Effects of Different Planting Years on Soil Physicochemical Indexes, Microbial Functional Diversity and Fruit Quality of Pear Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Sample Collection
2.2. Determination of the Fruit Quality Index
2.3. Soil Physicochemical Index Determination
2.4. Soil Microbiomass Carbon and Nitrogen Determination
2.5. Determination of Functional Diversity of Microbial Communities in Rhizosphere Soil
2.6. Data Availability
3. Results
3.1. Fruit Quality Analysis
3.2. Soil Physicochemical Index Analysis
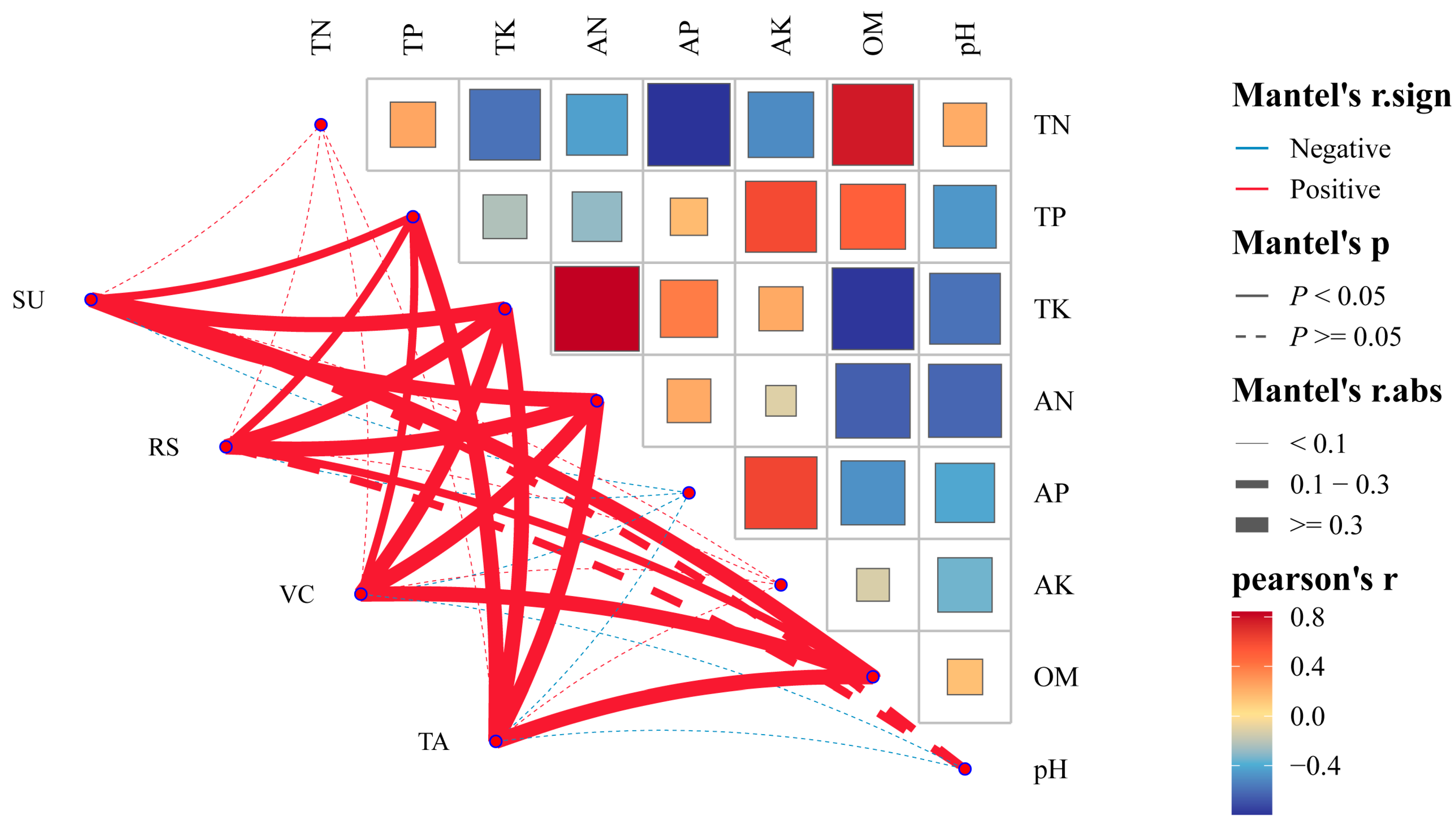
3.3. Correlation Network Heat Map Analysis
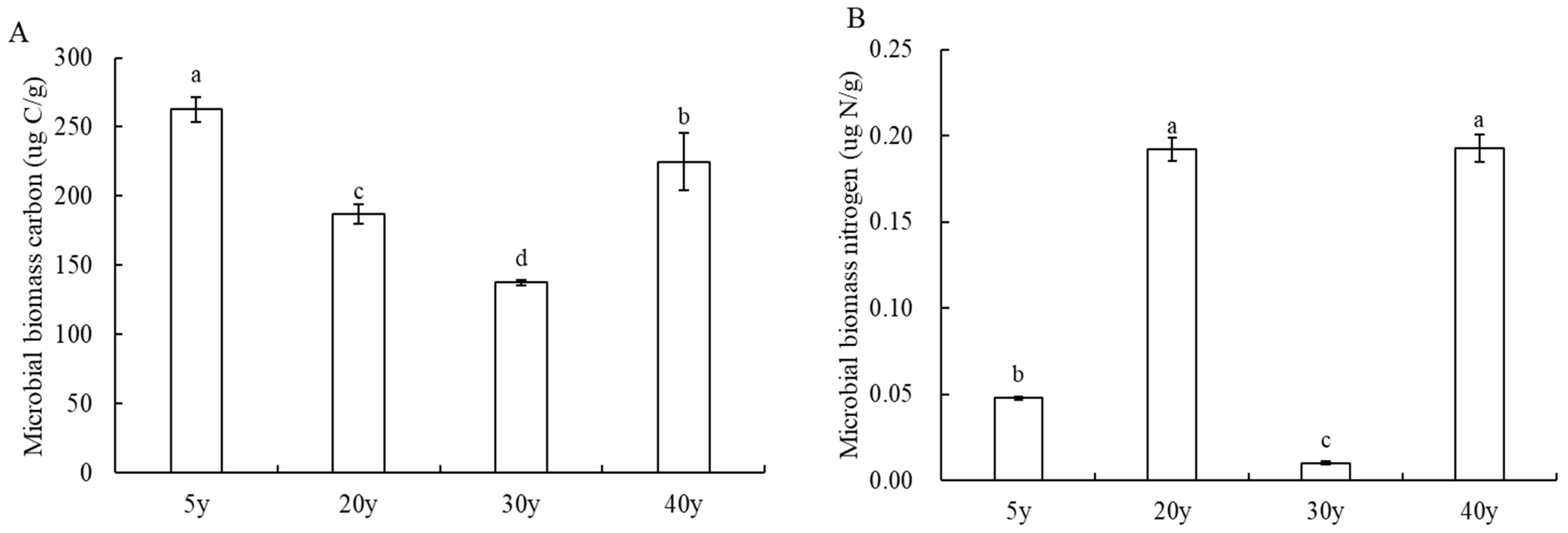
3.4. Analysis of Microbiomass Carbon and Nitrogen Content of Pear Trees in Different Planting Years
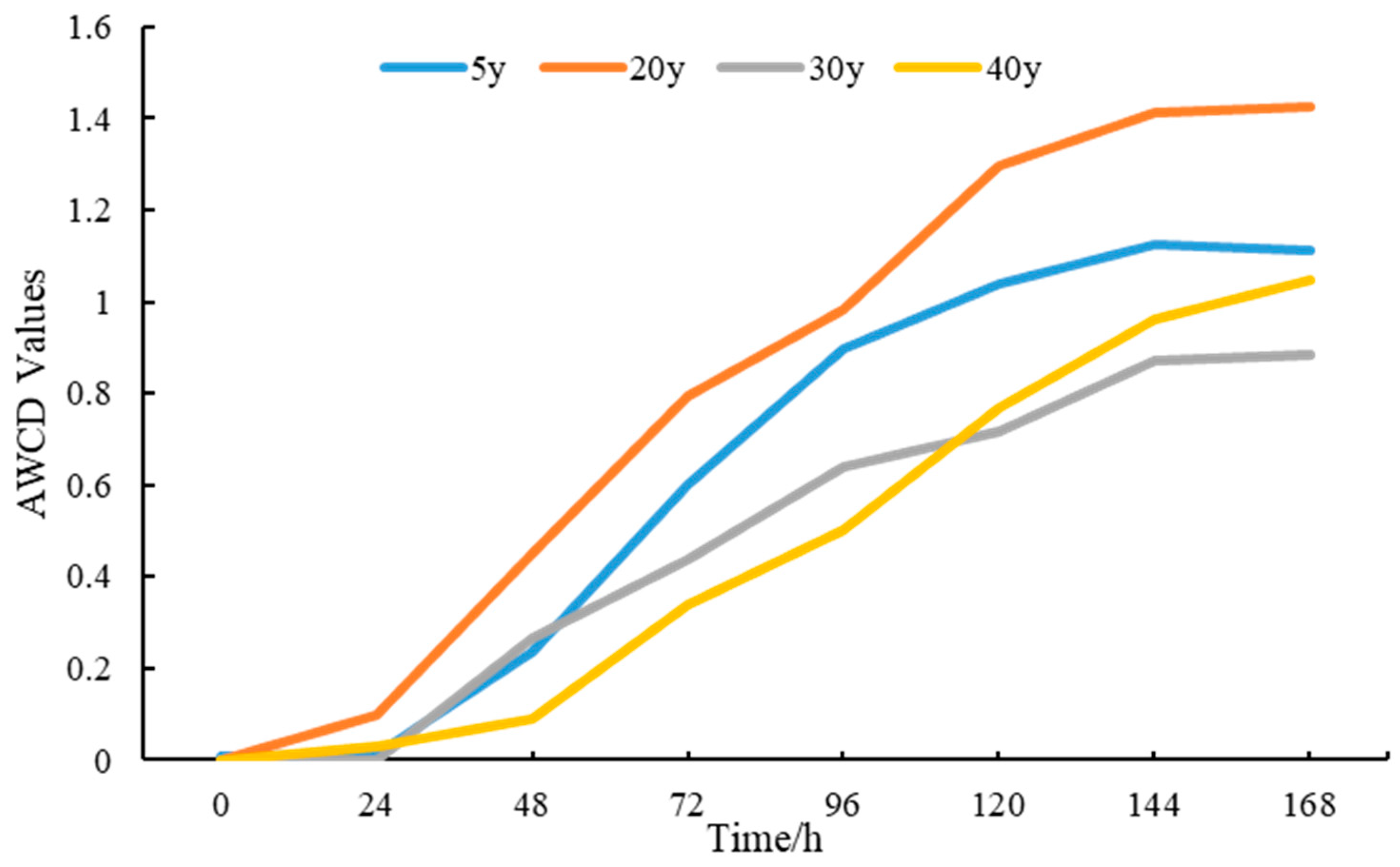
3.5. Functional Diversity Analysis of Rhizosphere Soil Bacterial Communities in Pear Trees of Different Planting Years
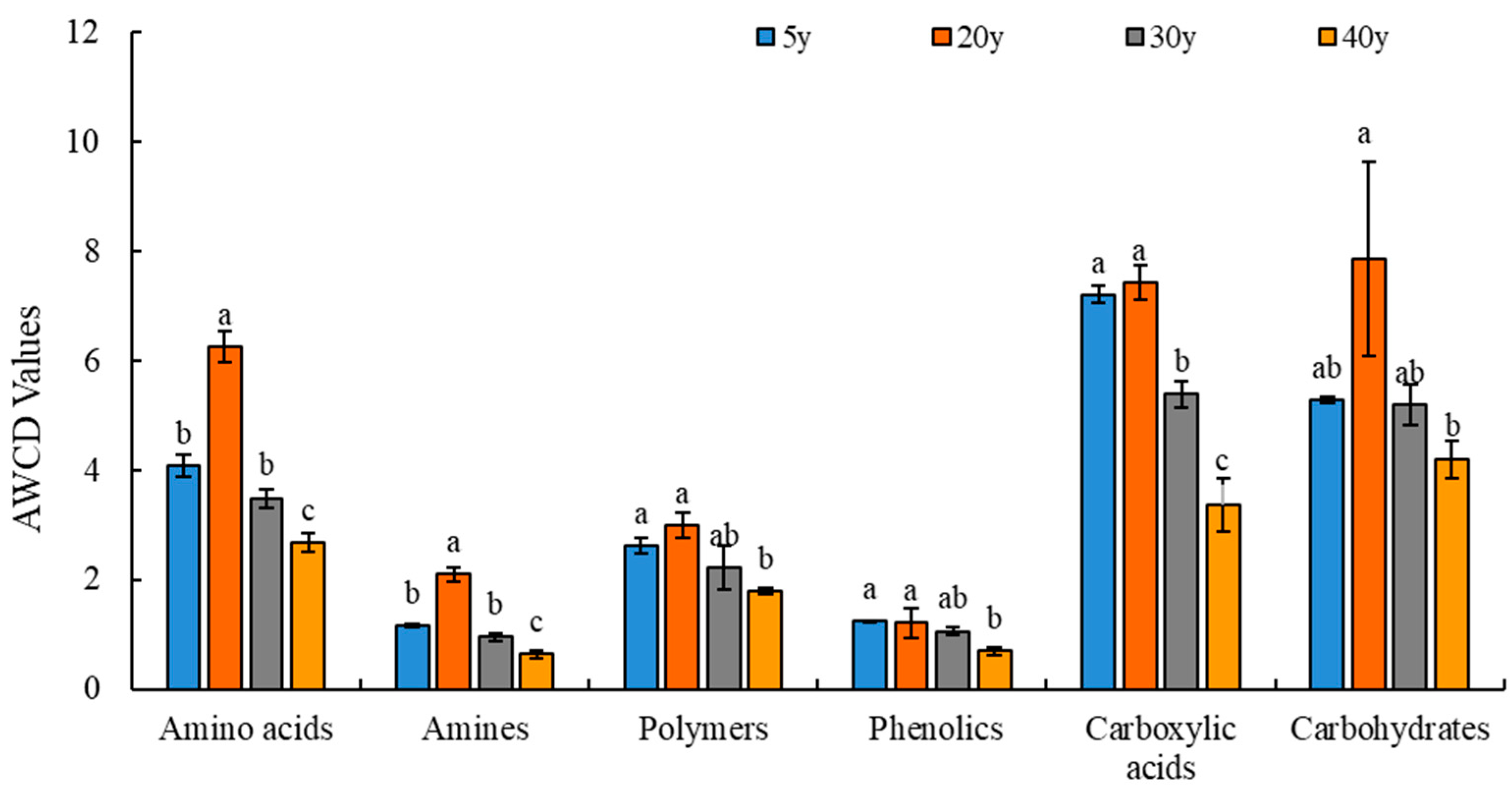
3.6. Differential Carbon Source Screening
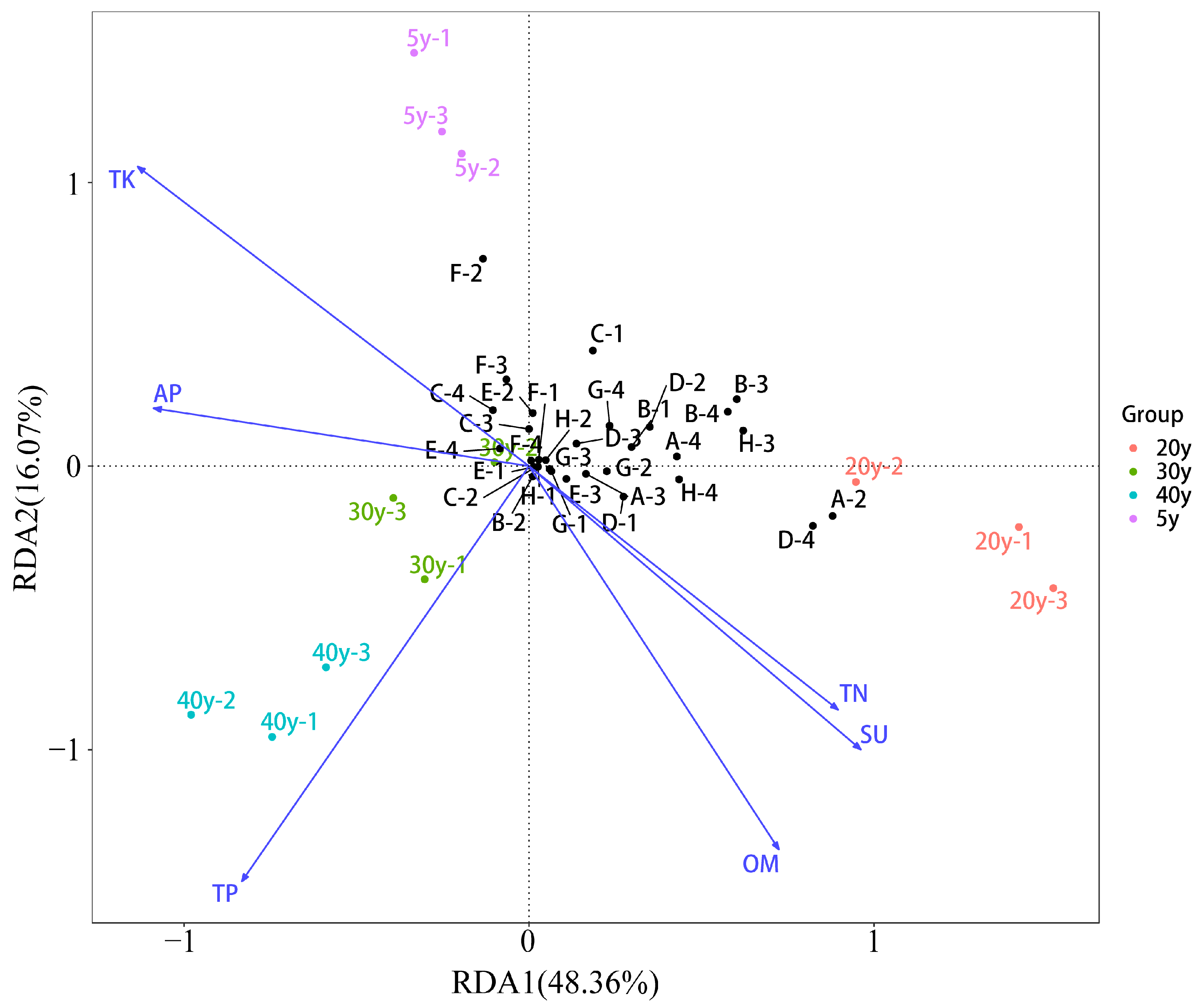
3.7. Redundancy Analysis
4. Discussion
4.1. Physicochemical Indexes of Rhizosphere Soil in Pear Trees of Different Years
4.2. Specific Microbial Communities in Pear Trees of Different Years
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hong, S.Y.; Lansky, E.; Kang, S.S.; Yang, M. A review of pears (Pyrus spp.), ancient functional food for modern times. BMC Complement. Med. Ther. 2021, 21, 219. [Google Scholar] [CrossRef]
- Attanzio, A.; Tesoriere, L.; Vasto, S.; Pintaudi, A.M.; Livrea, M.A.; Allegra, M. Short-term cactus pear [Opuntia ficus-indica (L.) Mill] fruit supplementation ameliorates the inflammatory profile and is associated with improved antioxidant status among healthy humans. Food Nutr. Res. 2018, 62, 1262. [Google Scholar] [CrossRef]
- Wang, T.; Chen, W.; Chen, D.; Huang, X.; Jin, W. Study the Changes of Light intensities and Temperatures and the Rules of Growth and Development of ‘Cui Guan’ Pear Cultivated in Plastic House. Anhui Agric. Sci. Bull. 2007, 13, 78–80. [Google Scholar]
- Gao, Y.; Yang, Q.; Yan, X.; Wu, X.; Yang, F.; Li, J.; Wei, J.; Ni, J.; Ahmad, M.; Bai, S.; et al. High-quality genome assembly of ‘Cuiguan’ pear (Pyrus pyrifolia) as a reference genome for identifying regulatory genes and epigenetic modifications responsible for bud dormancy. Hortic. Res. 2021, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bai, M.; Li, Y.; Zhang, B.; Wu, X.; Shi, Y.; Liu, H. Evaluating the Combined Effects of Water and Fertilizer Coupling Schemes on Pear Vegetative Growth and Quality in North China. Agronomy 2023, 13, 867. [Google Scholar] [CrossRef]
- Zhang, Q.; Pang, X.; Chen, X.; Ye, J.; Lin, S.; Jia, X. Rain-shelter cultivation influence rhizosphere bacterial community structure in pear and its relationship with fruit quality of pear and soil chemical properties. Sci. Hortic. 2020, 269, 109419. [Google Scholar] [CrossRef]
- Zargar, M.; Tumanyan, A.; Ivanenko, E.; Dronik, A.; Tyutyuma, N.; Pakina, E. Impact of foliar fertilization on apple and pear trees in reconciling productivity and alleviation of environmental concerns under arid conditions. Commun. Integr. Biol. 2019, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bai, B.; Chen, L.; Liu, H.; Jin, Q.; Wang, L.; Feng, T. Comparative analysis of the quality in ripe fruits of cuiguan pear from different regions. Molecules 2023, 28, 1733. [Google Scholar] [CrossRef] [PubMed]
- Musacchi, S.; Iglesias, I.; Neri, D. Training systems and sustainable orchard management for European pear (Pyrus communis L.) in the Mediterranean area: A review. Agronomy 2021, 11, 1765. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, Z.; Peijnenburg, W.J.; Liu, W.; Lu, T.; Hu, B.; Chen, J.; Chen, J.; Lin, Z.; Qian, H. Rhizosphere microbiome assembly and its impact on plant growth. J. Agric. Food Chem. 2020, 68, 5024–5038. [Google Scholar] [CrossRef]
- Jiménez-Gómez, A.; Celador-Lera, L.; Fradejas-Bayón, M.; Rivas, R. Plant probiotic bacteria enhance the quality of fruit and horticultural crops. AIMS Microbiol. 2017, 3, 483–501. [Google Scholar] [CrossRef]
- Alawiye, T.T.; Babalola, O.O. Bacterial diversity and community structure in typical plant rhizosphere. Diversity 2019, 11, 179. [Google Scholar] [CrossRef]
- Akinola, S.A.; Babalola, O.O. The fungal and archaeal community within plant rhizosphere: A review on their contribution to crop safety. J. Plant Nutr. 2021, 44, 600–618. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Bakker, M.G.; Sugiyama, A.; Manter, D.K.; Vivanco, J.M. Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS ONE 2013, 8, e55731. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Du, B.; Li, H. Effect of biochar applied with plant growth-promoting rhizobacteria (PGPR) on soil microbial community composition and nitrogen utilization in tomato. Pedosphere 2021, 31, 872–881. [Google Scholar] [CrossRef]
- Pang, Z.; Dong, F.; Liu, Q.; Lin, W.; Hu, C.; Yuan, Z. Soil metagenomics reveals effects of continuous sugarcane cropping on the structure and functional pathway of rhizospheric microbial community. Front. Microbiol. 2021, 12, 627569. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Netherway, T.; Hildebrand, F.; Pritsch, K.; Drenkhan, R.; Loit, K.; Anslan, S.; Bork, P.; Tedersoo, L. Plant nutrient-acquisition strategies drive topsoil microbiome structure and function. New Phytol. 2020, 227, 1189–1199. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, Y.; Li, X.; Zhu, Z.; Fu, W.; Hao, G.; Geng, Z.; Chen, S.; Li, Y.; Han, D. Assembly of rhizosphere microbial communities in Artemisia annua: Recruitment of plant growth-promoting microorganisms and inter-kingdom interactions between bacteria and fungi. Plant Soil 2021, 470, 127–139. [Google Scholar] [CrossRef]
- Ji, W.; Han, K.; Cai, Y.; Mu, Y.; Zhao, L.; Zhang, M.; Hou, C.; Gao, M.; Zhao, Q. Characterization of rhizosphere bacterial community and berry quality of Hutai No. 8 (Vitis vinifera L.) with different ages, and their relations. J. Sci. Food Agric. 2019, 99, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, Y.; Hu, C.; Zhan, T.; Zhang, S.; Cai, M.; Zhao, X. Soil applied Ca, Mg and B altered phyllosphere and rhizosphere bacterial microbiome and reduced Huanglongbing incidence in Gannan Navel Orange. Sci. Total Environ. 2021, 791, 148046. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Chen, Y.; Cheng, C.; Ma, R.; Zhao, D.; Li, Z.; Li, Y.; An, X.; Yang, X. Long-term soil management practices influence the rhizosphere microbial community structure and bacterial function of hilly apple orchard soil. Appl. Soil Ecol. 2022, 180, 104627. [Google Scholar] [CrossRef]
- Laurentin, A.; Edwards, C.A. Amicrotiter modification of theanthrone-sulfuric acid colorimetric assay for glucose-based carbohydrates. Anal. Biochem. 2003, 315, 143–145. [Google Scholar] [CrossRef]
- Ghnimi, S.; Al-Shibli, M.; Al-Yammahi, H.R.; Al-Dhaheri, A.; Al-Jaberi, F.; Jobe, B.; Kamal-Eldin, A. Reducing sugars, organic acids, size, color, and texture of 21 Emirati date fruit varieties (Phoenix dactylifera, L.). NFS J. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Valente, A.; Albuquerque, T.G.; Sanches-Silva, A.; Costa, H.S. Ascorbic acid content in exotic fruits: A contribution to produce quality data for food composition databases. Food Res. Int. 2011, 44, 2237–2242. [Google Scholar] [CrossRef]
- Mohammed, S.; Edna, M.; Siraj, K. The effect of traditional and improved solar drying methods on the sensory quality and nutritional composition of fruits: A case of mangoes and pineapples. Heliyon 2020, 6, e04163. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.S. Soil Agrochemical Analysis Methods; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Li, F.D.; Yu, Z.N.; He, S.J. Agricultural Microbiology Laboratory Manual; China Agricultural Press: Beijing, China, 1996. [Google Scholar]
- Choi, K.H.; Dobbs, F.C. Comparison of two kinds of Biolog microplates (GN and ECO) in their ability to distinguish among aquatic microbial communities. J. Microbiol. Meth. 1999, 36, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Chinthalapudi, D.P.; Pokhrel, S.; Kingery, W.L.; Shankle, M.W.; Ganapathi Shanmugam, S. Exploring the Synergistic Impacts of Cover Crops and Fertilization on Soil Microbial Metabolic Diversity in Dryland Soybean Production Systems Using Biolog EcoPlates. Appl. Biosci. 2023, 2, 328–346. [Google Scholar] [CrossRef]
- Teng, Z.; Fan, W.; Wang, H.; Cao, X.; Xu, X. Monitoring soil microorganisms with community-level physiological profiles using Biolog EcoPlates™ in Chaohu lakeside wetland, East China. Eurasian Soil Sci. 2020, 53, 1142–1153. [Google Scholar] [CrossRef]
- Kovács, C.; Balling, P.; Bihari, Z.; Nagy, A.; Sándor, E. Incidence of grapevinetrunk diseases is influenced by soil, topology and vineyard age, but not by Diplodia seriata infection rate in the Tokaj wine region, Hungary. Phytoparasitica 2017, 45, 21–32. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, S.; Xie, Y.; Chen, W.L. Control of Antagonistic bacteria FJ1 on Pear Scab. J. Anhui Agri. Sci. 2012, 40, 17567–17568+17574. [Google Scholar]
- Chalmers, D.J.; Mitchell, P.D.; Jerie, P.H. The physiology of growth control of peach and pear trees using reduced irrigation. Acta Horticul. 1984, 146, 143–150. [Google Scholar] [CrossRef]
- Meena, N.K.; Asrey, R. Tree age affects physicochemical, functional quality and storability of Amrapali mango (Mangifera indica L.) fruits. J. Sci. Food Agri. 2018, 98, 3255–3262. [Google Scholar] [CrossRef]
- Nakorn, S.; Chalumpak, C. Effect of tree age and fruit age on fruit development and fruit quality of Pummelo var. Tabtimsiam. J. Agric. Technol. 2016, 12, 637–645. [Google Scholar]
- Asrey, R.; Pal, R.K.; Sagar, V.R.; Patel, V.B. Impact of tree age and canopy position on fruit quality of guava. Acta Hortic. 2007, 735, 259–262. [Google Scholar] [CrossRef]
- Ahmed, I.; Dennis, F.G. Effect of apple tree age and flower position on cell size and cell number. Pak. J. Agric. Res. 1992, 13, 161–164. [Google Scholar]
- Bhatt, M.K.; Labanya, R.; Joshi, H.C. Influence of long-term chemical fertilizers and organic manures on soil fertility—A review. Univers. J. Agric. Res. 2019, 7, 177–188. [Google Scholar]
- Zhang, M.; Zhang, L.; Riaz, M.; Xia, H.; Jiang, C. Biochar amendment improved fruit quality and soil properties and microbial communities at different depths in citrus production. J. Clean. Prod. 2021, 292, 126062. [Google Scholar] [CrossRef]
- Singh, S.R.; Sharma, A.K.; Sharma, M.K. Influence of NPK combinations at different altitudes and aspects on fruit yield, quality and leaf nutrient status of apple cv. Red Delicious. Indian J. Hortic. 2009, 66, 175–182. [Google Scholar]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Liu, T.; Niu, Y. Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi. Open Chem. 2020, 18, 537–545. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, D.; Niu, Z.; Yan, J.; Zhou, X.; Kang, X. Effects of combined organic/inorganic fertilizer application on growth, photosynthetic characteristics, yield and fruit quality of Actinidia chinesis cv ‘Hongyang’. Glob. Ecol. Conserv. 2020, 22, e00997. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Mladenović, J. The influence of organic, organo-mineral and mineral fertilizers on tree growth, yielding, fruit quality and leaf nutrient composition of apple cv.‘Golden Delicious Reinders’. Sci. Hortic. 2022, 297, 110978. [Google Scholar] [CrossRef]
- Grigg, D.; Methven, D.; De Bei, R.; Rodríguez López, C.M.; Dry, P.; Collins, C. Effect of vine age on vine performance of Shiraz in the Barossa Valley, Australia. Aust. J. Grape. Wine R 2018, 24, 75–87. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahman, M.M.; Hossain, M.M.; Khaliq, Q.A.; Moniruzzaman, M. Effect of planting time and genotypes growth, yield and quality of strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2014, 167, 56–62. [Google Scholar] [CrossRef]
- Del Bubba, M.; Giordani, E.; Ancillotti, C.; Petrucci, W.A.; Ciofi, L.; Morelli, D.; Marinelli, C.; Checchini, L.; Furlanetto, S. Morphological, nutraceutical and sensorial properties of cultivated Fragaria vesca L. berries: Influence of genotype, plant age, fertilization treatment on the overall fruit quality. Agric. Food Sci. 2016, 25, 187–201. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Manici, L.M.; Insam, H.; Stres, B. Rhizosphere bacteria and fungi associated with plant growth in soils of three replanted apple orchards. Plant Soil 2015, 395, 317–333. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Doornbos, R.F.; Van Loon, L.C.; Bakker, P.A. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron. Sustain. Dev. 2012, 32, 227–243. [Google Scholar] [CrossRef]
- Rutgers, M.; Wouterse, M.; Drost, S.M.; Breure, A.M.; Mulder, C.; Stone, D.; Creamer, R.E.; Winding, A.; Bloem, J. Monitoring soil bacteria with community-level physiological profiles using Biolog™ ECO-plates in the Netherlands and Europe. Appl. Soil Ecol. 2016, 97, 23–35. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.C.; Bowen, P.A.; Bogdanoff, C.P.; Lowery, T.D.; Shaposhnikova, O.; Smith, S.; Hart, M.M. Evaluating the diversity of soil microbial communities in vineyards relative to adjacent native ecosystems. Appl. Soil Ecol. 2016, 100, 91–103. [Google Scholar] [CrossRef]
- Rodrigo-Comino, J.; Brevik, E.C.; Cerdà, A. The age of vines as a controlling factor of soil erosion processes in Mediterranean vineyards. Sci. Total Environ. 2018, 616, 1163–1173. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Sucrose (g/kg) | Reducing Sugar (g/kg) | Vitamin C (mg/kg) | Total Acid (g/kg) |
|---|---|---|---|---|
| 5 years | 5.53 ± 0.70 c | 40.1 ± 1.45 c | 51.67 ± 0.21 c | 1.77 ± 0.01 a |
| 20 years | 16.67 ± 0.75 a | 69.67 ± 1.00 a | 70.43 ± 0.70 a | 1.13 ± 0.06 c |
| 30 years | 16.07 ± 0.87 a | 70.23 ± 2.33 a | 70.00 ± 1.95 a | 1.14 ± 0.02 c |
| 40 years | 10.1 ± 0.75 b | 55.2 ± 1.15 b | 65.90 ± 2.12 b | 1.26 ± 0.02 b |
| Index | 5 Years | 20 Years | 30 Years | 40 Years |
|---|---|---|---|---|
| pH | 4.57 ± 0.09 c | 5.49 ± 0.15 a | 4.93 ± 0.06 b | 4.14 ± 0.06 d |
| Total N (g/kg) | 5.00 ± 0.50 c | 8.83 ± 0.17 a | 4.33 ± 0.17 c | 7.67 ± 0.17 b |
| Total P (g/kg) | 0.05 ± 0.01 d | 0.07 ± 0.01 c | 0.10 ± 0.01 b | 0.13 ± 0.01 a |
| Total K (g/kg) | 7.15 ± 0.84 a | 3.99 ± 0.11 c | 5.87 ± 0.21 ab | 5.65 ± 0.17 b |
| Available N (mg/kg) | 39.67 ± 2.33 a | 11.67 ± 2.33 c | 18.2 ± 2.65 bc | 25.67 ± 2.33 b |
| Available P (mg/kg) | 13.03 ± 2.00 a | 6.50 ± 0.23 b | 15.75 ± 2.27 a | 11.67 ± 9.95 ab |
| Available K (mg/kg) | 132.14 ± 5.33 c | 116.49 ± 5.33 d | 228.82 ± 1.33 a | 186.67 ± 2.02 b |
| Organic matter (%) | 12.75 ± 0.79 b | 21.85 ± 0.79 a | 15.93 ± 0.46 b | 20.94 ± 0.79 a |
| Water (%) | 14.32 ± 0.76 c | 18.36 ± 0.38 b | 20.61 ± 0.74 b | 23.32 ± 0.69 a |
| Treatment | Simpson Index | Shannon Index | Chao1 |
|---|---|---|---|
| 5 years | 0.990 ± 0.001 c | 4.270 ± 0.017 b | 0.861 ± 0.003 b |
| 20 years | 0.982 ± 0.001 c | 4.383 ± 0.050 a | 0.884 ± 0.010 a |
| 30 years | 1.007 ± 0.005 b | 4.413 ± 0.034 a | 0.890 ± 0.007 a |
| 40 years | 1.036 ± 0.013 a | 4.304 ± 0.030 b | 0.868 ± 0.006 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, X.; Chen, M.; Miao, P.; Cheng, W.; Zhou, Z.; Zhang, Y.; Zhang, Q.; Ye, J.; Jia, X.; Wang, H. Effects of Different Planting Years on Soil Physicochemical Indexes, Microbial Functional Diversity and Fruit Quality of Pear Trees. Agriculture 2024, 14, 226. https://doi.org/10.3390/agriculture14020226
Pang X, Chen M, Miao P, Cheng W, Zhou Z, Zhang Y, Zhang Q, Ye J, Jia X, Wang H. Effects of Different Planting Years on Soil Physicochemical Indexes, Microbial Functional Diversity and Fruit Quality of Pear Trees. Agriculture. 2024; 14(2):226. https://doi.org/10.3390/agriculture14020226
Chicago/Turabian StylePang, Xiaomin, Meihui Chen, Pengyao Miao, Weiting Cheng, Zewei Zhou, Ying Zhang, Qi Zhang, Jianghua Ye, Xiaoli Jia, and Haibin Wang. 2024. "Effects of Different Planting Years on Soil Physicochemical Indexes, Microbial Functional Diversity and Fruit Quality of Pear Trees" Agriculture 14, no. 2: 226. https://doi.org/10.3390/agriculture14020226
APA StylePang, X., Chen, M., Miao, P., Cheng, W., Zhou, Z., Zhang, Y., Zhang, Q., Ye, J., Jia, X., & Wang, H. (2024). Effects of Different Planting Years on Soil Physicochemical Indexes, Microbial Functional Diversity and Fruit Quality of Pear Trees. Agriculture, 14(2), 226. https://doi.org/10.3390/agriculture14020226






