Active-Modified Atmosphere Packaging of Ready-to-Eat Pomegranate (Punica granatum L.) Arils at Ambient Temperature for Extending Shelf-Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Processing, Packaging, and Treatment
2.3. Chemical Analysis
2.3.1. Titratable Acidity and pH
2.3.2. Anthocyanins
2.3.3. Ascorbic Acid
2.3.4. Reducing Sugars
2.4. Quality Analysis
2.4.1. Weight Loss
2.4.2. Hardness
2.4.3. Total Soluble Solids (TSS) Content
2.5. Package Headspace Gas Composition (O2 and CO2)
2.6. Microbiological Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis
3.1.1. Titratable Acidity and pH
3.1.2. Anthocyanins
3.1.3. Ascorbic Acid
3.1.4. Reducing Sugars
3.2. Quality Analysis
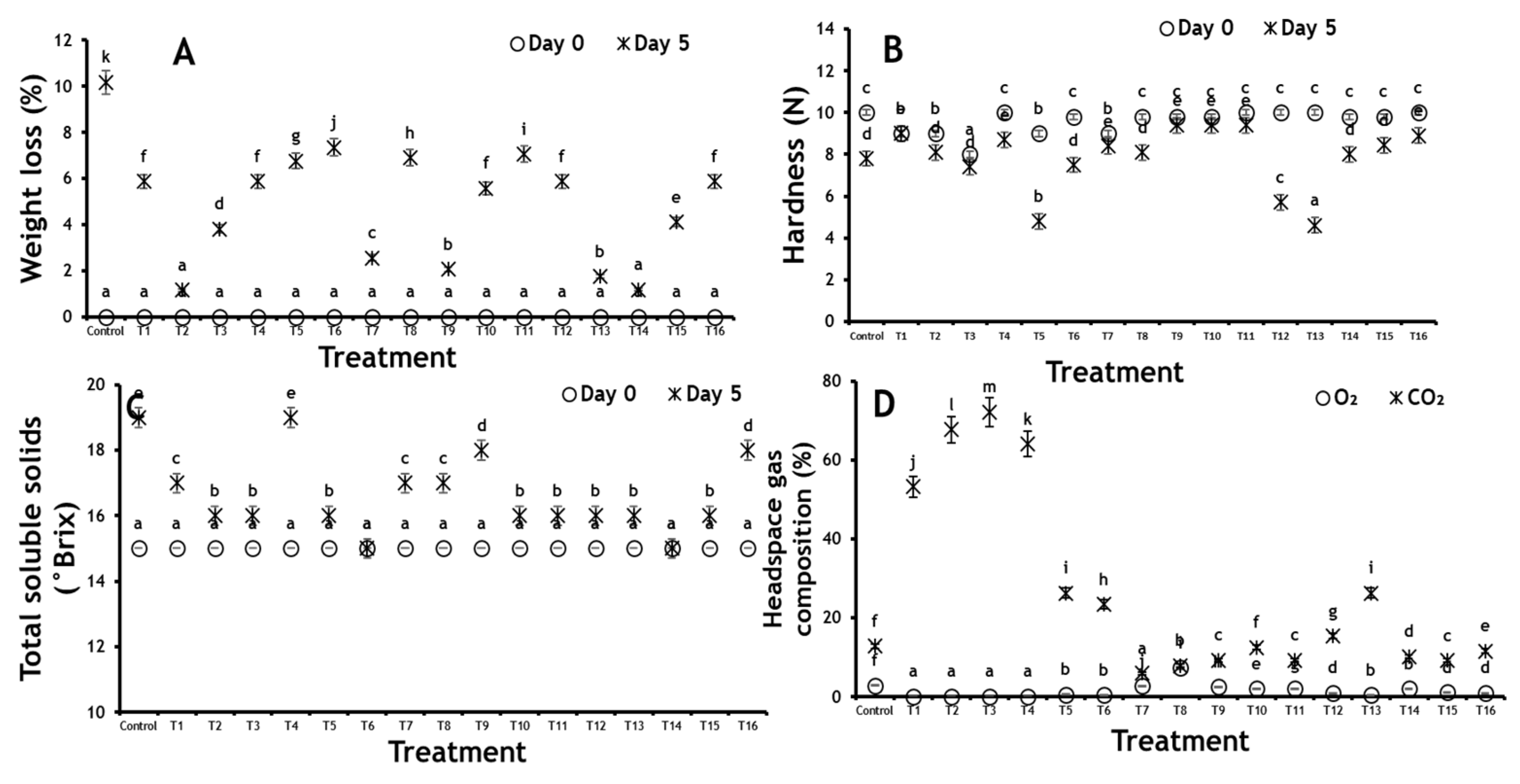
3.2.1. Weight Loss
3.2.2. Hardness
3.2.3. Total Soluble Solids (TSS)
3.3. Package Headspace Gas Composition (O2 and CO2)
3.4. Microbiological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opara, I.K.; Fawole, O.A.; Opara, U.L. Postharvest losses of pomegranate fruit at the packhouse and implications for sustainability indicators. Sustainability 2021, 13, 5187. [Google Scholar] [CrossRef]
- Palma, A.; Continella, A.; Malfa, S.L.; Gentile, A.; D’Aquino, S. Overall quality of ready-to-eat pomegranate arils processed from cold stored fruit. Postharvest Biol. Technol. 2015, 109, 1–9. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Moreira, M.d.R.; Roura, S.I.; Ayala-Zavala, J.F.; González-Aguilar, G.A. Using natural antimicrobials to enhance the safety and quality of fresh and processed fruits and vegetables: Types of antimicrobials. In Handbook of Natural Antimicrobials for Food Safety and Quality; Taylor, T.M., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 287–313. [Google Scholar]
- Bhatia, K.; Asrey, R. Minimal processing of pomegranates (Punica granatum L.)—A review on processing, quality, and shelf life. J. Food Processing Preserv. 2019, 43, e14281. [Google Scholar] [CrossRef]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2019, 59, 411–422. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Dorostkar, M. Effect of vacuum and modified atmosphere packaging on the quality attributes and sensory evaluation of fresh jujube fruit. Int. J. Fruit Sci. 2021, 21, 82–94. [Google Scholar] [CrossRef]
- Banda, K.; Caleb, O.J.; Jacobs, K.; Opara, U.L. Effect of active-modified atmosphere packaging on the respiration rate and quality of pomegranate arils (cv. Wonderful). Postharvest Biol. Technol. 2015, 109, 97–105. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, S.; Yang, J.; Zou, M.; Xin, L. Improving pomegranate fruit quality by short-term hypobaric treatment combined with modified atmosphere packaging storage. ISHS Acta Hortic. 2019, 1319, 217–222. [Google Scholar] [CrossRef]
- Golkarian, M.; Şen, F.; Okşar, R. Effects of pre-cooling and modified atmosphere packaging on storability of pomegranate (Punica granatum ’Hicaznar’) fruit. ISHS Acta Hortic. 2018, 1275, 237–244. [Google Scholar] [CrossRef]
- Fang, Y.; Wakisaka, M. A review on the modified atmosphere preservation of fruits and vegetables with cutting-edge technologies. Agriculture 2021, 11, 992. [Google Scholar] [CrossRef]
- Czerwiński, K.; Rydzkowski, T.; Wróblewska-Krepsztul, J.; Thakur, V.K. Towards impact of modified atmosphere packaging (MAP) on shelf-life of polymer-film-packed food products: Challenges and sustainable developments. Coatings 2021, 11, 1504. [Google Scholar] [CrossRef]
- Tinebra, I.; Scuderi, D.; Sortino, G.; Inglese, P.; Farina, V. Effects of argon-based and nitrogen-based modified atmosphere packaging technology on the quality of pomegranate (Punica granatum L. cv. Wonderful) Arils. Foods 2021, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Moradinezhad, F.; Ansarifar, E.; Mohammadian Moghaddam, M. Extending the shelf life and maintaining quality of minimally-processed pomegranate arils using ascorbic acid coating and modified atmosphere packaging. J. Food Meas. Charact. 2020, 14, 3445–3454. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; Manley, M.; Opara, U.L. Evaluation of parameters affecting modified atmosphere packaging engineering design for pomegranate arils. Int. J. Food Sci. Technol. 2013, 48, 2315–2323. [Google Scholar] [CrossRef]
- Caleb, O.J.; Opara, U.L.; Witthuhn, C.R. Modified atmosphere packaging of pomegranate fruit and arils: A review. Food Bioprocess Technol. 2012, 5, 15–30. [Google Scholar] [CrossRef]
- Tavasoli-Talarposhti, S.; Barzegar, M.; Hamidi-Esfahani, Z. Effect of modified atmosphere packaging on aril physico-chemical and microbial properties of two pomegranate cultivars (Punica granatum L.) grown in Iran. Nutr. Food Sci. Res. 2016, 3, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, D.; Xu, W.; Fu, Y.; Liao, R.; Shi, J.; Chen, Y. Application of passive modified atmosphere packaging in the preservation of sweet corns at ambient temperature. LWT 2021, 136, 110295. [Google Scholar] [CrossRef]
- Flores-Martínez, D.; Urías-Orona, V.; Hernández-García, L.; Rubio-Carrasco, W.; Silva-Gutiérrez, K.; Guevara-Zambrano, M.; Prieto-Cadena, J.; Serna-Méndez, T.; Muy-Rangel, D.; Niño-Medina, G. Physicochemical parameters, mineral composition, and nutraceutical properties of ready-to-drink flavored-colored commercial teas. J. Chem. 2018, 2018, 2861541. [Google Scholar] [CrossRef]
- Choi, I.; Chun, J.; Choi, H.-S.; Park, J.; Kim, N.-G.; Lee, S.-K.; Park, C.-H.; Jeong, K.-H.; Nam, J.-W.; Cho, J.; et al. Starch characteristics, sugars and thermal properties of processing potato (Solanum tuberosum L.) cultivars developed in Korea. Am. J. Potato Res. 2020, 97, 308–317. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Sellamuthu, P.S.; Emmanuel, R.S. Use of modified atmosphere packaging combined with essential oils for prolonging post-harvest shelf life of mango (cv. Banganapalli and cv. Totapuri). LWT 2021, 148, 111662. [Google Scholar] [CrossRef]
- Sheikhi, A.; Mirdehghan, S.H.; Karimi, H.R.; Ferguson, L. Effects of passive- and active-modified atmosphere packaging on physio-chemical and quality attributes of fresh in-hull pistachios (Pistacia vera L. cv. Badami). Foods 2019, 8, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Romero, D.; Castillo, S.; Guillén, F.; Díaz-Mula, H.M.; Zapata, P.J.; Valero, D.; Serrano, M. Aloe vera gel coating maintains quality and safety of ready-to-eat pomegranate arils. Postharvest Biol. Technol. 2013, 86, 107–112. [Google Scholar] [CrossRef]
- Ayhan, Z.; Eştürk, O. Overall quality and shelf life of minimally processed and modified atmosphere packaged “ready-to-eat” pomegranate arils. J. Food Sci. 2009, 74, C399–C405. [Google Scholar] [CrossRef]
- Sarhadi, H.; Sadeghizadeh, Y. The effect of modified atmosphere packaging on physicochemical, microbial, and sensorial properties of Iranian mazafati date. J. Food Qual. Hazards Control 2019, 6, 73–78. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Zareh, S.; Shiri, M.A.; Javdani, Z. The arils characterization of five different pomegranate (Punica granatum) genotypes stored after minimal processing technology. J. Food Sci. Technol. 2015, 52, 2023–2032. [Google Scholar] [CrossRef] [Green Version]
- O’Grady, L.; Sigge, G.; Caleb, O.J.; Opara, U.L. Effects of storage temperature and duration on chemical properties, proximate composition and selected bioactive components of pomegranate (Punica granatum L.) arils. LWT 2014, 57, 508–515. [Google Scholar] [CrossRef]
- Patanè, C.; Malvuccio, A.; Saita, A.; Rizzarelli, P.; Siracusa, L.; Rizzo, V.; Muratore, G. Nutritional changes during storage in fresh-cut long storage tomato as affected by biocompostable polylactide and cellulose based packaging. LWT 2019, 101, 618–624. [Google Scholar] [CrossRef]
- Selcuk, N.; Erkan, M. Impact of passive modified atmosphere packaging on physicochemical properties, bioactive compounds, and quality attributes of sweet pomegranates. Turkish J. Agric. For. 2016, 40, 475–488. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Gökmen, V. Extending the shelf-life of pomegranate arils with chitosan-ascorbic acid coating. LWT 2017, 76, 172–180. [Google Scholar] [CrossRef]
- Hussein, Z.; Caleb, O.J.; Jacobs, K.; Manley, M.; Opara, U.L. Effect of perforation-mediated modified atmosphere packaging and storage duration on physicochemical properties and microbial quality of fresh minimally processed ‘Acco’ pomegranate arils. LWT 2015, 64, 911–918. [Google Scholar] [CrossRef]
- Khorshidi, S.; Davarynejad, G.; Tehranifar, A.; Fallahi, E. Effect of modified atmosphere packaging on chemical composition, antioxidant activity, anthocyanin, and total phenolic content of cherry fruits. Hortic. Environ. Biotechnol. 2011, 52, 471–481. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, M.; Wei, X.; Xu, C.; Luo, Z.; Mao, L. Consolidated cold and modified atmosphere package system for fresh strawberry supply chains. LWT 2019, 109, 207–215. [Google Scholar] [CrossRef]
- Erkan, M.; Pekmezci, M.; Gübbük, H.; Karaşahİn, I. Effects of controlled atmosphere storage on scald development and postharvest physiology of granny smith apples. Turkish J. Agric. For. 2004, 28, 43–48. [Google Scholar]
- Caleb, O.J.; Opara, U.L.; Mahajan, P.V.; Manley, M.; Mokwena, L.; Tredoux, A.G.J. Effect of modified atmosphere packaging and storage temperature on volatile composition and postharvest life of minimally-processed pomegranate arils (cvs. ‘Acco’ and ‘Herskawitz’). Postharvest Biol. Technol. 2013, 79, 54–61. [Google Scholar] [CrossRef]
- Belay, Z.A.; Caleb, O.J.; Mahajan, P.V.; Opara, U.L. Application of simplex lattice mixture design for optimization of active modified atmosphere for pomegranate arils (cv. Wonderful) based on microbial criteria. Food Packag. Shelf Life 2017, 14, 12–17. [Google Scholar] [CrossRef]
- NSW-Food-Authority. Microbiological Quality of Fresh Cut Vegetables: A Survey to Determine the Safety of Fresh Cut Leafy Salad Vegetables Sold in NSW. 2001. Available online: https://www.foodauthority.nsw.gov.au/sites/default/files/_Documents/scienceandtechnical/microbiological_quality_fresh_cut_vegetables.pdf (accessed on 17 January 2022).

| Treatment Code | Headspace Gas Composition (%) | |
|---|---|---|
| O2 | CO2 | |
| Control | 21 † | 0.03 † |
| T1 | 0.2 | 6.3 |
| T2 | 0.1 | 9.5 |
| T3 | 0.6 | 12 |
| T4 | 2.5 | 13 |
| T5 | 2.9 | 3.4 |
| T6 | 4.9 | 2.9 |
| T7 | 7.0 | 2.8 |
| T8 | 1.0 | 2.8 |
| T9 | 2.8 | 5.8 |
| T10 | 3.5 | 5.8 |
| T11 | 11 | 5.9 |
| T12 | 4.6 | 11 |
| T13 | 2.5 | 8.3 |
| T14 | 4.7 | 8.3 |
| T15 | 6.3 | 8.7 |
| T16 | 7.0 | 12 |
| Treatment | Yeast and Mold Counts (log CFU/g) | |
|---|---|---|
| Storage Time (Days) | ||
| 0 | 5 | |
| Control | <1 a,A | 6.50 ± 0.34 f,B |
| T1 | <1 a,A | 3.50 ± 0.01 a,B |
| T2 | <1 a,A | 5.00 ± 0.24 e,B |
| T3 | <1 a,A | 3.50 ± 0.02 a,B |
| T4 | <1 a,A | 5.00 ± 0.04 e,B |
| T5 | <1 a,A | 4.00 ± 0.14 b,B |
| T6 | <1 a,A | 4.50 ± 0.20 c,B |
| T7 | <1 a,A | 4.00 ± 0.18 b,B |
| T8 | <1 a,A | 5.00 ± 0.22 d,B |
| T9 | <1 a,A | 4.50 ± 0.37 c,B |
| T10 | <1 a,A | 4.00 ± 0.15 b,B |
| T11 | <1 a,A | 4.50 ± 0.03 c,B |
| T12 | <1 a,A | 4.50± 0.16 c,B |
| T13 | <1 a,A | 4.50 ± 0.28 c,B |
| T14 | <1 a,A | 5.00 ± 0.28 e,B |
| T15 | <1 a,A | 4.50 ± 0.25 c,B |
| T16 | <1 a,A | 4.50 ± 0.05 c,B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokalla, P.; Inbaraj, B.S.; Dikkala, P.K.; Sridhar, K.; Dasi, D.S.; Koka, L.; Munakala, R.; Galipothula, R.; Chelli, K.S.R.; Kalletlapally, N.K. Active-Modified Atmosphere Packaging of Ready-to-Eat Pomegranate (Punica granatum L.) Arils at Ambient Temperature for Extending Shelf-Life. Agriculture 2022, 12, 155. https://doi.org/10.3390/agriculture12020155
Rokalla P, Inbaraj BS, Dikkala PK, Sridhar K, Dasi DS, Koka L, Munakala R, Galipothula R, Chelli KSR, Kalletlapally NK. Active-Modified Atmosphere Packaging of Ready-to-Eat Pomegranate (Punica granatum L.) Arils at Ambient Temperature for Extending Shelf-Life. Agriculture. 2022; 12(2):155. https://doi.org/10.3390/agriculture12020155
Chicago/Turabian StyleRokalla, Preethi, Baskaran Stephen Inbaraj, Praveen Kumar Dikkala, Kandi Sridhar, Daniel Smith Dasi, Lalitha Koka, Ramalakshmi Munakala, Ranjith Galipothula, Kavitha Swarupa Rani Chelli, and Naveen Kumar Kalletlapally. 2022. "Active-Modified Atmosphere Packaging of Ready-to-Eat Pomegranate (Punica granatum L.) Arils at Ambient Temperature for Extending Shelf-Life" Agriculture 12, no. 2: 155. https://doi.org/10.3390/agriculture12020155
APA StyleRokalla, P., Inbaraj, B. S., Dikkala, P. K., Sridhar, K., Dasi, D. S., Koka, L., Munakala, R., Galipothula, R., Chelli, K. S. R., & Kalletlapally, N. K. (2022). Active-Modified Atmosphere Packaging of Ready-to-Eat Pomegranate (Punica granatum L.) Arils at Ambient Temperature for Extending Shelf-Life. Agriculture, 12(2), 155. https://doi.org/10.3390/agriculture12020155







