Positive Interaction of Selenium Nanoparticles and Olive Solid Waste on Vanadium-Stressed Soybean Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Setup
2.2. Application of Treatments
2.3. Sampling
2.3.1. Determination of Plant Biomass, Photosynthetic Capacity and Pigments
2.3.2. Assessment of Stress Biomarkers, Antioxidant Metabolites, and Antioxidant Enzyme Activities
2.4. Statistical Analysis
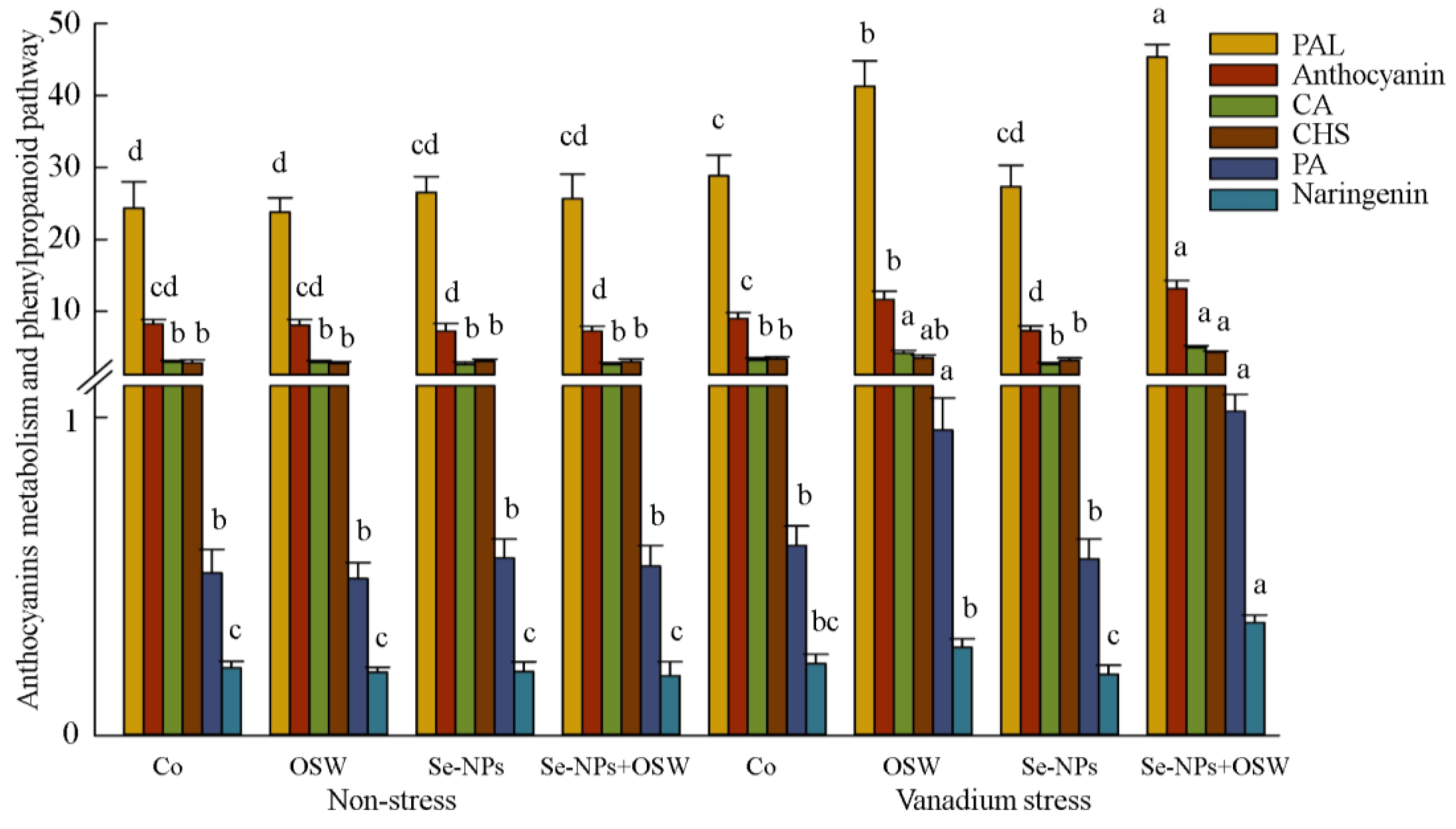
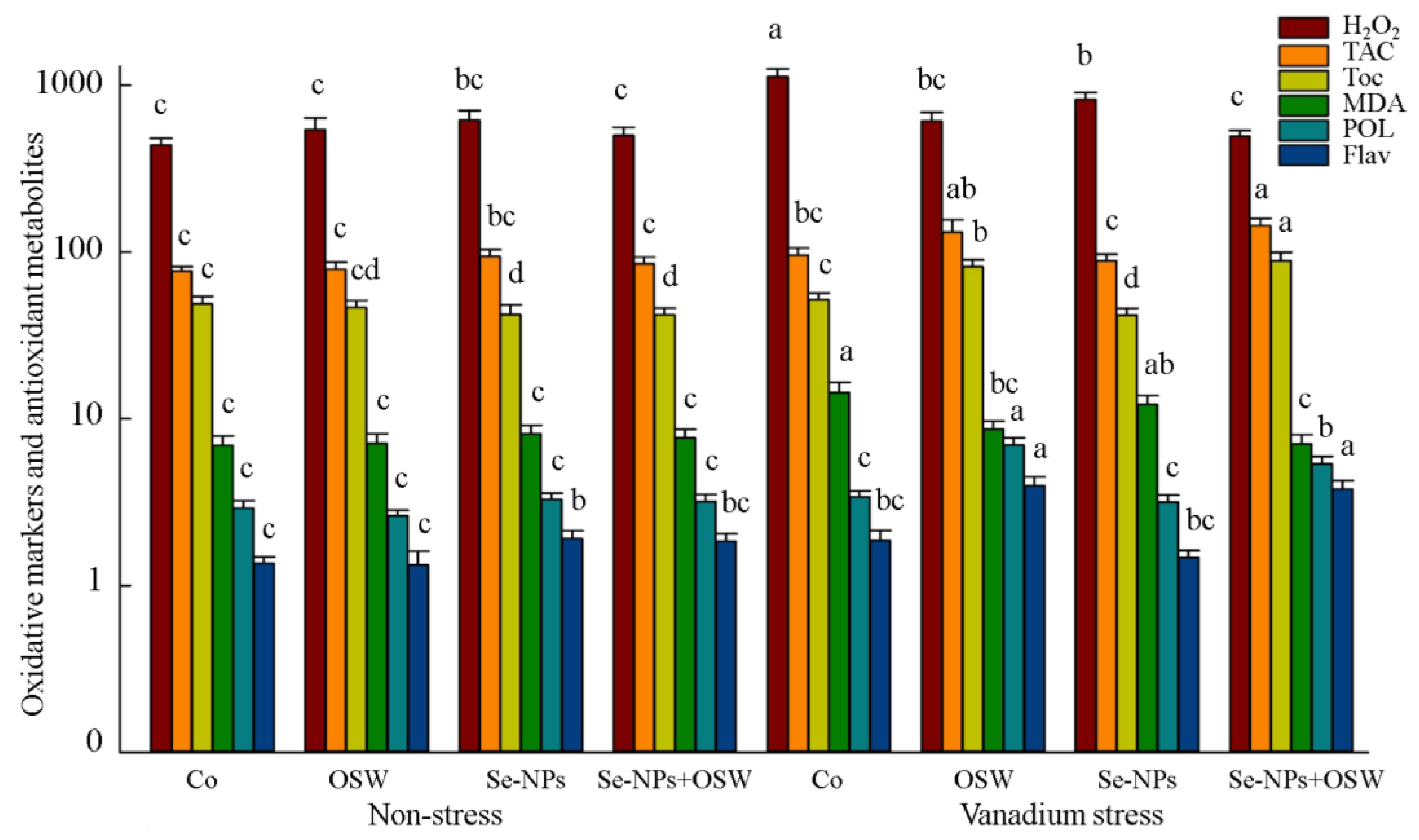
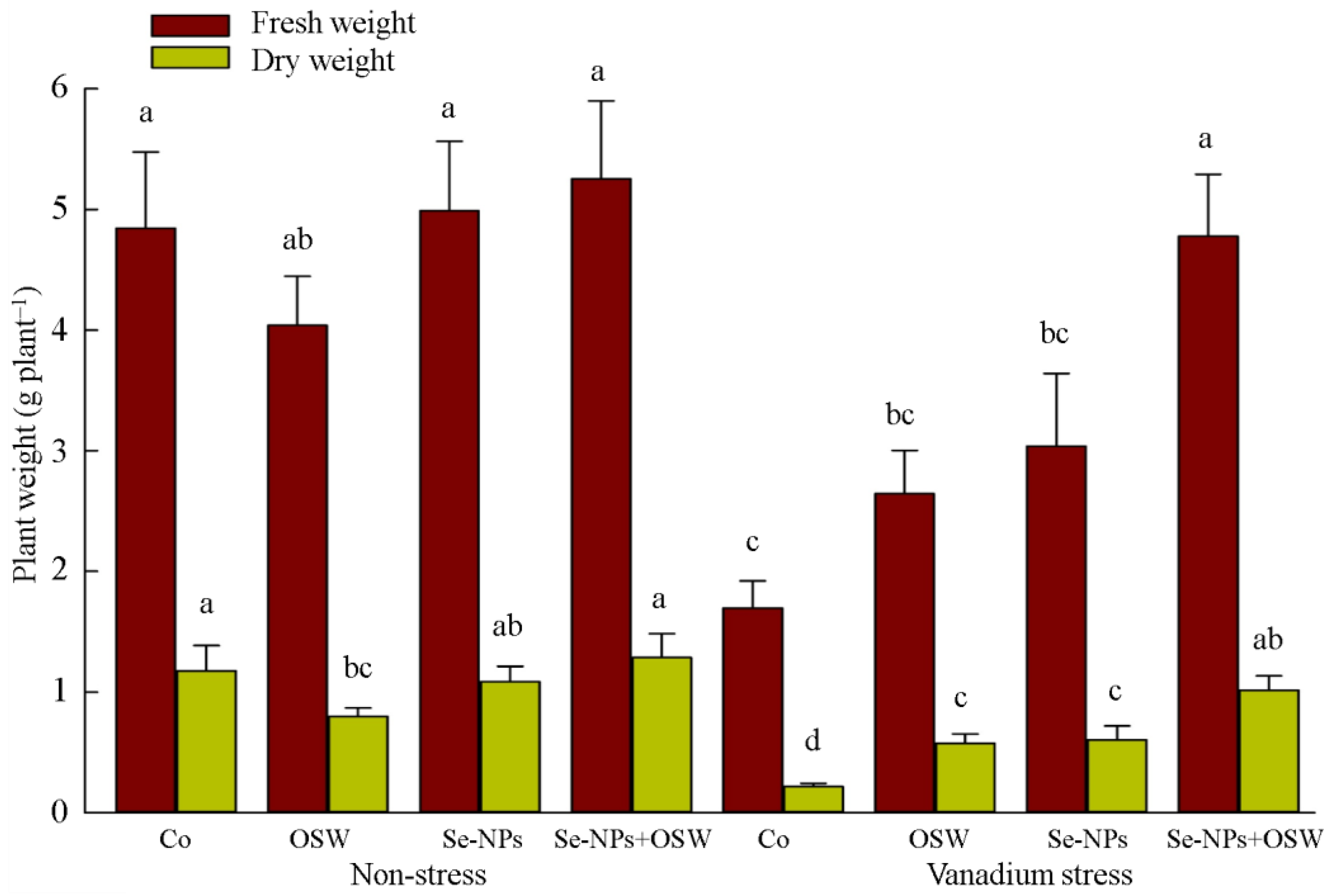
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Wang, M.; Jia, Y.; Gou, M.; Zeyer, J. Toxicity of vanadium in soil on soybean at different growth stages. Environ. Pollut. 2017, 231, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Alsherif, E.A.; Yaghoubi Khanghahi, M.; Crecchio, C.; Korany, S.M.; Sobrinho, R.L.; AbdElgawad, H. Understanding the Active Mechanisms of Plant (Sesuvium portulacastrum L.) Against Heavy Metal Toxicity. Plants 2023, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Terzano, R.; Rascio, I.; Allegretta, I.; Porfido, C.; Spagnuolo, M.; Yaghoubi-Khanghahi, M.; Crecchio, C.; Sakellariadou, F.; Gattullo, C.E. Fire effects on the distribution and bioavailability of potentially toxic elements (PTEs) in agricultural soils. Chemosphere 2021, 281, 130752. [Google Scholar] [CrossRef] [PubMed]
- Aihemaiti, A.; Jiang, J.; Gao, Y.; Meng, Y.; Zou, Q.; Yang, M.; Xu, Y.; Han, S.; Yan, W.; Tuerhong, T. The effect of vanadium on essential element uptake of Setaria viridis’ seedlings. J. Environ. Manag. 2019, 237, 399–407. [Google Scholar] [CrossRef]
- Imtiaz, M.; Ashraf, M.; Rizwan, M.S.; Nawaz, M.A.; Rizwan, M.; Mehmood, S.; Yousaf, B.; Yuan, Y.; Ditta, A.; Mumtaz, M.A.; et al. Vanadium toxicity in chickpea (Cicer arietinum L.) grown in red soil: Effects on cell death, ROS and antioxidative systems. Ecotoxicol. Environ. Saf. 2018, 158, 139–144. [Google Scholar] [CrossRef]
- Roychoudhury, A. Vanadium uptake and toxicity in plants. SF J. Agri. Crop. Manag. 2020, 1, 1010. [Google Scholar]
- Imtiaz, M.; Mushtaq, M.A.; Rizwan, M.S. Comparison of antioxidant enzyme activities and DNA damage in chickpea (Cicer arietinum L.) genotypes exposed to vanadium. Environ. Sci. Pollut. Res. 2016, 23, 19787–19796. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2019, 131, 108940. [Google Scholar] [CrossRef]
- Foti, P.; Romeo, F.V.; Russo, N.; Pino, A.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive mill wastewater as renewable raw materials to generate high added-value ingredients for agro-food industries. Appl. Sci. 2021, 11, 7511. [Google Scholar] [CrossRef]
- Yaghoubi Khanghahi, M.; Pirdashti, H.; Rahimian, H.; Nematzadeh, G.A.; Ghajar Sepanlou, M. The role of potassium solubilizing bacteria (KSB) inoculations on grain yield, dry matter remobilization and translocation in rice (Oryza sativa L.). J. Plant Nutr. 2019, 42, 1165–1179. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Asfi, M. Determination of Olive soild wastetoxic effects on three mint species grown in hydroponic culture. J. Plant Nutr. 2012, 35, 726–738. [Google Scholar] [CrossRef]
- Chouchene, A.; Jeguirim, M.; Trouvé, G. Biosorption performance, combustion behavior, and leaching characteristics of olive solid waste during the removal of copper and nickel from aqueous solutions. Clean Technol. Environ. Policy 2014, 16, 979–986. [Google Scholar] [CrossRef]
- Galliou, F.; Markakis, N.; Fountoulakis, M.S.; Nikolaidis, N.; Manios, T. Production of organic fertilizer from olive mill wastewater by combining solar greenhouse drying and composting. Waste Manag. 2018, 75, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qi, W.Y.; Chen, H.; Song, C.; Li, Q.; Wang, S.G. Selenium Nanoparticles as an Innovative Selenium Fertilizer Exert Less Disturbance to Soil Microorganisms. Front. Microbiol. 2021, 12, 746046. [Google Scholar] [CrossRef]
- Duborská, E.; Šebesta, M.; Matulová, M.; Zvěřina, O.; Urík, M. Current srategies for selenium and iodine biofortification in crop plants. Nutrients 2022, 14, 4717. [Google Scholar] [CrossRef]
- Rosenfeld, C.E.; Kenyon, J.A.; James, B.R.; Santelli, C.M. Selenium (IV,VI) reduction and tolerance by fungi in an oxic environment. Geobiology 2017, 15, 441–452. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.-O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef]
- Cieschi, M.T.; Polyakov, A.Y.; Lebedev, V.A.; Volkov, D.S.; Pankratov, D.A.; Veligzhanin, A.A.; Perminova, I.V.; Lucena, J.J. Eco-friendly iron-humic nanofertilizers synthesis for the prevention of iron chlorosis in soybean (Glycine max) grown in calcareous soil. Front. Plant Sci. 2019, 10, 413. [Google Scholar] [CrossRef]
- Belaqziz, M.; El-Abbassi, A.; Agrafioti, E.; Galanakis, C.M. Agronomic application of olive mill wastewater: Effects on maize production and soil properties. J. Environ. Manag. 2016, 171, 158–165. [Google Scholar] [CrossRef]
- El Hassani, F.Z.; Fadile, A.; Faouzi, M.; Zinedine, A.; Merzouki, M.; Benlemlih, M. The long term effect of olive mill wastewater (OMW) on organic matter humification in a semi-arid soil. Heliyon 2020, 6, e03181. [Google Scholar] [CrossRef]
- Selim, S.; Akhtar, N.; El Azab, E.; Warrad, M.; Alhassan, H.H.; Abdel-Mawgoud, M.; Al Jaouni, S.K.; Abdelgawad, H. Innovating the synergistic assets of β-Amino Butyric Acid (BABA) and selenium nanoparticles (SeNPs) in improving the growth, nitrogen metabolism, biological activities, and nutritive value of Medicago interexta sprouts. Plants 2022, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Selim, S.; Abuelsoud, W.; Alsharari, S.S.; Alowaiesh, B.F.; Al-Sanea, M.M.; Al Jaouni, S.; Madany, M.M.Y.; AbdElgawad, H. Improved mineral acquisition, sugars metabolism and redox status after mycorrhizal inoculation are the basis for tolerance to vanadium stress in C3 and C4 grasses. J. Fungi. 2021, 7, 915. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi Khanghahi, M.; Leoni, B.; Crecchio, C. Photosynthetic responses of durum wheat to chemical/microbiological fertilization management under salt and drought stresses. Acta Physiol. Plant. 2021, 43, 123. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef]
- Yaghoubi Khanghahi, M.; AbdElgawad, H.; Verbruggen, E.; Korany, S.M.; Alsherif, E.A.; Beemster, G.T.S.; Crecchio, C. Biofertilisation with a consortium of growth-promoting bacterial strains improves the nutritional status of wheat grain under control, drought, and salinity stress conditions. Physiol. Plant. 2022, 174, e13800. [Google Scholar] [CrossRef]
- AbdElgawad, H.; De Vos, D.; Zinta, G. Grassland species differentially regulate proline concentrations under future climate conditions: An integrated biochemical and modelling approach. New Phytol. 2015, 208, 354–369. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.L.; Reid, D.M. Leaf senescence and lipid peroxidation: Effects of some phytohormones, and scavengers of free radicals and singlet oxygen. Physiol. Plant. 1982, 56, 453–457. [Google Scholar] [CrossRef]
- Kumar, K.B.; Khan, P.A. Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Murshed, S.M.S.; Tan, S.H.; Nguyen, N.T. Temperature dependence of interfacial properties and viscosity of nanofluids for droplet-based microfluidics. J. Phys. D Appl. Phys. 2008, 41, 085502. [Google Scholar] [CrossRef]
- Drotar, A.; Phelps, P.; Fall, R. Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci. 1985, 42, 35–40. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Zhao, J.; Wang, X.; White, J.C.; Xing, B. CuO nanoparticle interaction with Arabidopsis thaliana: Toxicity, parentprogeny transfer, and gene expression. Environ. Sci. Technol. 2016, 50, 6008–6016. [Google Scholar] [CrossRef]
- Li, J.; Mu, Q.; Du, Y.; Luo, J.; Liu, Y.; Li, T. Growth and photosynthetic inhibition of cerium oxide nanoparticles on soybean (Glycine max). Bull. Environ. Contam. Toxicol. 2020, 105, 119–126. [Google Scholar] [CrossRef]
- Ghidaoui, J.S.; Bargougui, L.; Chaieb, M.; Mekki, A. Study of the phytotoxic potential of olive mill wastewaters on a leguminous plant ‘Vicia faba L.’. Water Sci. Technol. 2019, 80, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Magdich, S.; Abid, W.; Boukhris, M.; Rouina, B.B.; Ammar, E. Effects of long-term olive mill wastewater spreading on the physiological and biochemical responses of adult Chemlali olive trees (Olea europaea L.). Ecol. Eng. 2016, 97, 122–129. [Google Scholar] [CrossRef]
- Kamali-Andani, N.; Fallah, S.; Peralta-Videa, J.R.; Golkar, P. A comprehensive study of selenium and cerium oxide nanoparticles on mung bean: Individual and synergistic effect on photosynthesis pigments, antioxidants, and dry matter accumulation. Sci. Total Environ. 2022, 830, 154837. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. South Afr. J. Bot. 2018, 119, 1–10. [Google Scholar] [CrossRef]
- MacDonald, M.J.; D’Cunha, G.B. A modern view of phenylalanine ammonia lyase. Biochem. Cell Biol. 2007, 85, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Khademi-Astaneh, R.; Bolandnazar, S.; Zaare-Nahandi, F.; Oustan, S. Effect of selenium application on phenylalanine ammonia-lyase (PAL) activity, phenol leakage and total phenolic content in garlic (Allium sativum L.) under NaCl stress. Inf. Process. Agric. 2018, 5, 339–344. [Google Scholar] [CrossRef]
- Garousi, F. Toxicity of selenium, application of selenium in fertilizers, selenium treatment of seeds, and selenium in edible parts of plants. Acta Univsapientiae Aliment. 2017, 10, 61–74. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- El-Badri, A.M.; Hashem, A.M.; Batool, M.; Sherif, A.; Nishawy, E.; Ayaad, M.; Hassan, H.M.; Elrewainy, I.M.; Wang, J.; Kuai, J.; et al. Comparative efficacy of bio-selenium nanoparticles and sodium selenite on morpho-physiochemical attributes under normal and salt stress conditions, besides selenium detoxification pathways in Brassica napus L. J. Nanobiotechnology 2022, 20, 163. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Mohawesh, O.; Albalasmeh, A.; AlHamaiedeh, H.; Qaraleh, S.; Maaitah, O.; Bawalize, A.; Almajali, D. Controlled land application of olive mill wastewater (OMW): Enhance soil indices and barley growth performance in arid environments. Water Air Soil Pollut. 2020, 231, 1–12. [Google Scholar] [CrossRef]

| Basic characeristics | |
| Water content (%) | 117.15 ± 4.11 |
| Dry matter (%) | 12.49 ± 1.1 |
| pH | 5.06 ± 0.10 |
| Electric conductivity (mS cm−1) | 18.1± 0.23 |
| Chemical oxygen demand (g L−1) | 111.08 ± 7.1 |
| Biochemical oxygen demand (g L−1) | 60.02 ± 4.14 |
| Organic matter (g L−1) | 65.02 ± 8.04 |
| Salinity (g L−1) | 3.09 ± 0.2 |
| Minerals | |
| Nitrogen (g L−1) | 5.22 ± 0.1 |
| Phosphorus (g L−1) | 3.86 ± 0.21 |
| Potassium (g L−1) | 11.51 ± 1.01 |
| Calcium (g L−1) | 1.50 ± 0.04 |
| Magnesium (g L−1) | 1.23 ± 0.32 |
| Sodium (g L−1) | 2.30 ± 0.1 |
| Chloride (g L−1) | 2.08 ± 0.41 |
| Fe (g L−1) | 1.10 ± 0.12 |
| Zn (g L−1) | 0.60 ± 0.1 |
| Antioxidants | |
| Antioxidant activity (FRAP) | 51.92 ± 2.10 |
| Antioxidant activity DPPH (%) | 47.38 ± 1.07 |
| Total phenols (g L−1) | 10.21 ± 1.09 |
| Flavonoids (g L−1) | 1.05 ± 0.09 |
| ASC | APX | DHAR | MDHAR | GSH | GR | GPX | ||
|---|---|---|---|---|---|---|---|---|
| Non-stress | Control | 0.22 ± 0.02 c | 0.35 ± 0.06 c | 0.16 ± 0.02 b | 0.05 ± 0.00 c | 0.04 ± 0.00 c | 0.10 ± 0.02 cd | 0.52 ± 0.07 b |
| OSW | 0.20 ± 0.02 c | 0.32 ± 0.05 c | 0.15 ± 0.01 b | 0.05 ± 0.00 c | 0.04 ± 0.00 c | 0.13 ± 0.02 c | 0.50 ± 0.03 b | |
| Se-NPs | 0.19 ± 0.02 c | 0.38 ± 0.05 c | 0.19 ± 0.02 b | 0.06 ± 0.00 bc | 0.05 ± 0.01 c | 0.08 ± 0.01 d | 0.53 ± 0.04 b | |
| Se-NPs + OSW | 0.21 ± 0.02 c | 0.40 ± 0.07 c | 0.20 ± 0.02 b | 0.04 ± 0.00 c | 0.05 ± 0.00 c | 0.09 ± 0.01 d | 0.53 ± 0.07 b | |
| Vanadium stress | Control | 0.24 ± 0.02 bc | 0.42 ± 0.07 c | 0.21 ± 0.02 b | 0.05 ± 0.00 bc | 0.05 ± 0.01 c | 0.13 ± 0.02 c | 0.60 ± 0.07 b |
| OSW | 0.32 ± 0.03 a | 1.31 ± 0.11 a | 0.35 ± 0.04 a | 0.09 ± 0.00 a | 0.12 ± 0.01 a | 0.55 ± 0.05 a | 1.39 ± 0.24 a | |
| Se-NPs | 0.20 ± 0.02 c | 0.38 ± 0.05 c | 0.17 ± 0.02 b | 0.04 ± 0.00 c | 0.04 ± 0.00 c | 0.08 ± 0.01 d | 0.57 ± 0.08 b | |
| Se-NPs + OSW | 0.37 ± 0.03 a | 0.90 ± 0.08 b | 0.30 ± 0.03 a | 0.08 ± 0.00 a | 0.10 ± 0.02 ab | 0.49 ± 0.06 b | 1.41 ± 0.19 a |
| POX | CAT | SOD | ||
|---|---|---|---|---|
| Non-stress | Control | 1.41 ± 0.21 c | 7.86 ± 0.83 c | 217.64 ± 25.10 c |
| OSW | 1.30 ± 0.09 c | 7.81 ± 0.84 c | 220.16 ± 17.38 c | |
| Se-NPs | 0.97 ± 0.11 c | 8.91 ± 0.91 c | 242.66 ± 18.87 c | |
| Se-NPs + OSW | 1.30 ± 0.23 c | 8.40 ± 0.87 c | 231.69 ± 27.67 c | |
| Vanadium stress | Control | 1.52 ± 0.21 c | 9.50 ± 0.97 c | 264.64 ± 25.71 c |
| OSW | 1.44 ± 0.22 c | 8.79 ± 0.95 c | 255.70 ± 30.36 c | |
| Se-NPs | 2.43 ± 0.31 b | 21.22 ± 3.04 b | 404.53 ± 29.19 a | |
| Se-NPs + OSW | 3.43 ± 0.35 a | 27.73 ± 3.53 a | 337.50 ± 32.74 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albqmi, M.; Yaghoubi Khanghahi, M.; Selim, S.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; Al Jaouni, S.K.; Hussein, S.; Warrad, M.; AbdElgawad, H. Positive Interaction of Selenium Nanoparticles and Olive Solid Waste on Vanadium-Stressed Soybean Plant. Agriculture 2023, 13, 426. https://doi.org/10.3390/agriculture13020426
Albqmi M, Yaghoubi Khanghahi M, Selim S, Al-Sanea MM, Alnusaire TS, Almuhayawi MS, Al Jaouni SK, Hussein S, Warrad M, AbdElgawad H. Positive Interaction of Selenium Nanoparticles and Olive Solid Waste on Vanadium-Stressed Soybean Plant. Agriculture. 2023; 13(2):426. https://doi.org/10.3390/agriculture13020426
Chicago/Turabian StyleAlbqmi, Mha, Mohammad Yaghoubi Khanghahi, Samy Selim, Mohammad M. Al-Sanea, Taghreed S. Alnusaire, Mohammed S. Almuhayawi, Soad K. Al Jaouni, Shaimaa Hussein, Mona Warrad, and Hamada AbdElgawad. 2023. "Positive Interaction of Selenium Nanoparticles and Olive Solid Waste on Vanadium-Stressed Soybean Plant" Agriculture 13, no. 2: 426. https://doi.org/10.3390/agriculture13020426
APA StyleAlbqmi, M., Yaghoubi Khanghahi, M., Selim, S., Al-Sanea, M. M., Alnusaire, T. S., Almuhayawi, M. S., Al Jaouni, S. K., Hussein, S., Warrad, M., & AbdElgawad, H. (2023). Positive Interaction of Selenium Nanoparticles and Olive Solid Waste on Vanadium-Stressed Soybean Plant. Agriculture, 13(2), 426. https://doi.org/10.3390/agriculture13020426








