genotypes by plant growth promoting endophytes: Involvement of ACC deaminase and antioxidant enzymes. Plant Physiol. Biochem. 2019, 139, 569–577. [Google Scholar] [CrossRef]
- Lubyanova, A.R.; Allagulova, C.R.; Lastochkina, O.V. The Effects of seed pretreatment with endophytic bacteria Bacillus subtilis on the water balance of spring and winter wheat seedlings under short-time water deficit. Plants 2023, 12, 2684. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi Khanghahi, M.; Strafella, S.; Crecchio, C. Changes in photo-protective energy dissipation of photosystem II in response to beneficial bacteria consortium in durum wheat under drought and salinity stresses. Appl. Sci. 2020, 10, 5031. [Google Scholar] [CrossRef]
- Yandigeri, M.S.; Meena, K.K.; Singh, D.; Malviya, N.; Singh, D.P.; Solanki, M.K.; Yadav, A.K.; Arora, D.K. Drought-tolerant endophytic actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant Growth Regul. 2012, 68, 411–420. [Google Scholar] [CrossRef]
- Aizaz, M.; Lubna; Ahmad, W.; Khan, I.; Asaf, S.; Bilal, S.; Jan, R.; Asif, S.; Waqas, M.; Khan, A.L.; et al. Exploring the potential of halotolerant bacteria from coastal regions to mitigate salinity stress in wheat: Physiological, molecular, and biochemical insights. Front. Plant Sci. 2023, 14, 1224731. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff,, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hawamdeh, F.; Ayad, J.Y.; Alananbeh, K.M.; Akash, M.W. Bacterial Endophytes and Their Contributions to Alleviating Drought and Salinity Stresses in Wheat: A Systematic Review of Physiological Mechanisms. Agriculture 2024, 14, 769. https://doi.org/10.3390/agriculture14050769
Al-Hawamdeh F, Ayad JY, Alananbeh KM, Akash MW. Bacterial Endophytes and Their Contributions to Alleviating Drought and Salinity Stresses in Wheat: A Systematic Review of Physiological Mechanisms. Agriculture. 2024; 14(5):769. https://doi.org/10.3390/agriculture14050769
Chicago/Turabian StyleAl-Hawamdeh, Fayha, Jamal Y. Ayad, Kholoud M. Alananbeh, and Muhanad W. Akash. 2024. "Bacterial Endophytes and Their Contributions to Alleviating Drought and Salinity Stresses in Wheat: A Systematic Review of Physiological Mechanisms" Agriculture 14, no. 5: 769. https://doi.org/10.3390/agriculture14050769
APA StyleAl-Hawamdeh, F., Ayad, J. Y., Alananbeh, K. M., & Akash, M. W. (2024). Bacterial Endophytes and Their Contributions to Alleviating Drought and Salinity Stresses in Wheat: A Systematic Review of Physiological Mechanisms. Agriculture, 14(5), 769. https://doi.org/10.3390/agriculture14050769






