Abstract
Radix pseudostellariae is one of the well-known genuine medicinal herbs in Fujian province, China. However, the continuous cropping obstacles with respect to R. pseudostellariae have seriously affected the sustainable utilization of medicinal resources and the development of related industrial systems. The occurrence of continuous cropping obstacles is a comprehensive effect of multiple deteriorating biological and abiotic factors in the rhizosphere soil. Therefore, intensive ecological methods have been the key to abating such obstacles. In this study, four treatments were set up, i.e., fallow (RP-F), fallow + bacterial fertilizer (RP-F-BF), rice-paddy-upland rotation (RP-R), and rice-paddy-upland rotation + bacterial fertilizer (RP-R-BF), during the interval between two plantings of R. pseudostellariae, with a newly planted (NP) treatment as the control. The results show that the yield of R. pseudostellariae under the RP-F treatment decreased by 46.25% compared to the NP treatment. Compared with the RP-F treatment, the yields of the RP-F-BF, RP-R, and RP-R-BF treatments significantly increased by 14.11%, 27.79%, and 62.51%, respectively. The medicinal quality of R. pseudostellariae treated with RP-R-BF was superior to that achieved with the other treatments, with the total saponin and polysaccharide contents increasing by 8.54% and 27.23%, respectively, compared to the RP-F treatment. The ecological intensive treatment of RP-R-BF significantly increased the soil pH, content of organic matter, abundance of beneficial microbial populations, and soil enzyme activity, thus remediating the deteriorating environment of continuous cropping soil. On this basis, the ecological intensive treatment RP-R-BF significantly increased the activity of protective enzymes and the expression levels of genes related to disease and stress resistance in leaves and root tubers. Redundancy and Pearson correlation analyses indicated that rice-paddy-upland rotation improved the soil structure, promoted the growth of eutrophic r-strategy bacterial communities, enhanced compound oxidation and reduction, broke the relationship between the deteriorating environment and harmful biological factors, and eventually weakened the intensity of harmful factors. The subsequent application of bacterial fertilizer improved the beneficial biological and abiotic factors, activated various ecological functions of the soil, enhanced the ecological relationship between various biological and abiotic factors, and reduced the stress intensity of R. pseudostellariae, thereby improving its disease and stress resistance, and ultimately reflecting the recovery of yield and quality. The results indirectly prove that the intensive ecological amelioration of the soil environment was the main factor for the yield recovery of R. pseudostellariae under continuous cropping.
1. Introduction
In recent decades, the world’s population has grown rapidly, exceeding the capacity of natural resources at the current level of productivity. To support the massive global population, large-scale cropping has alleviated various pressures such as land resource scarcity, environmental restriction, and economic profit incentives [1,2]. However, single-crop farming has triggered a series of continuous cropping obstacles, including the obstruction of crop growth and development, the intensification of pests and diseases, and a decline in yield and quality [3]. Continuous cropping obstacles commonly exist in grain crops, cash crops, trees, vegetables, and flowers, including potato, sweet potato, soybean, strawberry, watermelon, tomato, cotton, peanut, tobacco, medicinal plants, etc. [4,5,6]. The continuous cropping obstacles with respect to medicinal plants such as ginseng, Radix pseudostellariae, American ginseng, Panax notoginseng, Rehmannia glutinosa, Platycodon grandiflorum, and Angelica sinensis are more serious than those of other plants [6]. As a well-known medicinal herb with an annual output value of CNY 3 billion in China, R. pseudostellariae reduces production by 30–50% in the second year of continuous cropping and over 70% in the third year of continuous cropping [7,8]. In addition, the quality of R. pseudostellariae declines seriously under continuous cropping; only after an interval of 3–4 years can the yield and quality be restored by replanting in the same field [9,10], which has become a bottleneck for the sustainable development of R. pseudostellariae.
Along with an understanding of the plant rhizosphere, most studies have found that the continuous cropping of plants alters the biological characteristics of the soil through the deposition of rhizosphere secretions and aboveground litter, which shapes the microbial community of the rhizosphere [11]. The process involves an increase in pathogenic microorganisms and a decrease in beneficial microorganisms, resulting in structural imbalances in the microbial community, which lead to disease outbreaks, hindered growth and development, and a sharp decline in yield and quality [6]. For example, with an increase in continuous cropping years, the abundance of pathogenic bacteria such as Fusarium and Talaromyces significantly increases in the rhizosphere soil of R. pseudostellariae, while the abundance of beneficial bacteria such as Bacillus, Burkholderia, Pseudomonas, Penicillium, and Streptomyces decreases [12]. It is thus clear that extensive land use will inevitably affect soil health and the diversity of the soil ecosystem [13,14,15].
The restoration and maintenance of soil health have become hot research topics among peers in China and abroad [16,17,18,19]. Based on the mechanism of continuous cropping obstacles, many scholars have explored environmentally friendly technologies to rebuild soil health, such as crop rotation, soil fumigation, and adding biochar, microbial fertilizer, organic fertilizer, and special element fertilizer [20,21,22,23,24]. Although these methods are effective in the current season, their effect is difficult to maintain in the next season due to the grave deterioration of the soil caused by long-term continuous cropping. Moreover, previous findings suggest that continuous cropping obstacles are a comprehensive manifestation of multiple factors such as soil acidification, nutrient sequestration, and soil-borne diseases [5]. Therefore, it is difficult to achieve lasting beneficial effects with a single technical measure.
At present, agricultural ecologists around the world are advocating for the development of sustainable agriculture through ecological intensification to improve crop yield and quality [25]. Ecological intensification emphasizes the sustainable use of ecological resources, which means improving resource utilization and land productivity without affecting the environment, and it is thus becoming the direction of modern agriculture [25]. A diversified crop portfolio, which involves arranging different functional plants or multiple species in a single crop cycle or different planting season, can result in highly compatible diversity on the surface and underground to achieve soil health, high crop yield, and high quality [26]. Similarly, other studies have shown that a combination of different tillage techniques can be used to strengthen soil remediation, accelerate the restoration of the soil environment, and consolidate the restoration effect over a long period of time [26]. The technology portfolios include soil fumigation with the addition of a microbial fertilizer [27,28], the combined application of biochar and microbial fertilizer [29], crop rotation with the addition of biochar [30,31], and so on. In multiple crop rotation patterns, many farmers have found that rice-paddy-upland rotation has a better effect on reducing continuous cropping obstacles with respect to R. pseudostellariae than other crop rotation techniques [32]. Further, due to the contradiction withrespect to land use for planting fruit, forests, and R. pseudostellariae, rice-paddy-upland rotation with R. pseudostellariae has gradually become the main planting model in Fujian province. This has achieved sustained high and stable yields, increased farmers’ incomes, and ensured the rational use of land resources [33]. In our study, paddy field–dryland rotation with the addition of a microbial fertilizer was used to abate continuous cropping obstacles with respect to R. pseudostellariae. Using new planting and continuous cropping treatments for R. pseudostellariae as the controls, the effects of ecological intensive treatments were evaluated from the perspectives of the physiological and biochemical characteristics of R. pseudostellariae and changes in the microecological environment, such as rhizosphere soil microorganisms. Through redundancy and Pearson correlation analyses, we aimed to explore the root ecological mechanisms and technical keys by which to alleviate continuous cropping obstacles with respect to R. pseudostellariae, in order to provide technical support for its sustainable ecological cultivation.
2. Materials and Methods
2.1. Experimental Design
The field experiment was conducted in the R. pseudostellariae-producing area of Ningde city, Fujian province, China, from December 2018 to June 2021. The experimental site was located in Daping village, Ningde, Fujian province (27.32° N, 119.71° E), which has a subtropical humid climate, an average elevation of 800 m, and an average annual rainfall of 2000 mm. The soil was red soil containing 1.164 g kg−1 of total nitrogen, 0.437 g kg−1 of total phosphorus, 0.866 g kg−1 of total potassium, 0.163 g kg−1 of available nitrogen, 0.105 g kg−1 of available phosphorus, and 0.133 g kg−1 of available potassium, and the proportion of clay was 10.6%. The tuberous roots of the “Zhesheng 2” variety were used as planting material, with 450 kg per hectare. The microbial fertilizer was made from microbial agents, soybean meal, fish meal, wheat bran, and chicken manure, and was mixed at a ratio of 3:1:1:1:3, with about 3% pure nitrogen, 6% P2O5, and 3% K2O. The microbial agents were developed by our research group in the early stage [34] and mainly composed of Bacillus and Burkholderia.
We set up five treatments (Figure S1), including newly planted (NP), keeping soil fallow after harvesting R. pseudostellariae (RP-F), applying microbial fertilizer in fallow soil before the next season of R. pseudostellariae (RP-F-BF), R. pseudostellariae rotated with rice (RP-R), and applying microbial fertilizer in the soil after harvesting the rice rotated with R. pseudostellariae (RP-R-BF). From 2018 to 2021, these treatments were conducted twice. For the field trial, we adopted a completely random design, and there were three experimental plots with 20 m2 per plot in each treatment. The rice variety in the rotation was Yexiangyoulishi, with conventional planting density and field management. The fertilizer dosage per 667 m2 was 20 kg of pure N, 10 kg of P2O5, and 12 kg of K2O throughout the growing season of R. pseudostellariae, and was consistent in different treatments. The amount of microbial fertilizer was 3000 kg per hectare. Chemical fertilizers were used to supplement the insufficient NPK in the treatments of RP-F-BF and RP-R-BF, and were applied on the soil surface at a distance of 3–5 cm from the plant in late March. The fertilization in other treatments was divided into base fertilizer (70%) and topdressing (30%). The base fertilizer was sprayed into the field before planting, and the deep ploughing depth was 25 to 30 cm. The topdressing was sprinkled on the soil surface, and was applied on the soil surface at a distance of 3–5 cm from the plant in late March.
The planting time of R. pseudostellariae is in December each year, and the harvest time is in July each year. In this study, the growth period of R. pseudostellariae was divided into four stages: the seedling stage (SS, from late February to late March), early stage of root expansion (EE, in April), middle stage of root expansion (ME, from early May to early June), and late stage of root expansion (LE, from middle June to early July). In 2021, plant and soil samples were collected in the middle of each growth period. The plant samples were used to analyze quality and gene expression. The soil samples were used to investigate physicochemical properties and changes in key microbial communities.
2.2. Yield Measurement and Quality Detection
During the harvest period of R. pseudostellariae, three sampling points were randomly selected for each treatment, with an area of 1 m × 1 m for each sampling point. Root tubers were excavated, washed, and dried to measure the yield. The dried root tubers were used to test the total saponin and polysaccharide contents. The total saponin and polysaccharide contents of R. pseudostellariae were determined, respectively, using the vanillin-acetic acid [35] and sulfuric acid-anthrone colorimetric spectrophotometry methods [36].
2.3. Determination of Soil Physicochemical Properties and Enzyme Activity
The soil pH value was tested using a pH meter (PHS-3C; Weijia, Jiangshu, China). The contents of total nitrogen and phosphorus were determined using a Smartchem 450 automatic chemical analyzer (AMS Alliance, Paris, France). The content of available nitrogen was determined via the alkaline hydrolysis diffusion method. The Mo-Sb colorimetric method was used to determine the content of available phosphorus. Flame photometry was used to determine the total potassium and available potassium [37]. The soil organic matter content was determined using the sulfuric acid potassium dichromate method [37].
Five soil enzyme activities were determined using the methods described by Guan [38]. The determination of the soil catalase activity was carried out using the potassium permanganate titration method. Urease activity was measured using sodium phenol-sodium hypochlorite colorimetry. Cellulase activity was measured using anthrone colorimetry. Sucrase activity was determined using nitrosalicylic acid colorimetry. Acid phosphatase activity was determined using the disodium p-nitrophenyl phosphate colorimetric method.
2.4. High-Throughput Sequencing Analysis of Rhizosphere Soil Microorganisms
DNA was extracted using a soil DNA extraction kit (Bori, Hangzhou, China) according to the manufacturer’s instructions. The V3–V4 variable region of bacteria and internal transcriptional spacer 1 of fungi were the target sequences of the PCR (T100 Thermal Cycler; BIO RAD, Hercules, CA, USA). The primers and program reported by Wu et al. [39] were used to amplify 16S rRNA genes and ITS1 fragments. Purified amplicons were sequenced using an Illumina HiSeq 2500 (Illumina, San Diego, CA, USA) at Aoweisen Gene Technology Co., Ltd. (Beijing, China). Qualified sequences were clustered into operational taxonomic units (OTUs) at a similarity threshold of 97% using the Uparse [40] algorithm of Vsearch [41] (v2.7.1).
2.5. Determination of Protective Enzyme Activity and Gene Expression Related to Disease Resistance
The protective enzyme activity and gene expression related to disease resistance were determined in the third leaf to last and root tubers at different growth stages. A total of 0.5 g of the sample was ground with 0.05 mol/L PH 7.8 phosphate buffer and centrifuged at 5000 rpm for 10 min. The supernatant was used to detect the activity of protective enzymes. The activities of peroxidase (POD), catalase (CAT), superoxide dismutase (SOD), and malondialdehyde (MDA) were measured using guaiacol colorimetry, ultraviolet spectrography, photochemical reduction of nitrio blue tetrazolium, and thiobarbituric acid colorimetry, respectively [42].
An RT-PCR was used to explore the expression of disease-resistance-related genes in leaves and root tubers. The total RNA of leaves and root tubers at different stages was extracted using Omega’s Plant RNA extraction kit (Omega Bio-Tek, Dallas, TX, USA). cDNA was prepared using the Synthesis SuperMix reverse transcription kit (TAKARA-Bio, Kusatsu, Shiga, Japan) according to the manufacturer’s instructions. The reaction systems and programs described by Qin et al. [43] were used in the experiments. The primers are shown in Table 1. The amplification reaction was completed using a Bio RadCFX96 fluorescence quantitative RT-PCR instrument (Bio Rad, USA). Gene expression was analyzed using the 2−△△Ct calculation method [43].

Table 1.
Effects of different treatments on the diversity of microbial community in rhizosphere soil of R. pseudostellariae.
Table 1.
Effects of different treatments on the diversity of microbial community in rhizosphere soil of R. pseudostellariae.
| Chao1 | Observed_Species | Shannon | Simpson | ||
|---|---|---|---|---|---|
| Fungi | NP | 1067.65 ± 256.77 a | 824 ± 205.79 a | 5.28 ± 0.92 ab | 0.88 ± 0.08 ab |
| RP-F | 670.16 ± 272.04 b | 475.67 ± 169.47 b | 4.05 ± 0.92 c | 0.81 ± 0.11 b | |
| RP-F-BF | 917.54 ± 84.79 ab | 597 ± 35.34 ab | 5.03 ± 0.24 abc | 0.93 ± 0.01 a | |
| RP-R | 1048.44 ± 141.76 a | 760.97 ± 107.96 a | 5.91 ± 0.42 a | 0.96 ± 0.01 a | |
| RP-R-BF | 810.22 ± 165.94 ab | 514.9 ± 85.17 b | 4.37 ± 0.45 bc | 0.87 ± 0.04 ab | |
| Bacteria | NP | 3664.64 ± 95.05 ab | 2762.2 ± 21.64 a | 9.27 ± 0.01 a | 0.9947 ± 0.0005 a |
| RP-F | 3249.78 ± 24.59 c | 2381.93 ± 58.01 c | 9.01 ± 0.10 c | 0.9944 ± 0.0004 a | |
| RP-F-BF | 3793.65 ± 200.31 a | 2754.3333 ± 58.2789 a | 9.21 ± 0.05 ab | 0.995 ± 0.0002 a | |
| RP-R | 3475.52 ± 57.11 b | 2491.59 ± 68.73 b | 8.80 ± 0.12 d | 0.9902 ± 0.0015 b | |
| RP-R-BF | 3628.55 ± 126.86 ab | 2588.5 ± 68.61 b | 9.10 ± 0.09 bc | 0.9946 ± 0.0003 a |
Treatments are the same as those given in Figure 1. Different lowercase letters indicate significant differences between different treatments at p < 0.05.
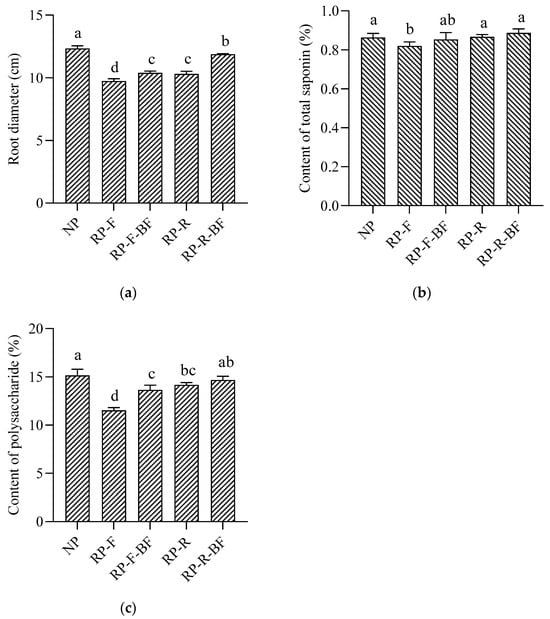
Figure 1.
Effects of different treatments on yield and quality of R. pseudostellariae. NP: the newly planting R. pseudostellariae; RP-F: the keeping soil fallow after harvesting R. pseudostellariae; RP-F-BF: applying bio-microbial fertilizer in fallow soil before the next planting period of R. pseudostellariae; RP-R: R. pseudostellariae rotated with rice; RP-R-BF: applying bio-microbial fertilizer in the soil after harvesting the rice rotated with R. pseudostellariae. Different lowercase letters indicate significant differences between different treatments at p < 0.05. (a) Root diameter of R. pseudostellariae under different treatments; (b) content of total saponin in root tubers of R. pseudostellariae; (c) content of polysaccharide in root tubers of R. pseudostellariae.
2.6. Data Analysis
The ribosomal RNA gene operon (rrn) copy numbers for bacterial OTUs were estimated according to the methods described by Stoddard et al. [44] and Nemergut et al. [45]. The community diversity of the rhizosphere microbes was analyzed using α- and β- diversity. Based on the I information, the richness and diversity indices were calculated using QIIME (v1.8.0) [41]. The PCA was analyzed using R (v3.6.0) to describe the dissimilarity between multiple samples. The β-diversity distance matrix between samples was calculated using the unweighted pair group method with an arithmetic mean (UPGMA) clustering tree. Redundancy analyses (RDAs) were used to explore the relationships between microbial abundance and environmental factors. The correlations between soil metabolites and microbial taxa were then evaluated using Spearman’s rank correlation test [46]. A one-way analysis of variance (ANOVA) was performed to assess the effects of the treatments on yield, gene expression, soil properties, and the alpha diversities and abundances of soil bacterial and fungal communities using the least significant difference (LSD, p < 0.05) in GraphPad Prism 7.1 software. The significance testing in the one-way ANOVA was based on the data from which the samples originated and followed a normal distribution [46,47].
3. Results
3.1. Yield and Quality of R. pseudostellariae under Different Treatments
The intensive ecological treatments significantly altered the appearance of root tubers in the second year of continuous planting of R. pseudostellariae (Supplementary Figure S2). Compared with the newly planted crop, the root length of R. pseudostellariae in the RP-F treatment decreased by 52.77%, and the diameter decreased by 21.12% (Figure 1b,c). The root length and diameter of R. pseudostellariae in the RP-F-BF, RP-R, and RP-R-BF treatments were also significantly lower than those of NP, but were higher than those of RP-F. The comparison between the four treatments was RP-R-BF > RP-F-BF > RP-R > RP-F.
In two identical trials, the yields of different treatments in the second test (December 2020–July 2021) were lower than those in the first test (December 2019–July 2020), but the changing trend between the two rounds of tests was consistent (Figure 1a). In the first field experiment, the yield of the RP-F treatment was significantly lower than that of the NP treatment, with a 46.25% drop in the yield. Compared with the RP-F treatment, RP-F-BF, RP-R, and RP-R-BF yields increased by 14.11%, 27.79%, and 62.51%. In the second trial, the RP-F yield declined by 67.38%. Compared with the RP-F treatment, the yields of RP-F-BF, RP-R, and RP-R-BF increased by 42.64%, 54.92%, and 140.52%, respectively. The yield of RP-R-BF increased by 55.25% as compared with RP-R.
The medicinal quality of R. pseudostellariae is an important indicator for measuring its value, and saponins and polysaccharides are its important quality indicators. The saponin and polysaccharide contents of R. pseudostellariae in the RP-F treatment were significantly lower than those of NP (Figure 1b,c). Compared to the RP-F treatment, the saponin content of R. pseudostellariae in the RP-F-BF, RP-R, and RP-R-BF treatments significantly increased by 3.66%, 6.10%, and 8.54% respectively. These three treatments significantly increased the polysaccharide contents by 18.30%, 22.81%, and 27.23%, respectively. There was no difference in the medicinal quality between the RP-R-BF and NP treatments.
3.2. Differential Physiological Responses of Leaves and Root Tubers under Different Treatments
The plants of R. pseudostellariae exhibited various physiological responses under different treatments. The content of malondialdehyde (MDA) and antioxidant enzyme activity in the leaves of R. pseudostellariae indicated its physiological state of resistance to the adverse environment. The MDA content in the leaves of different treatments showed an upward trend at different stages (Figure 2a). The MDA content in the leaves of RP-F was higher than that of other treatments in different growth stages, while the MDA content in the leaves of NP was significantly lower than that of other treatments. The peroxidase (POD, Figure 2b) activity in the leaves of different treatments was opposite to the variation tendency of MDA. The POD activity in the leaves of RP-F was lower than that of other treatments during the whole growth period. The leaf POD activity of RP-R-BF was significantly higher than that of RP-R and RP-F-BF in the middle and late stages of tuberous root expansion. The activity of superoxide dismutase (SOD, Figure 2c) in the leaves showed no significant difference in the middle stage of tuberous root expansion among different treatments. The SOD activity in the leaves of RP-R, RP-R-BF, and RP-F-BF was significantly higher than that of NP and RP-F in the early and late stages of tuberous root expansion. The leaf catalase (CAT, Figure 2d) activity showed an upward trend in RP-F and RP-R, and a downward trend in NP, RP-F-BF, and RP-R-BF. The CAT activity of RP-R-BF leaves was significantly lower than that of other treatments during the tuberous root expansion. In the later stage of tuberous root expansion, the CAT activity of RP-F leaves was significantly higher than that of other treatments.
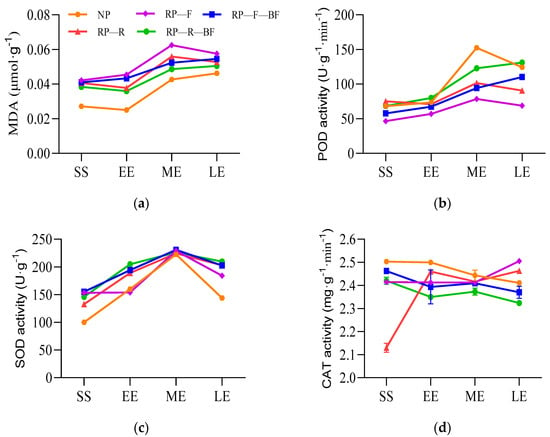
Figure 2.
Antioxidant enzyme activities in the leaves of continuously monocultured R. pseudostellariae under different treatments. Treatments are the same as those given in Figure 1. SS: the seedling stage; EE: the early expanding stage of tuber roots; ME: the middle expanding stage of tuber roots; LE: the late expanding stage of tuber roots. (a) Content of MDA in the leaves of R. pseudostellariae; (b) POD activity in the leaves of R. pseudostellariae; (c) SOD activity in the leaves of R. pseudostellariae; (d) CAT activity in the leaves of R. pseudostellariae.
The MDA content in the root tubers reached its maximum in the middle of root expansion (Figure 3a). The root MDA content of the RP-F treatment was significantly higher than that of the other treatments during the tuberous root expansion. The MDA content in the root tubers of NP was lower than that of other treatments in different growth stages. The POD activity of the root tubers in the RP-F treatment was significantly lower than that of other treatments throughout the entire expansion period. The POD activity of root tubers treated with RP-R-BF was significantly higher than that of the RP-R and RP-F-BF treatments in the middle and late stages of root expansion (Figure 3b). There was no significant difference in SOD activity among different treatments during the early stage of tuberous root expansion. The SOD activity in the root tubers of RP-F was significantly lower than that for RP-F-BF and RP-R treatments in the middle stage of expansion, but higher than that for other treatments in the later stage of expansion (Figure 3c). The CAT activity of RP-F root tubers was higher than that of other treatments during the root expansion period (Figure 3d).
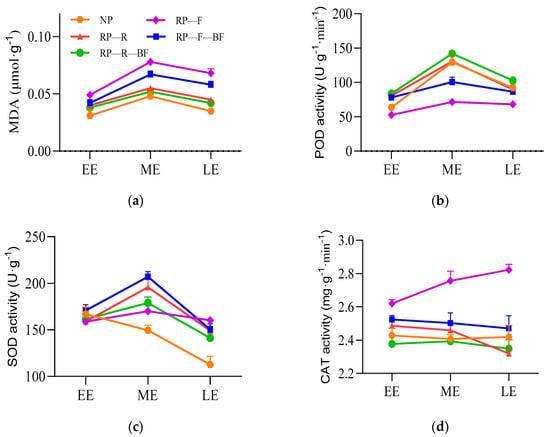
Figure 3.
Antioxidant enzyme activities in the tuber roots of continuously monocultured R. pseudostellariae under different treatments. Treatments are the same as those given in Figure 1. EE: the early expanding stage of tuber roots; ME: the middle expanding stage of tuber roots; LE: the late expanding stage of tuber roots. (a) Content of MDA in root tubers of R. pseudostellariae; (b) POD activity in root tubers of R. pseudostellariae; (c) SOD activity in root tubers of R. pseudostellariae; (d) CAT activity in root tubers of R. pseudostellariae.
3.3. Differential Expression of Disease Resistance Genes in Leaves and Tuberous Roots
In order to understand the defense status of R. pseudostellariae against continuous cropping obstacles under different treatments, we analyzed the differential expression of disease resistance genes in leaves (Supplementary Figure S3) and root tubers (Supplementary Figure S4) throughout the entire growth period. PAL1 and PAL3 expression levels in the leaves of NP were significantly lower than those of other treatments during the seedling stage, early root expansion stage, and middle expansion stage, but significantly higher than those of other treatments in the later expansion stage. In each stage, the expression levels of PAL1 and PAL3 in the leaves of RP-F were significantly lower than those of RP-F-BF, RP-R, and RP-R-BF. The expression abundance of PAL1 and PAL3 in the leaves of RP-R-BF was the highest compared to other treatments at different growth stages. Similarly, the expression abundances of CH1, CH4, and CH5 in the leaves of RP-F were significantly lower than those of RP-F-BF, RP-R, and RP-R-BF during the whole growth period. CH1, CH4, and CH5 expression levels in the NP leaves were significantly lower than those in the RP-F-BF, RP-R, and RP-R-BF treatments at the seedling, early, and middle stages of root expansion. The expression abundance of chitinase genes in leaves treated with RP-F-BF and RP-R-BF was significantly higher than that of RP-F and RP-R. The expression pattern of pathogenesis-related genes in the leaves of different treatments was opposite to that of phenylalanine ammonia lyase and chitinase genes. The expression abundances of PR1a, PR4, PR10, and PRS in the leaves of RP-F were significantly higher than those of other treatments at the seedling, early, and middle stages of root expansion, but significantly lower than those of other treatments in the later stage of root expansion. The expression level of disease-related protein genes in the NP leaves was significantly higher than that of other treatments only in the later stage of root expansion.
The gene expression patterns of phenylalanine ammonia lyase, chitinase, and pathogenesis-related genes in the root tubers of different treatments were similar to those of the leaves. PAL1 and PAL3 expression abundances in the root tubers of R. pseudostellariae treated with RP-F were significantly lower than those of RP-F-BF, RP-R, and RP-R-BF during the entire root expansion period, but they were higher than those of NP in the early stage of root expansion. PAL1 and PAL3 expression levels in the RP-R-BF treatment were the highest at each stage of root expansion. Similarly, compared with other treatments, the expression levels of CH1, CH4, and CH5 in the root tubers of RP-R-BF were the highest. However, PR1a, PR4, and PRS expression levels in the RP-F treatment were significantly higher than those of other treatments throughout the entire root expansion, while the expression level of the PR10 gene was only significantly higher than that of other treatments in the early stage of root expansion.
3.4. Differences in Soil Physicochemical Properties under Different Treatments
We measured the soil pH and NPK contents to clarify the dynamic changes in soil physicochemical properties under different treatments. The results show that after the new planting of R. pseudostellariae, the soil pH value significantly decreased (Figure 4a). The addition of microbial fertilizer, rice rotation, and the combination of the two methods resulted in a significant increase in soil pH under different treatments before the next planting. Although the soil pH of each treatment showed a decreasing trend in the continuous planting of R. pseudostellariae, the drop in soil pH under the RP-R-BF treatment was slower than that of other treatments, and there was no significant difference in soil pH between RP-R-BF and NP at the later stage of root expansion. The soil pH of RP-F was significantly lower than that of other treatments at different growth stages, and it showed the highest degree of acidification in the later stage of root expansion.
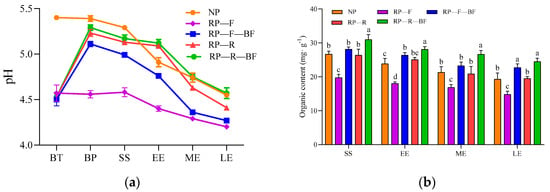
Figure 4.
Soil pH value and organic content under different treatments. Treatments are the same as those given in Figure 1. BT: before treatments; BP: before the planting of second crop; SS: the seedling stage; EE: the early expanding stage of tuber roots; ME: the middle expanding stage of tuber roots; LE: the late expanding stage of tuber roots. Different lowercase letters indicate significant differences between different treatments at p < 0.05. (a) Soil pH value under different treatments; (b) soil organic content under different treatments.
The organic matter (OM) content of the RP-F treatment was the lowest, and the decrease throughout the entire growth period was greater than that of other treatments (Figure 4b). The OM contents of RP-F-BF and RP-R-BF were significantly higher than that of NP in the middle and late stages of root expansion, and they maintained a high level in the later stage of root expansion. The contents of available phosphorus, available potassium, total phosphorus, and total potassium were significantly higher than those of NP soil (Figure 5b,c,e,f). Available phosphorus, available potassium, and total phosphorus in the soil treated with RP-R and RP-R-BF were also significantly lower than those under the RP-F treatment (Figure 5b,c,e).
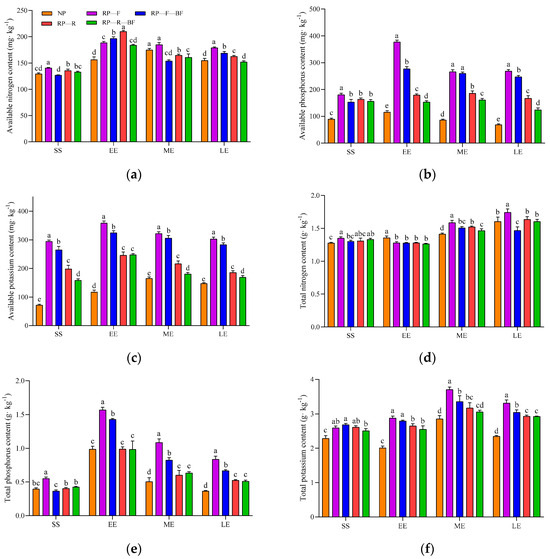
Figure 5.
Soil nitrogen, phosphorus, and potassium contents of R. pseudostellariae under different treatments. Treatments are the same as those given in Figure 1. SS: the seedling stage; EE: the early expanding stage of tuber roots; ME: the middle expanding stage of tuber roots; LE: the late expanding stage of tuber roots. Different lowercase letters indicate significant differences between different treatments at p < 0.05. (a) Soil-available nitrogen under different treatments; (b) soil-available phosphorus under different treatments; (c) soil-available potassium under different treatments; (d) total soil nitrogen under different treatments; (e) total soil phosphorus under different treatments; (f) total soil potassium under different treatments.
3.5. Differences in Microbial Community Structure in the Rhizosphere Soil of R. pseudostellariae under Different Treatments
The diversity indexes of the rhizosphere microbial community are summarized in Table 1. The Chao1, observed_species, and Shannon indices of the fungal community in the rhizosphere soil of NP and RP-R are significantly higher than those of RP-F. The alpha indexes of fungal diversity in the RP-F rhizosphere soil are lower than those of the other treatments. Similarly, the Chao1, observed_species, and Shannon indices of the bacterial community in RP-F are significantly lower than those of NP, RP-F-BF, and RP-R-BF.
The analysis of UPGMA and PCA based on I shows the differences in fungal and bacterial communities among the different treatments (Supplementary Figure S5a–d). The cluster of the fungal community shows RP-F, RP-F-BF, and RP-R-BF gathering together to form a group, and the NP and RP-R treatments are each grouped separately. The principal component and cluster analyses of bacterial communities in the rhizosphere soil under different treatments are divided into three groups, with RP-F and RP-F-BF belonging to one group, RP-R and RP-R-BF belonging to another group, and NP being a separate group.
The ribosomal RNA operon (rrn) copy number is a genomic feature related to bacterial growth rates and nutritional needs, and can effectively predict the bacterial growth rate and nutrient utilization efficiency. Due to the large number of ribosomes required for rapid bacterial growth, r-strategy (eutrophic) bacteria will hold more ribosomal RNA operons. Therefore, K-strategy (oligotrophic) and r-strategy bacteria can be distinguished based on the number of ribosomal RNA operons. As shown in Figure 6a, bacterial species with rrn ≤ 2 (K-strategy) account for 56.49%, species with rrn >2 and <5 (r-K intermediate type) account for 27.25%, and species with rrn ≥ 5 (r-strategy) account for 5.72%. The relative abundance of K-strategy bacteria in the new planting is the smallest. There is no significant difference in the abundance of K-strategy bacteria between the RP-F, RP-F-BF, and RP-R-BF treatments. The abundance of K-strategy bacteria under the RP-R treatment is significantly lower than that of RP-F, RP-F-BF, and RP-R-BF (Figure 6c). The abundance of r-strategy bacteria and the r/K ratio in RP-F are significantly lower than those of other treatments, while they are the highest for RP-R (Figure 6b). Thus, it can be seen that under different treatments, the evolution of rhizosphere soil microorganisms is related to the differences in life-history strategies adopted. The RP-R treatment has a good effect on the subsequent planting of R. pseudostellariae, which may be related to the evolution of rhizosphere microorganisms using the r-strategy, and the formation of eutrophic structural characteristics.
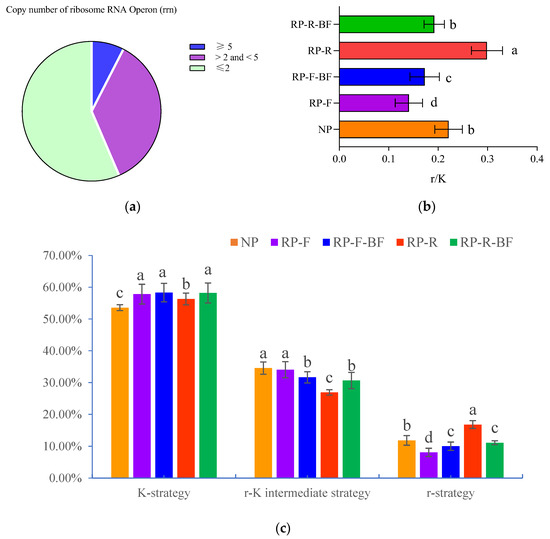
Figure 6.
Analysis of r-K survival strategies of rhizosphere soil microorganisms under different soil intensification treatments. Treatments are the same as those given in Figure 1. (a) The proportion of species with different ribosome RNA operon copy numbers; (b) shows the proportion of r-strategy bacteria and K-strategy bacteria under different treatments; (c) shows the relative abundance of r-strategy bacteria, K-strategy bacteria, and r-K intermediate strategy bacteria under different treatments. Different lowercase letters indicate significant differences between different treatments at p < 0.05.
3.6. Correlation Analysis between Rhizosphere Microbial Community and Environmental Factors
The RDA analysis showed that there was a significant correlation between fungal communities and factors including pH, available NPK, catalase, urease, and cellulose (Figure 7a, Table 2). Environmental factors including pH, urease, available PK, and total P significantly affected bacterial communities (Figure 7b, Table 2).
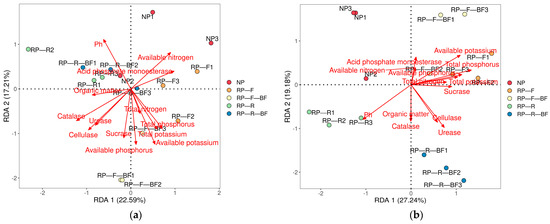
Figure 7.
Redundancy analysis between microbial communities and environmental factors in the rhizosphere soil of R. pseudostellariae under different treatments: (a) showed the redundancy analysis between fungal communities and environmental factors, and (b) showed the redundancy analysis between bacterial communities and environmental factors.

Table 2.
Effects of environmental factors on the rhizosphere microbial community of R. pseudostellariae.
A Pearson correlation analysis showed a significant correlation between soil environmental factors and key microorganisms (Figure 8). Total NPK in the soil was positively correlated with Acidobacterium and Burkholderia, while it was negatively correlated with Nitrosospira, Nitrospirae, Pseudomonas, Rhizobium, and Glomeromycota. The available NPK was positively correlated with Fusarium, Acidobacterium, and Didymella, but negatively correlated with Nitrosospira, Nitrospirae, Penicillium, Pseudomonas, and Rhodobacter. Sucrase and acid phosphate monoesterase were positively correlated with potentially beneficial microorganisms such as Trichoderma, Streptomyces, Pseudomonas, Nitrosospira, and Bacillus. There was a positive correlation between catalase and Penicillium. Urease, cellulase, catalase, and organic matter were negatively correlated with the pathogenic bacteria Didymella. Acid phosphate monoesterase was negatively correlated with the pathogenic bacteria Fusarium and Talaromyces. Soil pH was negatively correlated with Acidobacterium, Fusarium, and Talaromyces, while it was positively correlated with Nitrospirae and Rhizobium.
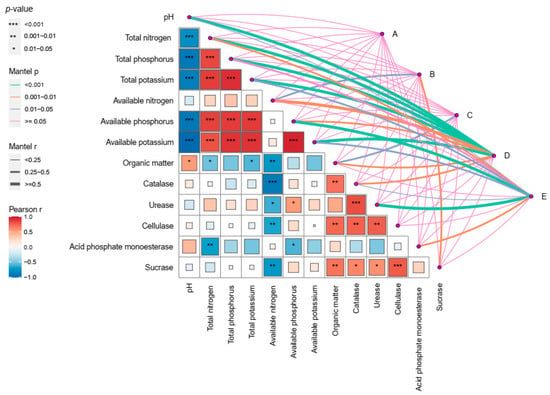
Figure 8.
The correlation analysis demonstrated the relationships between soil environmental factors and microbial communities. A: Acidobacterium, Acidobacteria, Actinobacterium, Actinobacteria; B: Bacillus, Pseudomonas, Burkholderia-Paraburkholderia, Streptomyces, Penicillium, Trichoderma, Glomeromycota; C: Cellulomonas; D: Fusarium, Talaromyces, Aspergillus, Didymella; E: Rhizobacter, Nitrospira, Nitrosospira, Nitrospirae.
4. Discussion
Continuous cropping obstacles are an unavoidable outcome in large-scale intensive agricultural production, and they have become one of the main limiting factors for soil health and the sustainable stability of productivity. The occurrence of continuous cropping obstacles in medicinal plants is a comprehensive reflection of the superimposed deterioration of multiple factors in the rhizosphere environment. Therefore, restoration of the soil environment through diversified intensive ecological methods is the key to abating continuous cropping obstacles.
Crop rotation, intercropping, and the application of bacterial fertilizers are effective measures by which to abate obstacles to continuous cropping [4,5,6]. Rice rotation has shown that residual rice stalks with an abundant silicon content can improve the diversity of soil fungi, promote the growth of Coniochaeta prunicola and Thermothelomyces hinnuleus, and inhibit the growth of pathogenic fungi such as Fusarium equiseti [48,49]. In this study, we found that after harvesting the first planting of R. pseudostellariae, treatments involving rice rotation, the addition of bacterial fertilizer, and a combination of the two methods significantly increased the organic matter content and pH value and slowed the drop in pH value during the second planting of R. pseudostellariae. These results are consistent with other research, which has shown that several medicinal plants such as Ligusticum sinense, Fritillaria hunbergia, Alisma orientalis, and Pinellia ternate rotated with cereal crops such as rice or wheat can significantly increase soil pH and effectively alleviate soil acidification in continuous cropping [50]. Other studies have shown that the continuous cropping of most medicinal plants exhibits the highest degree of acidification in rhizosphere soil, and changes in soil acidity significantly affect the microbial community structure [51]. For example, a decrease in soil pH caused by continuous cropping is beneficial for the proliferation of pathogenic fungi such as Fusarium oxysporum, while being disadvantageous to beneficial microorganisms such as Burkholderia [12]. The results of this study show that intensive ecological treatment, i.e., a combination of rice rotation and the addition of microbial fertilizer, significantly increases the diversity indexes of fungal and bacterial communities, reduces the population abundance of pathogenic fungi such as Fusarium and Aspergillus, and increases the population abundance of beneficial bacteria such as Penicillium and Trichoderma (Supplementary Figure S6). Research on the abatement of soybean continuous cropping obstacles has also indicated that maize/soybean rotation can significantly improve the abundance and diversity of bacteria in rhizosphere soil compared to soybean continuous cropping [52,53]. Similarly, compared to 3-year continuous cropping of pepper, the abundance of bacteria and actinomycetes in the rhizosphere soil after garlic/pepper rotation significantly increased, while the number of pathogenic fungi significantly decreased [54]. Thus, rotation can effectively improve the diversity and stability of microbial communities and ameliorate the soil environment and quality.
In addition, bacterial fertilizers are widely used due to their environmentally friendly, disease resistant, and soil improvement characteristics [55,56,57]. Bacterial fertilizer plays a significant role in reducing continuous cropping obstacles for plants such as tomato [58,59], cucumber [60,61], watermelon [62,63], strawberry [27,64], etc. The results of this study indicate that the combination of paddy-upland rotation and the application of bacterial fertilizer effectively improved the yield and quality of replanted R. pseudostellariae. This is because the intensive ecological treatment before the continuous planting of R. pseudostellariae significantly increased the soil organic matter content and promoted the activities of sucrase, acid phosphate monoesterase, catalase, urease, and cellulose. The results of the Spearman correlation analysis show that the soil enzyme activities were positively correlated with beneficial bacteria such as Trichoderma, Streptomyces, Pseudomonas, Nitrosospira, and Penicillium. It is thus clear that the intensive ecological methods for continuous cropping soil increase the abundance of beneficial microorganisms, which can not only degrade harmful substances, inhibiting rhizosphere pathogenic microorganisms and improving stress resistance, but also efficiently decompose and activate ineffective nutrients in the soil, improve soil physical and chemical properties, promote crop absorption of nutrients, stimulate crop growth, and thus improve the yield and quality of continuous cropping [56,57].
The results also show that, under intensive ecological soil treatment, the peroxidase and superoxide dismutase activities in the leaves and tuberous roots of R. pseudostellariae were significantly higher than those of the RP-F treatment, and the MDA content was significantly reduced. This indicates that the ecological intensive treatment significantly improved the protective enzyme activity in the continuous planting of R. pseudostellariae, quickly removing and reducing the damage caused by reactive oxygen. In addition, the expression levels of defense-related genes, including phenylalanine ammonia lyase and chitinase genes, in the leaves and tuberous roots of replanted R. pseudostellariae under the intensive ecological soil treatment were significantly higher than those of the RP-F and RP-R treatments, while the expression levels of pathogenesis-related genes were significantly lower than those of the RP-F treatment. In addition, CAT activity in RP-R at the seedling stage was significantly lower than that in other treatments, which may also have been caused by the low expression abundance of chitinase gene in RP-R. Studies have shown that when bacteria produce chitinase, its hydrogen peroxide content is significantly higher [65]. In this study, CAT activity in RP-R gradually increased, while the activity of CAT in other treatments decreased to remove excess reactive oxygen species (ROS), especially H2O2 [66]. The above results indicate that, under continuous cropping stress, the replanted R. pseudostellariae under the RP-F treatment was in a state of stress response throughout the entire growth period, disrupting normal physiological functions and even excessively improving disease resistance, which led to the premature aging and death of plant tissues and organs, thereby reducing overall stress resistance, hindering normal growth, and seriously affecting the yield and quality of R. pseudostellariae [9]. Qin et al. [43] found that after being infected by the pathogenic fungus Fusarium oxysporum, R. pseudostellariae accumulates a large amount of calcium ions in the infected cells in a short time, then activates the calcium signal sensing system to induce hypersensitivity reactions, and promotes the secretion of more phenolic acids by the roots, which causes colonization by the pathogenic fungus F. oxysporum around the roots of R. pseudostellariae, resulting in a sharp increase in the number of pathogenic F. oxysporum fungi in the rhizosphere soil. Ultimately, this leads to the deterioration of soil health. Therefore, with the increment of continuous cropping years, R. pseudostellariae exhibits weak stems and abnormal leaves, and significantly reduces aboveground and underground biomass [9,10]. In this study, the combination of rice-paddy-upland rotation and bacterial fertilizer application not only improved the stress resistance of R. pseudostellariae, but also significantly increased the length and diameter of root tubers, as well as the yield. The intensive ecological treatment also significantly increased the polysaccharide and saponin contents, achieving good results in alleviating continuous cropping obstacles with respect to R. pseudostellariae.
5. Conclusions
In summary, this study suggests that continuous cropping obstacles are the result of multiple factors that are interrelated, superimposed, and amplified to hinder plant growth and development. Breaking down the association between the factors of continuous cropping obstacles, minimizing the effects of the obstacles, and conversely enhancing various benign ecological factors conducive to plant growth are the keys to effectively repairing the ecological relationship between various biological and abiotic factors in the rhizosphere ecosystem. Therefore, using intensive ecological methods to reconstruct healthy rhizosphere soil is an effective path by which to reduce continuous cropping obstacles. The results of this study indicate that the combination of rice-paddy-upland rotation and bacterial fertilizer application is the best intensive ecological treatment by which to abate the continuous cropping obstacles with respect to R. pseudostellariae. This is mainly due to the benign change in the soil environment caused by rice-paddy-upland rotation, which enhanced the oxidation and reduction of compounds, alleviated soil acidification, and improved soil conditions. Rice-paddy-upland rotation also disconnected the relationship between the deteriorating environment caused by continuous cropping and harmful biological factors in the early stage of replanting R. pseudostellariae, suppressed the intensity of harmful factors, and resulted in the diversity reconstruction of rhizosphere microorganisms with increasing eutrophic r-strategy bacterial communities. The follow-up application of bacterial fertilizer enhanced the improvement effect on the rhizosphere soil, which increased the soil organic matter and abundance of beneficial microbes, improved various soil enzyme activities, and activated ecological functions in the rhizosphere soil, thereby achieving the healthy growth, stable yield, and high quality of replanted R. pseudostellariae.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture14020326/s1, Figure S1. Schematic diagram of soil treatments of R. pseudostellariae in 2019 and 2020. Figure S2. Appearance and morphology of R. Pseudostellariae root tubers under different treatments. Figure S3. Differential expression of disease resistance and defense-related genes in the leaves of R. pseudostellariae under different treatments. Figure S4. Differential expression of disease resistance and defense-related genes in the root tubers of R. pseudostellariae under different treatments. Figure S5. Principal component analysis and cluster analysis of microbial communities in the rhizosphere soil of R. pseudostellariae under different treatments. Figure S6. The abundance of microbial species in the rhizosphere soil of R. pseudostellariae under different treatments. Table S1. Primer sequence of defense-related genes.
Author Contributions
S.L. and W.L. conceived and directed the project. S.L., Y.Y., T.C., Y.J., J.Y. and Z.C. performed all of the experiments. S.L. and Y.Y. and Y.J. performed the integrated data analysis. S.L. and T.C. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Key Research and Development Plan (2017YFE0121800), the National Science Foundation of China (31401950, 81573530), the Natural Science Foundation of Fujian Province (2022J01592), and the Putian Science and Technology Plan Project (2021NJJ005).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Files.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eisenstein, M. Natural solutions for agricultural productivity. Nature 2020, 588, 58–59. [Google Scholar] [CrossRef]
- Crippa, M.; Solazzo, E.; Guizzardi, D.; Monforti-Ferrario, F.; Tubiello, F.-N.; Leip, A. Food systems are responsible for a third of global anthropogenic GHG emissions. Nat. Food. 2021, 2, 198–209. [Google Scholar] [CrossRef]
- Lin, W.-X.; Chen, T. Transition of agricultural systems to ecologicalizaton and new vision of modern eco-agriculture development in China. Chinese J. Eco-Agri. 2019, 27, 169–176. [Google Scholar]
- Huang, L.-F.; Song, L.-X.; Xia, X.J.; Mao, W.-H.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q. Plant-soil feedbacks and soil sickness: From mechanisms to application in agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef]
- Wang, J.-H.; Chen, T.; Lin, W.-X. Plant allelopathy types and their application in agriculture. Chinese J. Eco-Agri. 2013, 21, 1173–1183. [Google Scholar] [CrossRef]
- Chen, T.; Lin, S.; Wu, L.-K.; Lin, W.-X.; Sampietro, D.-A. Soil Sickness: Current Status and Future Perspectives. Allelopathy J. 2015, 36, 167–195. [Google Scholar]
- Guo, L.-P.; Huang, L.-Q.; Jiang, Y.-X.; Lyu, D.-M. Soil deterioration during cultivation of medicinal plants and ways to prevent it. China J. Chin. Mater. Medica 2006, 31, 714–717. [Google Scholar]
- Gao, W.-W.; Zhao, Y.-J.; Wang, Y.-P.; Chen, S.-L. Review of research on sustainable use of medicinal plants cropland in China. China J. Chin. Mater. Medica 2006, 31, 1665–1669. [Google Scholar]
- Lin, M.-Z.; Zhang, Z.-X.; Lin, Z.-C.; Long, C.-H.; Zeng, L.-J.; Lin, W.-X. Analysis of differential expression of proteins in replanting disease of Pseudostellaria heterophylla. Acta Prataculturae Sin. 2010, 19, 197–207. [Google Scholar]
- Xia, P.-H.; Liu, Y. Study on Obstacle Effect of Succession Cropping on Pseudostellaria heterophylla. Acta Bot. Boreali-Occident. Sin. 2010, 30, 2240–2246. [Google Scholar]
- Zhang, F.-S.; Shen, J.-B.; Feng, G. Rhizosphere Ecology: Processes & Management; China Agricultural University Press: Beijing, China, 2009. [Google Scholar]
- Wu, H.-M.; Qin, X.-J.; Wang, J.-Y.; Wu, L.-K.; Chen, J.; Fan, J.-K.; Zheng, L.; Tangtai, H.-P.; Arafat, Y.; Lin, W.-W.; et al. Rhizosphere chemical language in the Radix pseudostellariae rhizosphere under continuous monoculture regimes. Agr. Ecosyst. Environ. 2019, 270, 19–31. [Google Scholar] [CrossRef]
- van der Putten, W.-H.; Bardgett, R.-D.; Bever, J.-D.; Bezemer, T.-M.; Casper, B.-B.; Fukami, T.; Kardol, P.; Klironomos, J.-N.; Kulmatiski, A.; Schweitzer, J.-A.; et al. Plant-soil feedback: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.-E.; Tringe, S.-G.; Sa, T.-M.; Singh, B.-K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; Yuan, J.; Hale, L.; Tao, W.; Huang, Q.-W.; Vivanco, J.-M.; Zhou, J.-Z.; Kowalchuk, G.-A.; Shen, Q.-R. Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ. 2021, 44, 613–628. [Google Scholar] [CrossRef]
- Tittonell, P. Ecological intensification of agriculture—Sustainable by nature. Curr. Opin. Env. Sust. 2014, 8, 53–61. [Google Scholar] [CrossRef]
- Kleijn, D.; Bommarco, R.; Fijen, T.-P.-M.; Garibaldi, L.-A.; Potts, S.-G.; van der Putten, W.-H. Ecological Intensification: Bridging the Gap between Science and Practice. Trends Ecol. Evol. 2019, 34, 154–166. [Google Scholar] [CrossRef]
- Jing, J.-Y.; Cong, W.-F.; Bezemer, T.-M. Legacies at work: Plant–soil–microbiome interactions underpinning agricultural sustainability. Trends Plant Sci. 2022, 8, 781–792. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Zhang, J.-Z.; Jia, J.-Y.; Fan, F.; Zhang, F.-S.; Zhang, J.-L. Research progresses on farmland soil ecosystem multifunctionality. Acta Pedol. Sin. 2022, 59, 1177–1189. [Google Scholar]
- Mao, L.-L.; Zhang, L.-Z.; ZHANG, S.-P.; Evers, J.-B.; van der Werf, W.; Wang, J.-J.; Sun, H.-Q.; Su, Z.-C.; Spiertz, H. Resource use efficiency, ecological intensification and sustainability of intercropping systems. J. Integr. Agr. 2015, 14, 1542–1550. [Google Scholar] [CrossRef]
- Wu, Q.-M.; Zhang, J.-M.; Li, Y.-Y.; Zhang, Y. Recent advances on the mechanism of beneficial microbial fertilizers in crops. Biotech. Bulletin. 2021, 37, 221–230. [Google Scholar]
- Bergstrand, K.-J. Organic fertilizers in greenhouse production systems-a review. Sci. Hortic. 2022, 295, 110855. [Google Scholar] [CrossRef]
- Cao, A.-C.; Fang, W.-S.; Li, Y.; Yan, D.-D.; Wang, Q.-X.; Guo, M.-X.; Huang, B.; Song, Z.-X.; Jin, X. Review on 60 years of soil fumigation and disinfestation in China. J. Plant Protect. 2022, 49, 325–335. [Google Scholar]
- Yadav, S.-P.-S.; Bhandari, S.; Bhatta, D.; Poudel, A.; Bhattarai, S.; Yadav, P.; Ghimire, N.; Paudel, P.; Paudel, P.; Shrestha, J.; et al. Biochar application: A sustainable approach to improve soil health. J. Agr. Food Res. 2023, 11, 100498. [Google Scholar]
- Dore, T.; Makowski, D.; Malezieux, E.; Munier, N.; Tchamitchian, M.; Tittonell, P. Facing up to the paradigm of ecological intensification in agronomy: Revisiting methods, concepts and knowledge. Eur. J. Agron. 2011, 34, 197–210. [Google Scholar] [CrossRef]
- Li, L. Intercropping enhances agroecosystem services and functioning: Current knowledge and perspectives. Chinese J. Eco.-Agri. 2016, 24, 403–415. [Google Scholar]
- Li, Q.J.; Zhang, D.Q.; Song, Z.X.; Ren, L.R.; Jin, X.; Fang, W.-S.; Yan, D.-D.; Li, Y.; Wang, Q.-X.; Cao, A.-C. Organic fertilizer activates soil beneficial microorganisms to promote strawberry growth and soil health after fumigation. Environ. Pollut. 2022, 295, 118653. [Google Scholar] [CrossRef]
- Pu, R.-F.; Wang, P.-P.; Guo, L.-P.; Li, M.-H.; Cui, X.-M.; Wang, C.-X.; Liu, Y.; Yang, Y. The remediation effects of microbial organic fertilizer on soil microorganisms after chloropicrin fumigation. Ecotox. Environ. Safe. 2022, 231, 113188. [Google Scholar] [CrossRef]
- Bai, S.-H.; Omidvar, N.; Gallart, M.; Kämper, W.; Tahmasbian, I.; Farrar, M.-B.; Singh, K.; Zhou, G.-Y.; Muqadass, B.; Xu, C.-Y.; et al. Combined effects of biochar and fertilizer applications on yield: A review and meta-analysis. Sci. Total Environ. 2022, 808, 152073. [Google Scholar] [CrossRef]
- Reyes-Cabrera, J.; Leon, R.-G.; Erickson, J.-E.; Rowland, D.-L.; Silveira, M.-L.; Morgan, K.-T. Differences in biomass and water dynamics between a cotton-peanut rotation and a sweet sorghum bioenergy crop with and without biochar and vinasse as soil amendments. Field Crop Res. 2017, 214, 123–130. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Sun, B.-H.; Wu, S.-F.; Feng, H.; Gao, M.-X.; Zhang, B.-B.; Liu, Y.-Y. After-effects of straw and straw-derived biochar application on crop growth, yield, and soil properties in wheat (Triticum aestivum L.)-maize (Zea mays L.) rotations: A four-year field experiment. Sci. Total Environ. 2021, 780, 146560. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Huang, D.-S. Efficient cultivation technique of Radix pseudostellariae and rice in rotation. Agric. Technol. Commun. 2009, 12, 171–172. [Google Scholar]
- Yang, Y. Techniques of rice rotation cultivation in Radix pseudostellariae. Mod. Rural Sci. Technol. 2017, 4, 15. [Google Scholar]
- Wei, X.-Y. Insight into the Mechanism of Panax notoginseng under Continuous Monoculture and Evaluation of Microial Fertilizer Remediation; Fujian Agriculture and Forestry University: Fuzhou, China, 2018. [Google Scholar]
- Xu, Q.; Wang, H.-F.; Zhou, X.-Y. Optimization of extraction technology of saponins from Radix pseudostellariae. Chin. Tradit. Herb. Drugs 2001, 32, 34–35. [Google Scholar]
- Liu, D.; Zhang, C.-H.; Chen, X.; Ling, Q.-Y.; Han, B.-X.; Chen, N.-F. Optimization of extraction process and contrastive study of polysoccharides from Radix Pseudostellarial. J. Anhui Agri. Sci. 2014, 42, 12469–12471. [Google Scholar]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Guan, S.-Y. Soil Enzyme and Study Method; Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Wu, H.-M.; Lin, M.-H.; Rensing, C.; Qin, X.-J.; Zhang, S.-K.; Chen, J.; Wu, L.-K.; Zhao, Y.-L.; Lin, S.; Lin, W.-X. Plant-mediated rhizospheric interactions in intraspecific intercropping alleviate the replanting disease of Radix pseudostellariae. Plant Soil 2020, 454, 411–430. [Google Scholar] [CrossRef]
- Edgar, R.-C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Wang, X.-K. Principles and Techniques of Plant Physiology and Biochemistry Experiment; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Qin, X.-J.; Wu, H.-M.; Chen, J.; Wu, L.-K.; Lin, S.; Khan, M.-U.; Boorboori, M.-R.; Lin, W.-X. Transcriptome analysis of Pseudostellaria heterophylla in response to the infection of pathogenic Fusarium oxysporum. BMC Plant Biol. 2017, 17, 155. [Google Scholar] [CrossRef]
- Stoddard, S.-F.; Smith, B.-J.; Hein, R.; Roller, B.-R.-K.; Schmidt, T.-M. rrnDB: Improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucleic Acids Res. 2015, 43, 593–598. [Google Scholar] [CrossRef]
- Nemergut, D.-R.; Knelman, J.-E.; Ferrenberg, S.; Bilinski, T.; Melbourne, B.; Lin, J.; Violle, C.; Darcy, J.-L.; Prest, T.; Schmidt, S.-K. Decreases in average bacterial community rRNA operon copy number during succession. Isme J. 2016, 10, 1147–1156. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Y.; Liang, C.; Luo, Y.; Xu, Q.; Han, C.; Zhao, Q.; Sun, B. Competitive interaction with keystone taxa induced negative priming under biochar amendments. Microbiome 2019, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.-A.; Hilton, A. Statistical Analysis in Microbiology: Statnotes; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Shou, N.-S.; Huang, D.-W.; Wu, Y.; Chen, R.-F.; Zhang, L.-Q.; Wu, X.-Y. Effects of Silicon on Microbial Community Structure of Rhizosphere Soil of Rice at Different Nitrogen Levels. Chin. J. Soil Sci. 2021, 52, 903–911. [Google Scholar]
- Etesami, H.; Schaller, J. Improving phosphorus availability to rice through silicon management in paddy soils: A review of the role of silicate-solubilizing bacteria. Rhizosphere 2023, 27, 1007. [Google Scholar] [CrossRef]
- Kang, C.-Z.; Lyu, C.-G.; Huang, L.-Q.; Wang, S.; Wang, H.-Y.; Zhang, W.-J.; Wang, R.-S.; Wang, T.-L.; Sun, J.-H.; Zhou, T.; et al. Pattern of ecological planting for Chinese materia medica based on regional distribution. China J. Chin. Mater. Medica 2020, 45, 1982–1989. [Google Scholar]
- Yuan, Y.-D.; Zuo, J.-J.; Zhang, H.-Y.; Zu, M.-T.; Liu, S.-A. The Chinese medicinal plants rhizosphere: Metabolites, microorganisms, and interaction. Rhizosphere 2022, 22, 100540. [Google Scholar] [CrossRef]
- Perez Brandan, C.; Arzeno, J.L.; Huidobro, D.J.; Conforto, E.C.; Grumberg, B.C.; Hilton, S.; Vargas Gil, S. The effect of crop sequences on soil microbial, chemical and physical indicators and its relationship with soybean sudden death syndrome (complex of Fusarium species). Spanish J. Agri. Res. 2014, 12, 252–264. [Google Scholar] [CrossRef]
- Pérez-Brandán, C.; Huidobro, J.; Grümberg, B.; Scandiani, M.M.; Luque, A.G.; Meriles, J.M.; Vargas-Gil, S. Soybean fungal soil-borne diseases: A parameter for measuring the effect of agricultural intensification on soil health. Canadian J. Microbiol. 2014, 60, 73–84. [Google Scholar] [CrossRef]
- Dong, Y.-F.; Lyu, X.-Z.; Zhang, Z.-K.; He, H.-J.; Yu, J.-Q.; Zhou, Y.-H. Effects of different cultivation patterns on soil microbial community and enzyme activity in continuous cropped pepper field. Acta Agri. Zhejiangensis 2019, 31, 1485–1492. [Google Scholar]
- Meena, V.-S.; Meena, S.-K.; Verma, J.-P.; Kumar, A.; Aeron, A.; Mishra, P.-K.; Bisht, J.-K.; Pattanayak, A.; Naveed, M.; Dotaniya, M.-L. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: A review. Ecol. Eng. 2017, 107, 8–32. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.-L.; Yadav, A.-N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.-S.; Saxena, A.-K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agri. Biotech. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Shahwar, D.; Mushtaq, Z.; Mushtaq, H.; Alqarawi, A.-A.; Park, Y.; Alshahrani, T.-S.; Faizan, S. Role of microbial inoculants as bio fertilizers for improving crop productivity: A review. Heliyon 2023, 9, e16134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-F.; Zhu, Y.-J.; Wang, Z.-R.; Zhang, H.-F.; Chen, M.-C.; Chen, Y.-P.; Wang, J.-P.; Liu, B. Effects of a novel bio-organic fertilizer on the composition of rhizobacterial communities and bacterial wilt outbreak in a continuously mono-cropped tomato field. Appl. Soil Ecol. 2020, 156, 103717. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Yuan, Z.-Y.; Feng, J.-J.; Chen, S.-H.; Sun, G.-Z.; Wei, Z.-H.; Hu, T.-T. Effects of microbial fertilizer and irrigation amount on growth, physiology and water use efficiency of tomato in greenhouse. Sci. Horticul. 2024, 323, 112553. [Google Scholar] [CrossRef]
- Ali, A.; Ghani, M.-I.; Ding, H.-Y.; Iqbal, M.; Cheng, Z.-H.; Cai, Z.-C. Arbuscular mycorrhizal inoculum coupled with organic substrate induces synergistic effects for soil quality changes, and rhizosphere microbiome structure in long-term monocropped cucumber planted soil. Rhizosphere 2021, 20, 100428. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Mao, X.-X.; Zhang, M.-S.; Yang, W.; Di, H.-J.; Ma, L.; Liu, W.-J.; Li, B.-W. The application of Bacillus Megaterium alters soil microbial community composition, bioavailability of soil phosphorus and potassium, and cucumber growth in the plastic shed system of North China. Agr. Ecosyst. Environ. 2021, 307, 107236. [Google Scholar] [CrossRef]
- Ling, N.; Deng, K.-Y.; Song, Y.; Wu, Y.-C.; Zhao, J.; Raza, W.; Huang, Q.-W.; Shen, Q.-R. Variation of rhizosphere bacterial community in watermelon continuous mono-cropping soil by long-term application of a novel bioorganic fertilizer. Microbiol. Res. 2014, 169, 570–578. [Google Scholar] [CrossRef]
- Ding, S.; Zhou, D.-P.; Wei, H.-W.; Wu, S.-H.; Xie, B. Alleviating soil degradation caused by watermelon continuous cropping obstacle: Application of urban waste compost. Chemosphere 2021, 262, 128387. [Google Scholar] [CrossRef]
- Zhang, D.-Q.; Cheng, H.-Y.; Hao, B.-Q.; Li, Q.-J.; Fang, W.-S.; Ren, L.-R.; Yan, D.-D.; Ouyang, C.-B.; Li, Y.; Wang, Q.-X.; et al. Effect of fresh chicken manure as a non-chemical soil fumigant on soil-borne pathogens, plant growth and strawberry fruit profitability. Crop Prot. 2021, 146, 105653. [Google Scholar] [CrossRef]
- He, Z.-K. Chitinolyticbacter Meiyuanensis Isolation, Identification, and Fermentation for Chintinase Production; Jiangnan University: Wuxi, China, 2011. [Google Scholar]
- Luo, Y. Effect of Low Temperature Stress and Cold Hardening on Physiology Character of Strawberry and Molecular Cloning of Class II Chitinase Gene; Sichuan Agricultural University: Chengdu, China, 2007. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).