Residual Effects of Different Cropping Systems on Physicochemical Properties and the Activity of Phosphatases of Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Field Experiment
- Conventional system—recommended mineral NPK doses (the doses of mineral fertilizer are presented in Table 2) and organic doses (pig manure at a rate of 30 t·ha−1 under sugar beet), seed dressing, fungicide, insecticide, herbicide, retardant and one-time mechanical weed control;
- Organic system—no chemical plant protection and mineral fertilization; application of pig manure at a dose of 40 t·ha−1 under sugar beet; ploughing in autumn of stubble crops (phacelia, faba bean and field pea) three times under oats and under sugar beet; 3-fold mechanical weeding of crops.
2.2. Weather Conditions
2.3. Sampling and Analyses
2.4. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Soil Biochemical Properties
| Variable | Between SS | df | Within SS | df | F | p |
|---|---|---|---|---|---|---|
| AcPh | 2.004 | 1 | 0.996 | 2 | 4.024 | 0.183 |
| AlPh | 2.898 | 1 | 0.102 | 2 | 57.078 | 0.017 * |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. World Population Prospects: Highlights; United Nations: New York, NY, USA, 2011; pp. 1–8. Available online: https://population.un.org/wpp (accessed on 14 March 2022).
- Borlaug, N.E. Feeding a world of 10 billion people: The miracle ahead. In Vitro Cellular & Developmental Biology. Plant 2002, 38, 221–228. [Google Scholar]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Domagała-Świątkiewicz, I.; Gąstoł, M. Soil chemical properties under organic and conventional crop management systems in south Poland. Biol. Agric. Hortic. 2013, 29, 12–28. [Google Scholar] [CrossRef]
- Fess, T.L.; Benedito, V.A. Organic versus conventional cropping sustainability: A comparative system analysis. Sustainability 2018, 10, 272. [Google Scholar] [CrossRef] [Green Version]
- Stephens, E.C.; Jones, A.D.; Parsons, D. Agricultural systems research and global food security in the 21st century: An overview and roadmap for future opportunities. Agric. Syst. 2018, 163, 1–6. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Schwenke, G.D.; Van Zwieten, L. Impact of agricultural inputs on soil organisms—A review. Aust. J. Soil Res. 2006, 44, 379–406. [Google Scholar] [CrossRef] [Green Version]
- FAO. Transforming Food and Agriculture to Achieve the SDGs: 20 Interconnected Actions to Guide Decision-Makers; FAO: Rome, Italy, 2018. [Google Scholar]
- FAO. Biodiversity For Sustainable Agriculture; FAO: Rome, Italy, 2018. [Google Scholar]
- Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015.
- Jezierska-Tys, S.; Wesołowska, S.; Gałązka, A. Biological activity and functional diversity in soil in different cultivation systems. J. Environ. Sci. Technol. 2020, 17, 4189–4204. [Google Scholar] [CrossRef]
- Medan, D.; Torretta, J.P.; Hodara, K.; de la Fuente, E.B.; Montaldo, N.H. Effects of agriculture expansion and intensification on the vertebrate and invertebrate diversity in the Pampas of Argentina. Biodivers. Conserv. 2011, 20, 3077–3100. [Google Scholar] [CrossRef]
- Bobulská, L.; Fazekašová, D.; Angelovičová, L.; Kotorová, D. Impact of ecological and conventional farming systems on chemical and biological soil quality indices in a cold mountain climate in Slovakia. Biol. Agric. Hortic. 2015, 26, 2–17. [Google Scholar] [CrossRef]
- FAO; WHO. Codex Alimentarius: Organically Produced Foods, 3rd ed.; FAO: Rome, Italy, 2007. [Google Scholar]
- Fließbach, A.; Oberholzer, H.R.; Gunst, L.; Mäder, P. Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric. Ecosyst. Environ. 2007, 118, 273–284. [Google Scholar] [CrossRef]
- Lal, R. Enhancing ecosystem services with no-till. Renew. Agric. Food Syst. 2013, 28, 102–114. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. 784 Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Tkaczyk, P.; Mocek-Płóciniak, A.; Skowrońska, M.; Bednarek, W.; Kuśmierz, S.; Zawierucha, E. The Mineral Fertilizer-Dependent Chemical Parameters of Soil Acidification under Field Conditions. Sustainability 2020, 12, 7165. [Google Scholar] [CrossRef]
- Alvarez, R.; Steinbach, H.S. A Review of the Effects of Tillage Systems on Some Soil Physical Properties, Water Content, Nitrate Availability and Crops Yield in the Argentine Pampas. Soil Till. Res. 2009, 104, 1–15. [Google Scholar] [CrossRef]
- Puglisi, E.; Del Re, A.A.M.; Rao, M.A.; Gianfreda, L. Development and validation of numerical indexes integrating enzyme activities of soils. Soil Biol. Biochem. 2006, 38, 1673–1681. [Google Scholar] [CrossRef]
- Pupin, B.; Silva, F.; Nahas, E. Microbial alterations of the soil influenced by induced compaction. Rev. Bras. Ciéncia Solo 2009, 33, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- Frąc, M.; Oszust, K.; Lipiec, J. Community level physiological profiles (CLPP), characterization and microbial activity of soil amended with dairy sewage sludge. Sensors 2012, 12, 3253–3268. [Google Scholar] [CrossRef] [Green Version]
- Green, V.S.; Stott, D.E.; Cruz, J.C.; Curi, N. Tillage impacts on soil biological activity and aggregation in a Brazilian Cerrado Oxisol. Soil Till. Res. 2007, 92, 114–121. [Google Scholar] [CrossRef]
- Melero, S.; Lüpez-Garrido, R.; Madejün, E.; Murillo, J.M.; Vanderlinden, K.; Ordüñez, R.; Moreno, F. Carbon fractions and enzymatic activities in two cultivated dryland soils under conservation tillage. In Proceedings of the 19th Congress Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Piotrowska-Długosz, A.; Kobierski, M.; Długosz, J. Enzymatic Activity and Physicochemical Properties of Soil Profiles of Luvisols. Materials 2021, 14, 6364. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. Soil Biol. 2011, 26, 215–243. [Google Scholar]
- Bastida, F.; Zsolnay, A.; Hernández, T.; García, C. Past, present and future of soil quality indices: A biological perspective. Geoderma 2008, 147, 159–171. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Futa, B.; Kraska, P.; Andruszczak, S.; Gierasimiuk, P.; Jaroszuk-Sierocińska, M. Impact of Subsurface Application of Compound Mineral Fertilizer on Soil Enzymatic Activity under Reduced Tillage. Agronomy 2021, 11, 2213. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. In International Soil Classification System for 690 Naming Soils and Creating Legends for Soil Maps; Report No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Polish Society of Soil Science. Particle size distribution and textural classes of soils and mineral materials—Classification of Polish Society of Soil Science 2008. Soil Sci. Ann. 2009, 60, 5–16. [Google Scholar]
- International Organization for Standardization. Soil Quality. In Sampling; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- International Organization for Standardization. Soil Quality. In Determination of pH; ISO, 10390; ISO: Geneva, Switzerland, 2005. [Google Scholar]
- International Organization for Standardization. Soil Quality. In Determination of Organic Carbon by Sulfochromic Oxidation; International Organization for Standardization: Geneva, Switzerland, 1998. [Google Scholar]
- International Organization for Standardization. Soil Quality. In Determination of Total Nitrogen Content by Dry Combustion; International Organization for Standardization: Geneva, Switzerland, 1998. [Google Scholar]
- Polish Committee for Standardization. Polish Standard: The Chemical and Agricultural Analysis of the Soil—Determination of the Content of Assailable Phosphorus in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1996. [Google Scholar]
- Soil Survey Laboratory Methods Manual. Soil Survey Investigation Report; United States Department of Agriculture, Natural Resources Conservation Service, National Soil Survey Center: Washington, DC, USA, 1996. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Malina, A. Multidimensional Analysis of the Spatial Differentiation of the Structure of the Polish Economy by Voivodship; Publishing House of the Cracow University of Economics, Wydawnictwo Akademii Ekonomicznej: Cracow, Poland, 2004. [Google Scholar]
- Pandey, D.; Agrawal, M.; Bohra, J.S. Effects of conventional tillage and no tillage permutations on extracellular soil enzyme activities and microbial biomass under rice cultivation. Soil Tillage Res. 2014, 136, 51–60. [Google Scholar] [CrossRef]
- Lal, R. Restoring soil quality to mitigate soil degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef] [Green Version]
- Bai, Z.; Casparia, T.; Ruiperez Gonzaleza, M.; Batjesa, N.H.; Mäderb, P.; Bünemannb, E.K.; de Goedec, R.; Brussaardc, L.; Xud, M.; Santos Ferreirae, C.S.; et al. Effects of agricultural management practices on soil quality: A review of long-term experiments for Europe and China. Agric. Ecosyst. Environ. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E. Chemical Properties of Soil in Four-Field Crop Rotations under Organic and Conventional Farming Systems. Agronomy 2020, 10, 1045. [Google Scholar] [CrossRef]
- Reganold, J.P.; Andrews, P.K.; Reeve, J.R.; Carpenter-Boggs, L.; Schadt, C.W.; Alldredge, J.R.; Zhou, J. Fruit and soil quality of organic and conventional strawberry agroecosystems. PLoS ONE 2010, 5, e12346. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E.; Feledyn-Szewczyk, B.; Antonkiewicz, J. Enzymatic Activity of Loess Soil in Organic and Conventional Farming Systems. Agriculture 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Benke, M.B.; Hao, X.; O’Donovan, J.T.; Clayton, G.W.; Lupwayi, N.Z.; Caffyn, P.; Hall, M. Livestock manure improves acid soil productivity under a cold northern Alberta climate. Can. J. Soil Sci. 2009, 90, 685–697. [Google Scholar] [CrossRef]
- Schoenau, J.J.; Davis, J.G. Optimizing soil and plant responses to land applied manure nutrients in the Great Plains of North America. Can. J. Soil Sci. 2006, 86, 587–595. [Google Scholar] [CrossRef]
- Simansky, V.; Szombathova, N. Basal, potential and relative respiration with dependence on applied crop residues and bio-stimulators in Haplic chernozems/Bazalna, potencialna a relativna respiracia a jej ovplyvnenie aplikaciou rastlinnych zvyskov a biotimulatorov rozkladu v cernozemi. J. Cent. Eur. Agric. 2011, 12, 702. [Google Scholar]
- Kaiser, M.; Ellerbrock, R.H.; Gerke, H.H. Cation exchange capacity and composition of soluble soil organic matter fractions. Soil Sci. Soc. Am. J. 2008, 72, 1278–1285. [Google Scholar] [CrossRef]
- Mayes, M.; Heal, K.R.; Brandt, C.C.; Philips, J.R.; Jardine, P. Relation between soil order and sorption of dissolved organic carbon in temperate subsoils. Soil Sci. Soc. Am. J. 2012, 76, 1027:1–1027:12. [Google Scholar] [CrossRef]
- Balota, E.L.; Kanashiro, M.; Filho, A.C.; Andrade, D.S.; Dick, R.P. Soil enzyme activities under long-term tillage and crop rotation systems in subtropical agroecosystems. Braz. J. Microbiol. 2004, 35, 300–306. [Google Scholar] [CrossRef]
- Gianfreda, L.; Rao, A.M.; Piotrowska, A.; Palumbo, G.; Colombo, C. Soil enzyme activities as affected by anthropogenic alterations: Intensive agricultural practices and organic pollution. Sci. Total Environ. 2005, 341, 265–279. [Google Scholar] [CrossRef]
- Marinari, S.; Mancinelli, R.; Campiglia, E.; Grego, S. Chemical and biological indicators of soil quality in organic and conventional farming systems in Central Italy. Ecol. Indic. 2006, 6, 701–711. [Google Scholar] [CrossRef]
- Lemanowicz, J. Dynamics of phosphorus content and the activity of phosphatase in forest soil in the sustained nitrogen compounds emissions zone. Environ. Sci. Pollut. Res. Int. 2018, 25, 33773–33782. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Martínez, V.; Bell, C.W.; Morris, B.E.L.; Zak, J.; Allen, V.G. Long-term soil microbial community and enzyme activity responses to an integrated cropping-livestock system in a semi-arid region. Agric. Ecosyst. Environ. 2010, 137, 231–240. [Google Scholar] [CrossRef]
- Sawicka, B.; Krochmal-Marczak, B.; Pszczółkowski, P.; Bielińska, E.J.; Wójcikowska-Kapusta, A.; Barbaś, P.; Skiba, D. Effect of Differentiated Nitrogen Fertilization on the Enzymatic Activity of the Soil for Sweet Potato (Ipomoea batatas L. [Lam.]) Cultivation. Agronomy 2020, 10, 1970. [Google Scholar] [CrossRef]
- Bielińska, E.J.; Mocek-Płóciniak, A. Impact of the tillage system on the soil enzymatic activity. Arch. Environ. Prot. 2012, 38, 75–82. [Google Scholar] [CrossRef]
- Bielińska, E.J.; Futa, B.; Chmielewski, S.; Patkowski, K.; Gruszecki, T. Quantification of biodiversity related to the active protection of grassland habitats in the eastern Lublin region of Poland based on the activity of soil enzymes. Pol. J. Soil Sci. 2017, 50, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Błońska, E.; Lasota, J.; Zwydak, M. The relationship between soil properties, enzyme activity and land use. Leśne Pr. Badaw. For. Res. Pap. 2017, 78, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Lemanowicz, J. Mineral fertilization as a factor determining selected sorption properties of soil against the activity of phosphatases. Plant Soil Environ. 2013, 59, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Kotroczó, Z.; Veres, Z.; Fekete, J.; Krakomperger, Z.; Tóth, J.A.; Lajtha, K.; Tóthmérész, B. Soil enzyme activity in response to long-term organic matter manipulation. Soil Biol. Biochem. 2014, 70, 237–243. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Tabatabai, M.A. Enzyme activities in a limed agricultural soil. Biol. Fertil. Soils 2000, 31, 85–91. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Siwik-Ziomek, A.; Koper, J. Effect of Spring Barley Nitrogen Fertilisation on the Changes in the Content of Phosphorus and the Activity of Alkaline and Acid Phosphatase in Soil. Ecol. Chem. Eng. 2012, 19, 1497–1507. [Google Scholar]
- Cabugao, K.G.; Yaffar, D.; Stenson, N.; Childs, J.; Phillips, J.; Mayes, M.A.; Yang, X.; Weston, D.J.; Norby, R.J. Bringing function to structure: Root-soil interactions shaping phosphatase activity throughout a soil profile in Puerto Rico. Ecol. Evol. 2021, 11, 1150–1164. [Google Scholar] [CrossRef]
- Aon, M.; Colaneri, A.C. Temporal and spatial evolution of anzymatic activities and psysicochemical properties on an agricultural soil. Appl. Soil Ecol. 2001, 18, 255–270. [Google Scholar] [CrossRef]


| Initial Soil Properties | Unit | Value |
|---|---|---|
| pHKCl | 5.46–5.65 | |
| Total organic carbon (TOC) | g∙kg−1 | 15.15–16.81 |
| Total nitrogen (TN) | 1.29–1.73 | |
| Available P | mg∙kg−1 | 138.7–156.5 |
| Available K | 226.8–237.9 | |
| Available Mg | 65.9–68.0 | |
| B | 2.16–2.38 | |
| Cu | 6.9–7.3 | |
| Mn | 181.0–198.2 | |
| Zn | 8.9–9.1 |
| Crop Plant | Mineral Fertilization in kg·ha−1 | Manure Fertilization in t·ha−1 | ||
|---|---|---|---|---|
| N | P | K | ||
| Sugar beet | 100 (prior to sowing) | 100 (prior to sowing) | 140 prior to sowing) | 30 (in autumn) |
| Spring barley | 90 (split dose *) | 70 (prior to sowing) | 90 (prior to sowing) | |
| Red clover | - | 80 (prior to sowing) | 100 (in spring) | |
| Winter wheat | 140 (split dose **) | 60 (prior to sowing) | 80 (prior to sowing) | |
| Oats | 70 (prior to sowing) | 70 (prior to sowing) | 110 (prior to sowing) | |
| Crop Plant | Date of Sowing | Date of Harvest |
|---|---|---|
| Sugar beet | 20–25 April | 16–19 October |
| Spring barley | 18–21 April | 10–12 August |
| Red clover | 18–21 April | Blooming phase |
| Winter wheat * | 20–22 September | 8–10 August next year |
| Oats ** | 10–12 April | 17–19 August |
| Month | March– August | ||||||
|---|---|---|---|---|---|---|---|
| March | April | May | June | July | August | ||
| Rainfall (in mm) | The sum | ||||||
| 2020 | 26.0 | 19.0 | 111.4 | 170.3 | 67.8 | 59.3 | 453.8 |
| 1963–2010 | 31.3 | 42.4 | 63.5 | 72.7 | 80.0 | 69.5 | 359.4 |
| Air temperature (in °C) | Mean | ||||||
| 2020 | 4.6 | 8.6 | 11.2 | 17.4 | 18.8 | 20.4 | 13.5 |
| 1963–2010 | 1.8 | 7.7 | 13.6 | 16.5 | 18.3 | 17.7 | 12.6 |
| Farming System Crop Plant | Soil Layers | pHKCl | HA | BC | CEC |
|---|---|---|---|---|---|
| cmol(+)kg−1 | |||||
| Conventional winter wheat (CW) | 0–30 cm | 6.16 ± 0.04 c | 1.60 ± 0.00 b | 13.30 ± 0.80 c | 14.90 ± 0.80 e |
| 30–50 cm | 5.55 ± 0.13 a | 1.40 ± 0.20 ab | 11.80 ± 0.10 bc | 13.30 ± 0.30 d | |
| Mean | 5.85 ± 0.09 b | 1.50 ± 0.10 ab | 12.55 ± 0.90 c | 14.10 ± 0.55 de | |
| Organic winter wheat (OW) | 0–30 cm | 6.45 ± 0.08 d | 2.00 ± 0.00 c | 10.30 ± 0.50 ab | 11.90 ± 0.50 c |
| 30–50 cm | 5.49 ± 0.03 a | 1.60 ± 0.00 b | 9.80 ± 0.20 ab | 11.80 ± 0.20 c | |
| Mean | 5.97 ± 0.06 c | 1.80 ± 0.00 bc | 10.05 ± 0.35 ab | 11.85 ± 0.70 c | |
| Conventional sugar beet (CS) | 0–30 cm | 6.76 ± 0.05 e | 2.20 ± 0.20 c | 9.40 ± 0.40 ab | 11.60 ± 0.60 bc |
| 30–50 cm | 5.74 ± 0.03 b | 1.60 ± 0.00 b | 8.50 ± 0.50 a | 10.10 ± 0.50 a | |
| Mean | 6.25 ± 0.04 d | 1.90 ± 0.10 bc | 8.95 ± 0.45 ab | 10.85 ± 0.55 b | |
| Organic sugar beet (OS) | 0–30 cm | 6.82 ± 0.03 e | 1.60 ± 0.00 b | 8.80 ± 1.00 ab | 10.40 ± 1.00 a |
| 30–50 cm | 5.83 ± 0.03 b | 1.20 ± 0.00 a | 8.70 ± 1.00 ab | 9.90 ± 1.00 a | |
| Mean | 6.33 ± 0.03 d | 1.40 ± 0.00 ab | 8.75 ± 1.00 ab | 10.15 ± 1.00 a |
| Farming System Crop Plant | Soil Layers | TOC | TN | TOC/TN | P |
|---|---|---|---|---|---|
| g·kg−1 | mg·kg−1 | ||||
| Conventional winter wheat (CW) | 0–30 cm | 7.98 ± 0.40 c | 0.66 ± 0.06 b | 12.09 ± 0.07 g | 63.94 ± 0.59 cd |
| 30–50 cm | 3.82 ± 0.05 a | 0.45 ± 0.06 a | 8.48 ± 0.01 a | 48.45 ± 0.74 a | |
| Mean | 5.90 ± 0.23 b | 0.56 ± 0.06 a | 10.29 ± 0.04 c | 56.20 ± 0.67 b | |
| Organic winter wheat (OW) | 0–30 cm | 11.82 ± 0.20 f | 1.02 ± 0.10 de | 11.59 ± 0.03 e | 69.98 ± 3.62 d |
| 30–50 cm | 7.05 ± 0.23 c | 0,77 ± 0.06 c | 9.16 ± 0.38 b | 62.06 ± 0.42 bc | |
| Mean | 9.44 ± 0.22 e | 0.90 ± 0.35 cd | 10.38 ± 0.22 c | 66.01 ± 2.02 cd | |
| Conventional sugar beet (CS) | 0–30 cm | 12.10 ± 0.36 f | 0.97 ± 0.12 d | 12.47 ± 0.05 f | 84.87 ± 0.02 e |
| 30–50 cm | 7.52 ± 0.14 c | 0.66 ± 0.06 b | 11.39 ± 0.03 de | 57.51 ± 1.83 b | |
| Mean | 9.81 ± 0.25 e | 0.82 ± 0.09 c | 11.93 ± 0.04 e | 71.19 ± 0.93 d | |
| Organic sugar beet (OS) | 0–30 cm | 8.70 ± 0.12 d | 0.77 ± 0.06 c | 11.30 ± 0.22 d | 79.03 ± 2.25 e |
| 30–50 cm | 7.65 ± 0.12 c | 0.68 ± 0.06 bc | 11.25 ± 0.38 d | 62.85 ± 4.43 bc | |
| Mean | 8.18 ± 0.12 cd | 0.73 ± 0.06 bc | 11.28 ± 0.30 d | 70.94 ± 3.34 d | |
| Variable | Between SS | df | Within SS | df | F | p |
|---|---|---|---|---|---|---|
| TOC | 2.571 | 2 | 0.429 | 5 | 2.993 | 0.378 ns |
| TN | 2.813 | 2 | 0.187 | 5 | 7.501 | 0.250 ns |
| TOC/TN | 2.490 | 2 | 0.510 | 5 | 2.441 | 0.412 ns |
| Farming System Crop Plant | Soil Layers | AcPh | AlPh | AlPh/AcPh |
|---|---|---|---|---|
| mmol PNP kg−1.h−1 | ||||
| Conventional winter wheat (CW) | 0–30 cm | 69.55 ± 2.41 e | 26.56 ± 3.74 bc | 0.38 ± 0.15 b |
| 30–50 cm | 34.66 ± 1.24 b | 15.92 ± 0.83 a | 0.45 ± 0.06 d | |
| Mean | 52.11 ± 1.83 cd | 21.24 ± 2.29 b | 0.42 ± 0.13 c | |
| Organic winter wheat (OW) | 0–30 cm | 88.54 ± 2.88 f | 30.85 ± 3.16 b | 0.35 ± 0.11 a |
| 30–50 cm | 46.40 ± 2.82 c | 19.51 ± 2.39 ab | 0.42 ± 0.08 c | |
| Mean | 67.47 ± 2.85 e | 25.18 ± 2.78 b | 0.39 ± 0.10 b | |
| Conventional sugar beet (CS) | 0–30 cm | 84.50 ± 1.28 f | 38.72 ± 1.31 c | 0.46 ± 0.11 d |
| 30–50 cm | 28.04 ± 1.32 b | 13.36 ± 1.92 a | 0.47 ± 0.14 d | |
| Mean | 56.27 ± 1.30 d | 26.04 ± 1.62 b | 0.47 ± 0.12 d | |
| Organic sugar beet (OS) | 0–30 cm | 69.86 ± 1.88 e | 30.30 ± 3.26 bc | 0.44 ± 0.05 cd |
| 30–50 cm | 20.57 ± 1.86 a | 10.07 ± 2.13 a | 0.49 ± 0.12 de | |
| Mean | 45.22 ± 1.87 c | 20.29 ± 2.70 ab | 0.47 ± 0.15 d | |
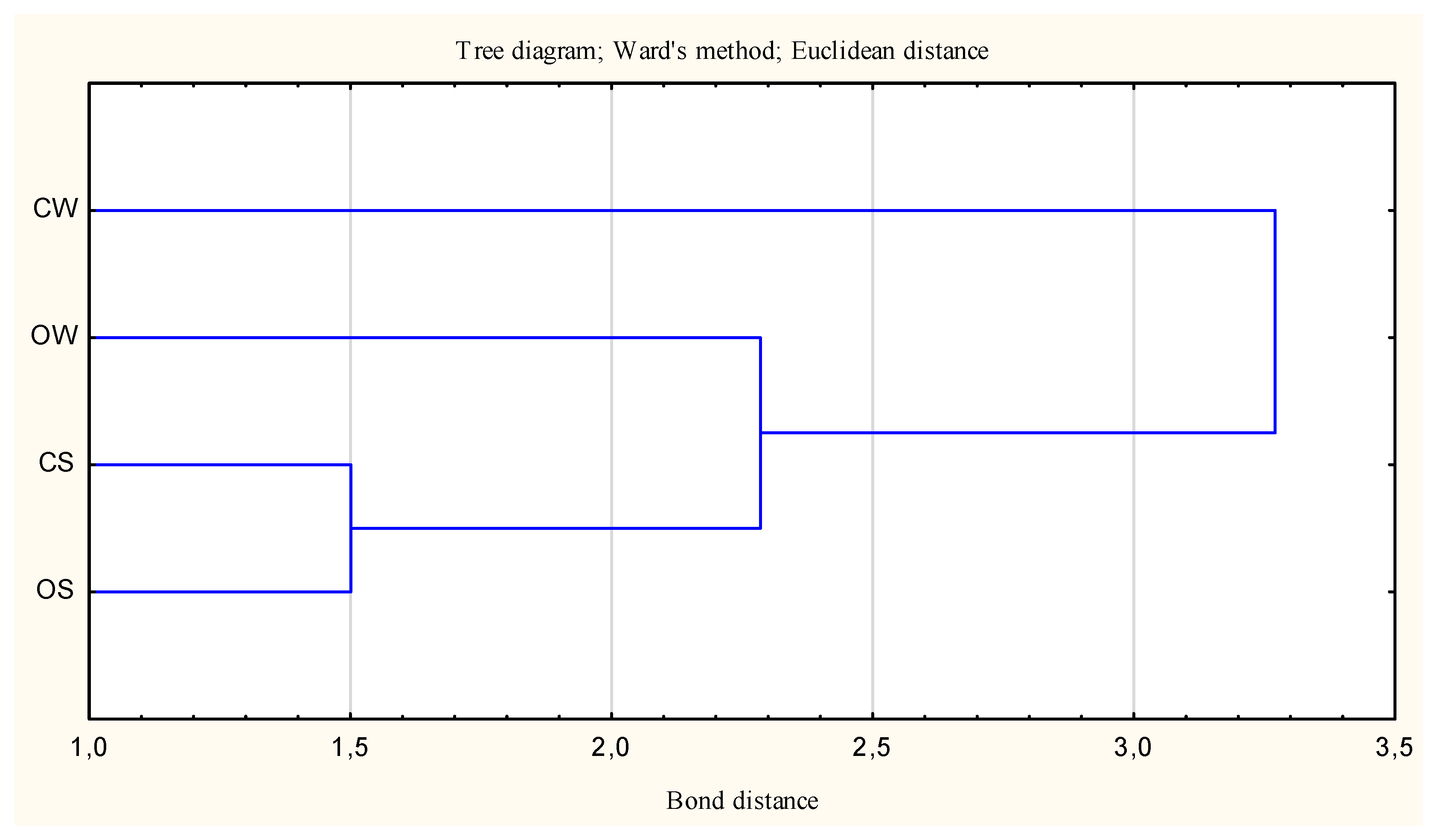
| Clusters | 1 | 2 |
|---|---|---|
| 1 | 0.00 | 1.57 |
| 2 | 0.00 |
| Variable | Mean Values of the Output Variables in the Clusters | |
|---|---|---|
| Clusters 1 (OW and CS) | Clusters 2 (CW and OS) | |
| AcPh | 61.87 | 48.67 |
| AlPh | 25.61 | 20.76 |
| Parameter | Farming System | pHKCl | TOC | TN | P |
|---|---|---|---|---|---|
| AcPh | Conventional | −0.94 ** | 0.79 ** | 0.77 ** | 0.87 ** |
| Organic | −0.69 * | 0.82 ** | 0.87 ** | 0.66 ** | |
| AlPh | Conventional | 0.87 ** | 0.84 ** | 0.85 ** | 0.83 ** |
| Organic | 0.77 ** | 0.70 * | 0.73 ** | 0.79 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesołowska, S.; Futa, B.; Myszura, M.; Kobyłka, A. Residual Effects of Different Cropping Systems on Physicochemical Properties and the Activity of Phosphatases of Soil. Agriculture 2022, 12, 693. https://doi.org/10.3390/agriculture12050693
Wesołowska S, Futa B, Myszura M, Kobyłka A. Residual Effects of Different Cropping Systems on Physicochemical Properties and the Activity of Phosphatases of Soil. Agriculture. 2022; 12(5):693. https://doi.org/10.3390/agriculture12050693
Chicago/Turabian StyleWesołowska, Sylwia, Barbara Futa, Magdalena Myszura, and Agata Kobyłka. 2022. "Residual Effects of Different Cropping Systems on Physicochemical Properties and the Activity of Phosphatases of Soil" Agriculture 12, no. 5: 693. https://doi.org/10.3390/agriculture12050693
APA StyleWesołowska, S., Futa, B., Myszura, M., & Kobyłka, A. (2022). Residual Effects of Different Cropping Systems on Physicochemical Properties and the Activity of Phosphatases of Soil. Agriculture, 12(5), 693. https://doi.org/10.3390/agriculture12050693







