Effects of Pruning on Growth, Rhizosphere Soil Physicochemical Indexes and Bacterial Community Structure of Tea Tree and Their Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Determination of Physichemical Properties of Soils
2.3. Determination of Tea Growth Indexes
2.4. 16S rDNA Amplicon Sequencing Analysis
2.5. Statistical Analysis
3. Results
3.1. Effect of Pruning on the Growth of Tea Tree
3.2. Effect of Pruning on Soil Physicochemical Properties
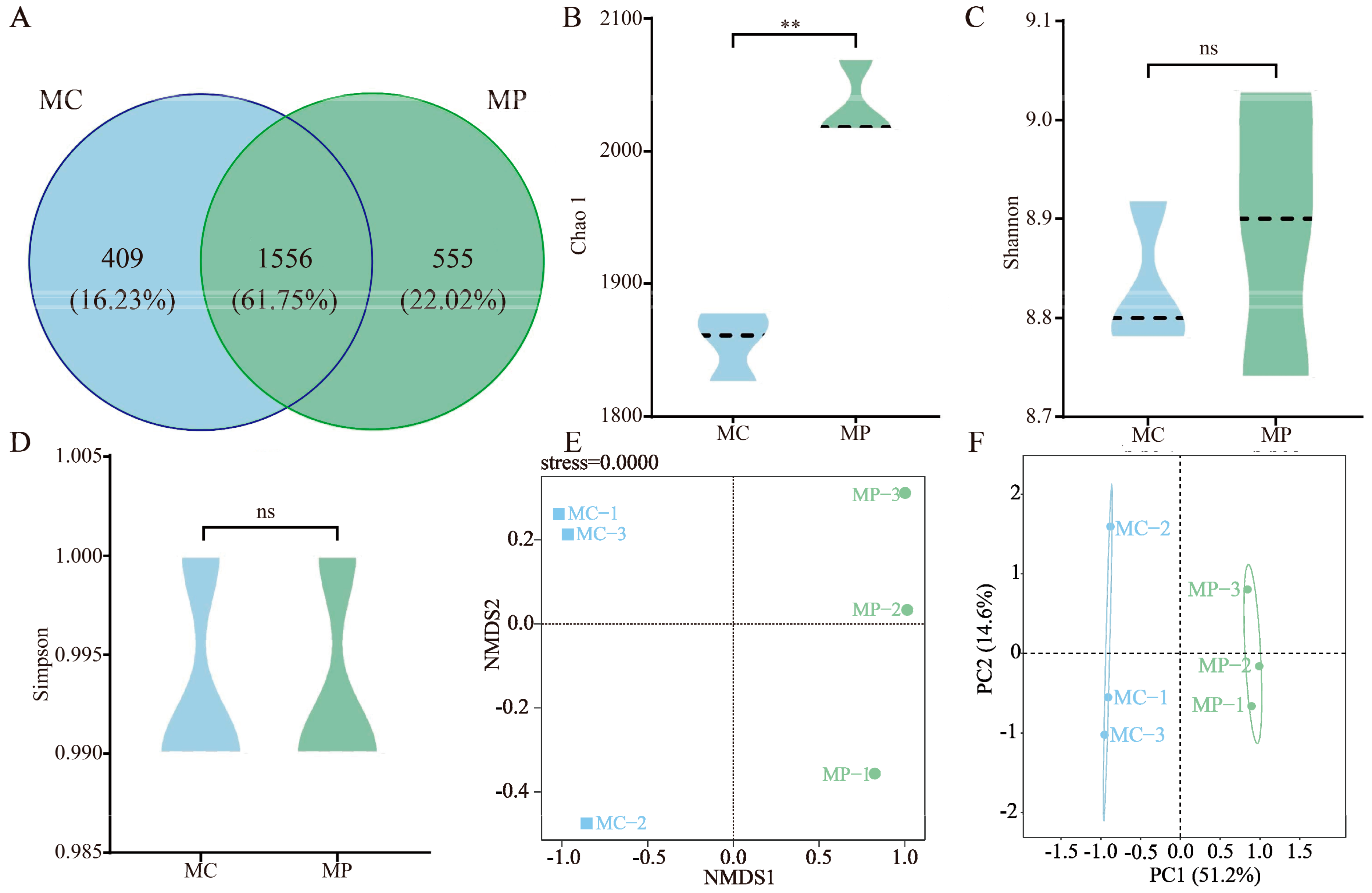
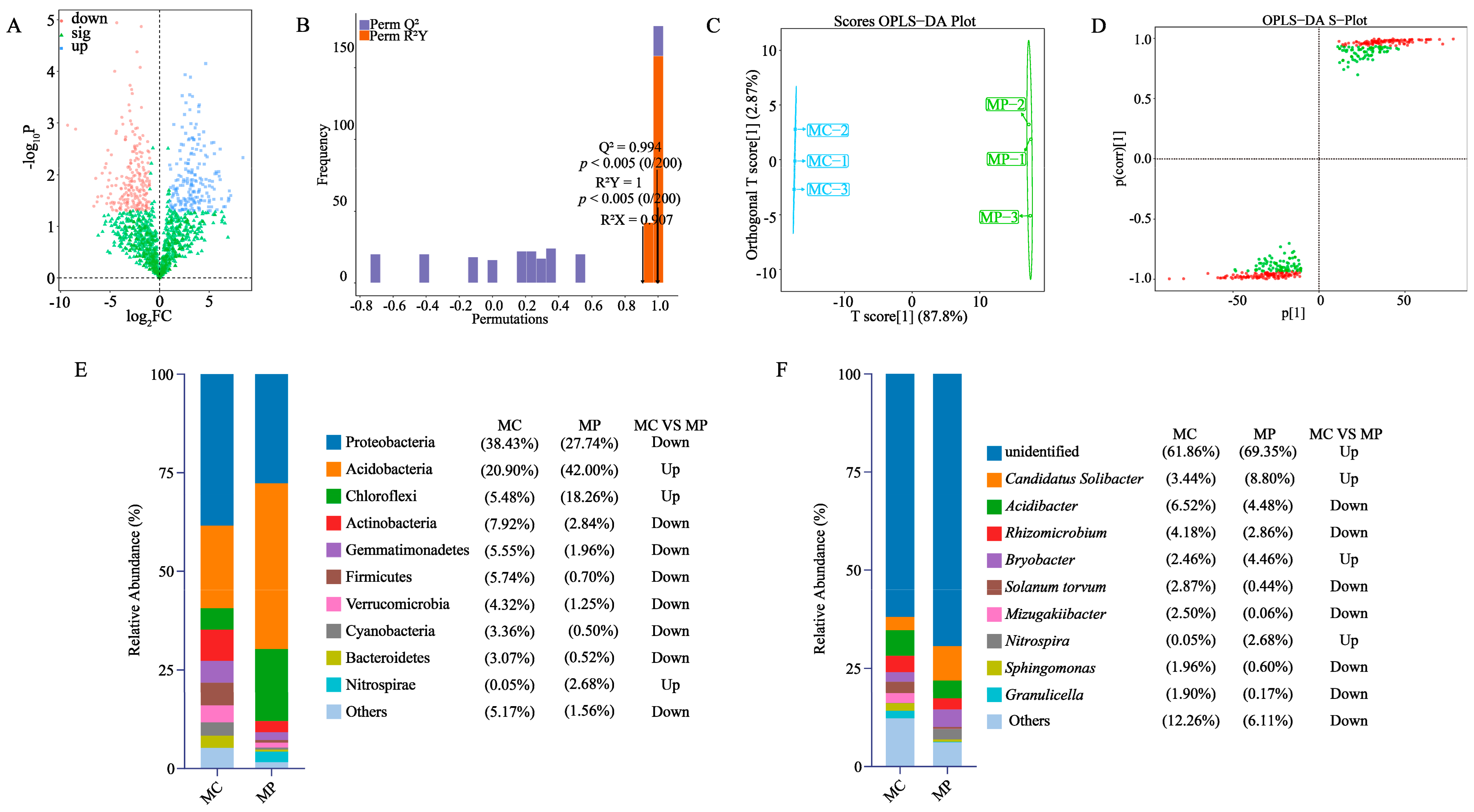
3.3. Soil Bacterial Community and Diversity Analysis
3.4. Screening of Key Differentiating Microorganisms
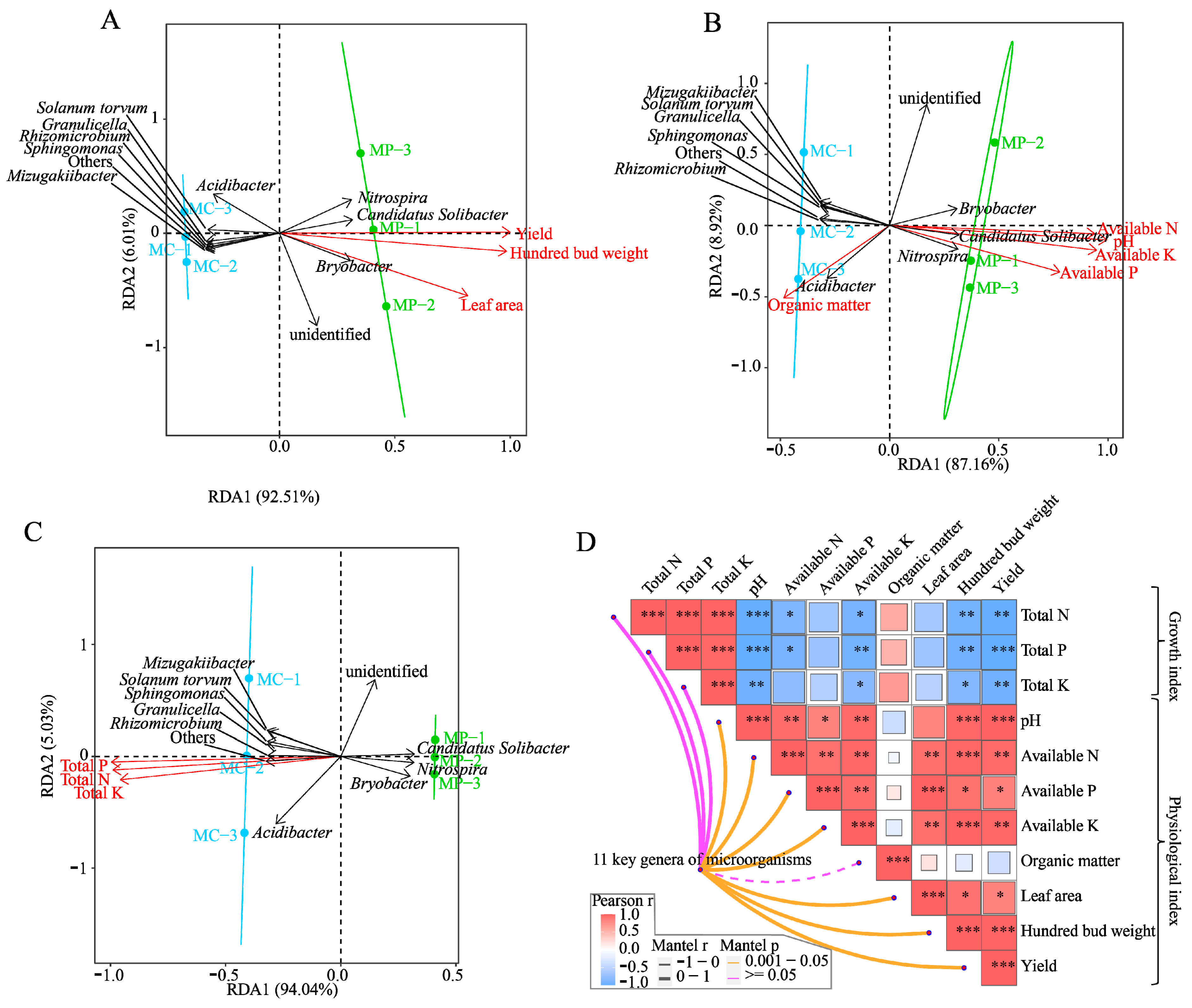
3.5. Analysis of Interaction between Key Microorganisms, Tea Tree Growth Indexes, and Soil Physicochemical Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Prosser, J.; Bohannan, B.; Curtis, T.; Ellis, R.; Firestone, M.; Freckleton, R.; Green, J.; Green, L.; Killham, K.; Lennon, J.; et al. The role of ecological theory in microbial ecology. Nat. Rev. Microbiol. 2007, 5, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, P.; Tian, H.; Xiao, Q.; Jiang, H. Pyrosequencing-based assessment of soil microbial community structure and analysis of soil properties with vegetable planted at different years under greenhouse conditions. Soil Tillage Res. 2019, 187, 1–10. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qiu, Z.; Ye, J.; Verma, J.; Li, J.; Singh, B. Effective colonisation by a bacterial synthetic community promotes plant growth and alters soil microbial community. J. Sustain. Agric. Environ. 2022, 1, 30–42. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; He, C.; Shi, Q.; Chen, S.; Reid, B.; Zhu, Y.; Sun, G. Organic carbon amendments affect the chemodiversity of soil dissolved organic matter and its association with soil microbial communities. Environ. Sci. Technol. 2019, 53, 50–59. [Google Scholar] [CrossRef]
- Rath, K.; Fierer, N.; Murphy, D.; Rousk, J. Linking bacterial community composition to soil salinity along environmental gradients. ISME J. 2019, 13, 836–846. [Google Scholar] [CrossRef]
- Schneider, A.; Gommeaux, M.; Duclercq, J.; Fanin, N.; Conreux, A.; Alahmad, A.; Lacoux, J.; Roger, D.; Spicher, F.; Ponthieu, M.; et al. Response of bacterial communities to Pb smelter pollution in contrasting soils. Sci. Total Environ. 2017, 605, 436–444. [Google Scholar] [CrossRef]
- Wan, W.; Tan, J.; Wang, Y.; Qin, Y.; He, H.; Wu, H.; Zou, W.; He, D. Responses of the rhizosphere bacterial community in acidic crop soil to pH: Changes in diversity, composition, interaction, and function. Sci. Total Environ. 2020, 700, 134418. [Google Scholar] [CrossRef]
- He, Z.H. Effects of different pruning methods on the yield and quality of organic tea. South China Agric. 2017, 11, 9–10. [Google Scholar] [CrossRef]
- Tian, R.; Lv, R.; Fang, L.; Shi, Y. Study on the influence of different pruning methods on tree crown formation in tea plantation with high quality tea machine. China Tea 2015, 37, 16–19. [Google Scholar] [CrossRef]
- Arkorful, E.; Yu, Y.; Chen, C.; Lu, L.; Hu, S.; Yu, H.; Ma, Q.; Thangaraj, K.; ajiv Periakaruppan, R.; Jeyaraj, A.; et al. Untargeted metabolomic analysis using UPLC-MS/MS identifies metabolites involved in shoot growth and development in pruned tea plants (Camellia sinensis (L.) O. Kuntz). Sci. Hortic. 2020, 264, 109164. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, X.; Fang, L.; Xiao, X.; Tang, X.; Shi, Y. The influence of different pruning treatments on the continuous mechanized picking effect of famous tea. China Tea 2020, 42, 32–35. [Google Scholar] [CrossRef]
- Jiang, Y.; Lin, X.; Khan, M.; Jiang, W.; Xu, Y.; Li, Z.; Lin, W. Tea pruning for the umbrella-shaped canopy can alleviate rhizosphere soil degradation and improve the ecosystem functioning of tea orchards. Catena 2023, 222, 106885. [Google Scholar] [CrossRef]
- Borgohain, A.; Sarmah, M.; Gogoi, B.B.; Konwar, K.; Handique, J.G.; Paul, R.K.; Yeasin, M.; Pandey, V.; Yadav, R.; Malakar, H.; et al. Can tea pruning litter biochar be a friend or foe for tea (Camellia sinensis L.) plants’ growth and growth regulators?: Feasible or fumes of fancy. Ind. Crops Prod. 2023, 195, 116394. [Google Scholar] [CrossRef]
- Sarmah, M.; Borgohain, A.; Gogoi, B.B.; Yeasin, M.; Paul, R.K.; Malakar, H.; Handique, J.G.; Saikia, J.; Deka, D.; Khare, P.; et al. Insights into the effects of tea pruning litter biochar on major micronutrients (Cu, Mn, and Zn) pathway from soil to tea plant: An environmental armour. J. Hazard. Mater. 2023, 442, 129970. [Google Scholar] [CrossRef]
- Bora, S.S.; Hazarika, D.J.; Gogoi, R.; Dullah, S.; Gogoi, M.; Barooah, M. Long-term pruning modulates microbial community structure and their functional potential in tea (Camellia sinensis L.) soils. Appl. Soil Ecol. 2022, 176, 104483. [Google Scholar] [CrossRef]
- Pramanik, P.; Phukan, M.; Ghosh, S.; Goswami, A. Pruned tea bushes secrete more root exudates to influence microbiological properties in soil. Arch. Agron. Soil Sci. 2018, 64, 1172–1180. [Google Scholar] [CrossRef]
- Arafat, Y.; Wei, X.; Jiang, Y.; Chen, T.; Saqib, H.; Lin, S.; Lin, W. Spatial distribution patterns of root-associated bacterial communities mediated by root exudates in different aged ratooning tea monoculture systems. Int. J. Mol. Sci. 2017, 18, 1727. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Y.; Lin, S.; Wang, Y.; Chen, P.; Hong, L.; Jia, X.; Kang, J.; Wu, Z.; Wang, H. Metabolomics analysis of the effect of acidification on rhizosphere soil microecosystem of tea tree. Front. Plant Sci. 2023, 14, 1137465. [Google Scholar] [CrossRef]
- Lu, R.S. Soil Agrochemical Analysis Methods; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Munyaka, P.; Eissa, N.; Bernstein, C.; Khafipour, E.; Ghia, J. Antepartum antibiotic treatment increases offspring susceptibility to experimental colitis: A role of the gut microbiota. PLoS ONE 2015, 10, e0142536. [Google Scholar] [CrossRef]
- Lu, H.; Yeh, Y.; Shiah, F.; Gong, G.; Hsieh, C. Evolutionary constraints on species diversity in marine bacterioplankton communities. ISME J. 2019, 13, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.; Wang, Q.; Cardenas, E.; Fish, J.; Kulam-Syed-Mohideen, A.; McGarrell, D.; Marsh, T.; Garrity, G.; Tiedje, J. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37 (Suppl. 1), D141–D145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sheng, H.; He, Y.; Wu, J.; Jiang, Y.; Tam, N.; Zhou, H. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 2012, 78, 8264–8271. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, Q.; Chen, M.; Wang, Y.; Lin, S.; Pan, Y.; Cheng, P.; Li, M.; Zhang, Y.; Ye, J.; et al. Analysis of the effect of different withering methods on tea quality based on transcriptomics and metabolomics. Front. Plant Sci. 2023, 14, 1235687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Y.; Miao, P.; Chen, M.; Du, M.; Pang, X.; Ye, J.; Wang, H.; Jia, X. Effects of pruning on tea tree growth, soil enzyme activity and microbial diversity. Agronomy 2023, 13, 1214. [Google Scholar] [CrossRef]
- Yin, L.; Xiao, X.; Liu, D.; Sun, Y.; Deng, S.; Luo, Q.; Xia, L.; Yang, Q.; Liang, M. Effect of light pruning in different period on tea yield and quality of Yunnan bigleaf varieties. Southwest China J. Agric. Sci. 2019, 32, 1034–1038. [Google Scholar] [CrossRef]
- Lu, L.; Luo, W.; Zheng, Y.; Jin, J.; Liu, R.; Lv, Y.; Ye, Y.; Ye, J. Effect of different pruning operations on the plant growth, phytohormones and transcriptome profiles of the following spring tea shoots. Beverage Plant Res. 2022, 2, 12. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.; Li, X.; Yan, P.; Zhang, L.; Han, W. Summer pruning improves the branch growth and tea quality of tea trees (Camellia sinensis). Acta Physiol. Plant 2021, 43, 65. [Google Scholar] [CrossRef]
- Jia, X.; Wang, Y.; Zhang, Q.; Lin, S.; Zhang, Y.; Du, M.; Chen, M.; Ye, J.; Wu, Z.; Wang, H. Reasonable deep application of sheep manure fertilizer to alleviate soil acidification to improve tea yield and quality. Front. Plant Sci. 2023, 14, 1179960. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Y.; Wang, Y.; Hong, L.; Kang, J.; Jia, Y.; Li, M.; Chen, Y.; Wu, Z.; Wang, H. Improvement of soil acidification and ammonium nitrogen content in tea plantation by long-term use of organic fertilizer. Plant Biol. 2023, 25, 994–1008. [Google Scholar] [CrossRef]
- Nielsen, M.N.; Winding, A. Microorganisms as Indicators of Soil Health; National Environmental Research Institute: Roskilde, Denmark, 2002. [Google Scholar]
- Arkorful, E.; Hu, S.; Zou, Z.; Yu, Y.; Chen, X.; Li, X. Metabolomic analyses provide new insights into signaling mechanisms for nutrient uptake by lateral roots of pruned tea plant (Camellia sinensis). J. Agric. Food Chem. 2020, 68, 7890–7903. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, Z.; Hu, H.; He, J. Niche specialization of comammox Nitrospira in terrestrial ecosystems: Oligotrophic or copiotrophic? Crit. Rev. Environ. Sci. Technol. 2023, 53, 161–176. [Google Scholar] [CrossRef]
- Zhang, R.; Rong, L.; Zhang, L. Soil nutrient variability mediates the effects of erosion on soil microbial communities: Results from a modified topsoil removal method in an agricultural field in Yunnan plateau, China. Environ. Sci. Pollut. Res. 2022, 29, 3659–3671. [Google Scholar] [CrossRef]
- Ren, H.; Wang, H.; Wang, Q.; Qi, X.; Zhang, S.; Yu, Z.; Ijaz, M.; Zhang, M.; Ahmed, T.; EI-Sharnouby, M.; et al. Effect of fungicides on bayberry decline disease by modulating rhizosphere soil properties, microflora, and metabolites. Agronomy 2022, 12, 677. [Google Scholar] [CrossRef]
- Yu, M.; Xiang, X.; Tan, H.; Zhang, Q.; Shan, Y.; Yang, H. Potential correlation between volatiles and microbiome of Xiang xi sausages from four different regions. Food Res. Int. 2021, 139, 109943. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Appiah, K.; Azizi, M.; Fujii, Y. Plant growth inhibitory activities and volatile active compounds of 53 spices and herbs. Plants 2020, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.; Zerillo, M.; Zühlke, D.; Kielak, A.; Pijl, A.; Riedel, K.; Kuramae, E. Responses of Acidobacteria granulicella sp. WH15 to high carbon revealed by integrated omics analyses. Microorganisms 2020, 8, 244. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Leaf Area (cm2) | Hundred-Bud Weight (g) | Yield (kg/ha) |
|---|---|---|---|
| MC | 20.81 ± 2.69 ns | 108.60 ± 5.24 ns | 2850 ± 72.45 ns |
| MP | 27.49 ± 3.88 * | 152.10 ± 7.81 * | 5250 ± 124.57 * |
| Soil Index | MC | MP | Soil Index | MC | MP |
|---|---|---|---|---|---|
| pH | 4.21 ± 0.01 * | 4.62 ± 0.01 * | Total N (g/kg) | 1.18 ± 0.02 * | 0.98 ± 0.02 * |
| Available N (mg/kg) | 116.67 ± 2.35 * | 124.50 ± 1.15 * | Total P (g/kg) | 0.92 ± 0.01 * | 0.67 ± 0.02* |
| Available P (mg/kg) | 11.73 ± 1.16 * | 15.20 ± 2.03 * | Total K (g/kg) | 6.78 ± 0.12 * | 6.02 ± 0.17 * |
| Available K (mg/kg) | 104.27 ± 0.62 * | 111.53 ± 1.88 * | Organic matter (g/kg) | 20.10 ± 1.25 ns | 19.24 ± 0.87 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Wang, Y.; Chen, Y.; Zhang, Y.; Chen, M.; Zou, J.; Miao, P.; Ye, J.; Pang, X.; Jia, X.; et al. Effects of Pruning on Growth, Rhizosphere Soil Physicochemical Indexes and Bacterial Community Structure of Tea Tree and Their Interaction. Agriculture 2023, 13, 1972. https://doi.org/10.3390/agriculture13101972
Zhang Q, Wang Y, Chen Y, Zhang Y, Chen M, Zou J, Miao P, Ye J, Pang X, Jia X, et al. Effects of Pruning on Growth, Rhizosphere Soil Physicochemical Indexes and Bacterial Community Structure of Tea Tree and Their Interaction. Agriculture. 2023; 13(10):1972. https://doi.org/10.3390/agriculture13101972
Chicago/Turabian StyleZhang, Qi, Yuhua Wang, Yiling Chen, Ying Zhang, Meihui Chen, Jishuang Zou, Pengyao Miao, Jianghua Ye, Xiaomin Pang, Xiaoli Jia, and et al. 2023. "Effects of Pruning on Growth, Rhizosphere Soil Physicochemical Indexes and Bacterial Community Structure of Tea Tree and Their Interaction" Agriculture 13, no. 10: 1972. https://doi.org/10.3390/agriculture13101972
APA StyleZhang, Q., Wang, Y., Chen, Y., Zhang, Y., Chen, M., Zou, J., Miao, P., Ye, J., Pang, X., Jia, X., & Wang, H. (2023). Effects of Pruning on Growth, Rhizosphere Soil Physicochemical Indexes and Bacterial Community Structure of Tea Tree and Their Interaction. Agriculture, 13(10), 1972. https://doi.org/10.3390/agriculture13101972





