Physiological Quality of Swingle Citrumelo Seed after Refrigerated Storage of Fruits and Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Plant Material
2.2. Fruit Harvest and Experimental Design
2.3. Evaluations
2.3.1. Physicochemical Evaluations of Swingle Citrumelo Fruits and Juice
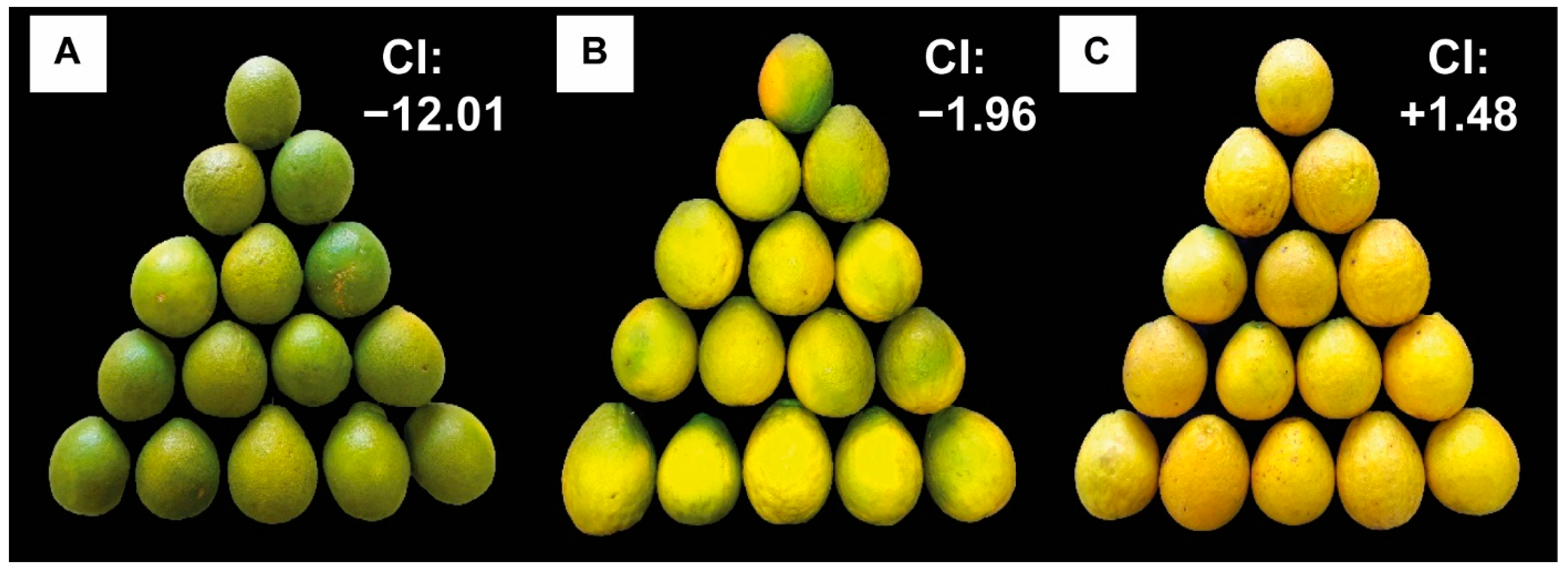
Fruit Colouring
Total Soluble Solids, Titratable Acidity, and Ratio
2.3.2. Evaluations on Swingle Citrumelo Seeds
Water Content
Seed Germination
Emergence in Greenhouse
2.4. Data Analysis
3. Results
3.1. Physicochemical Quality of Fruits
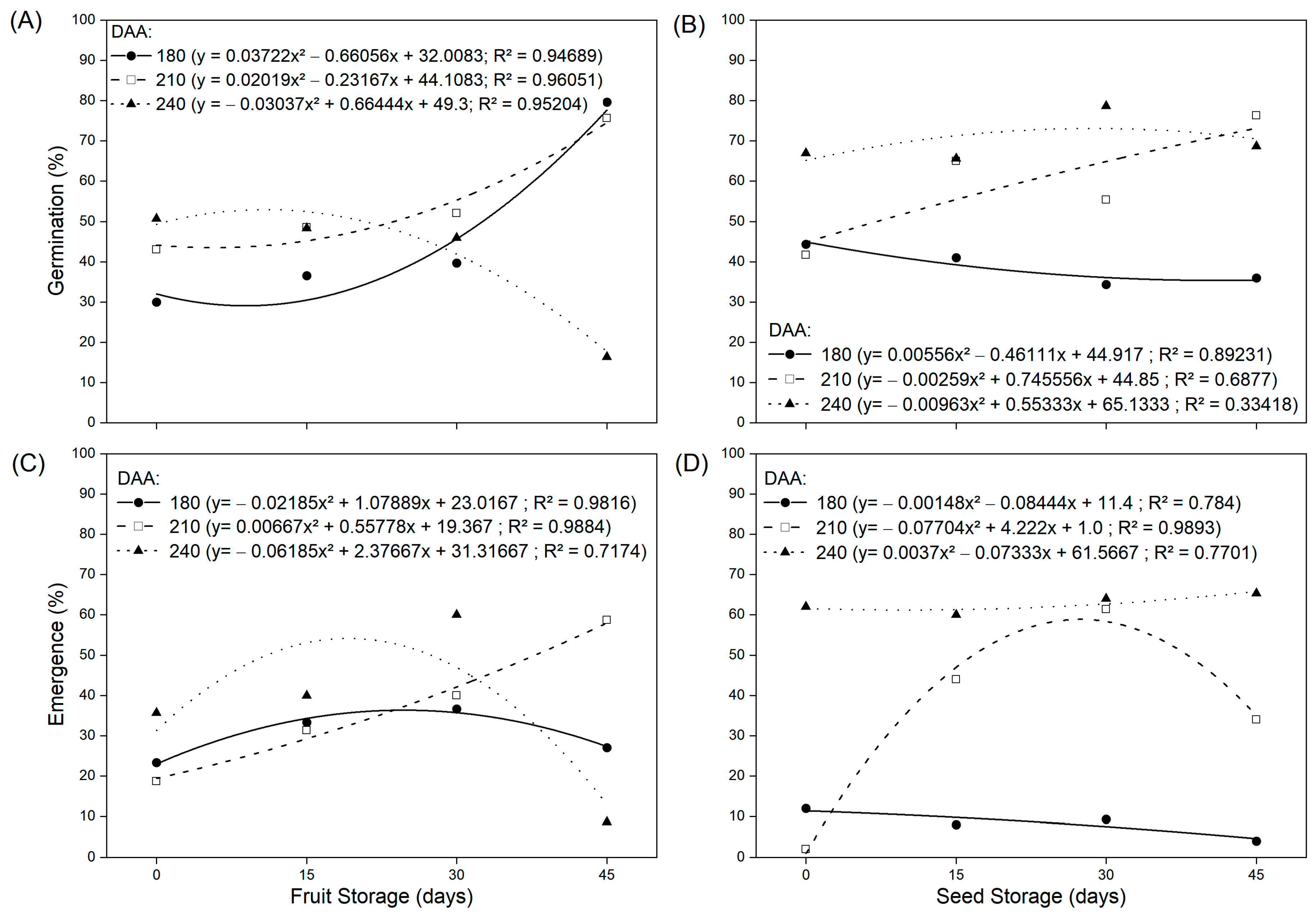
3.2. Water Content, Germination and Seed Emergence
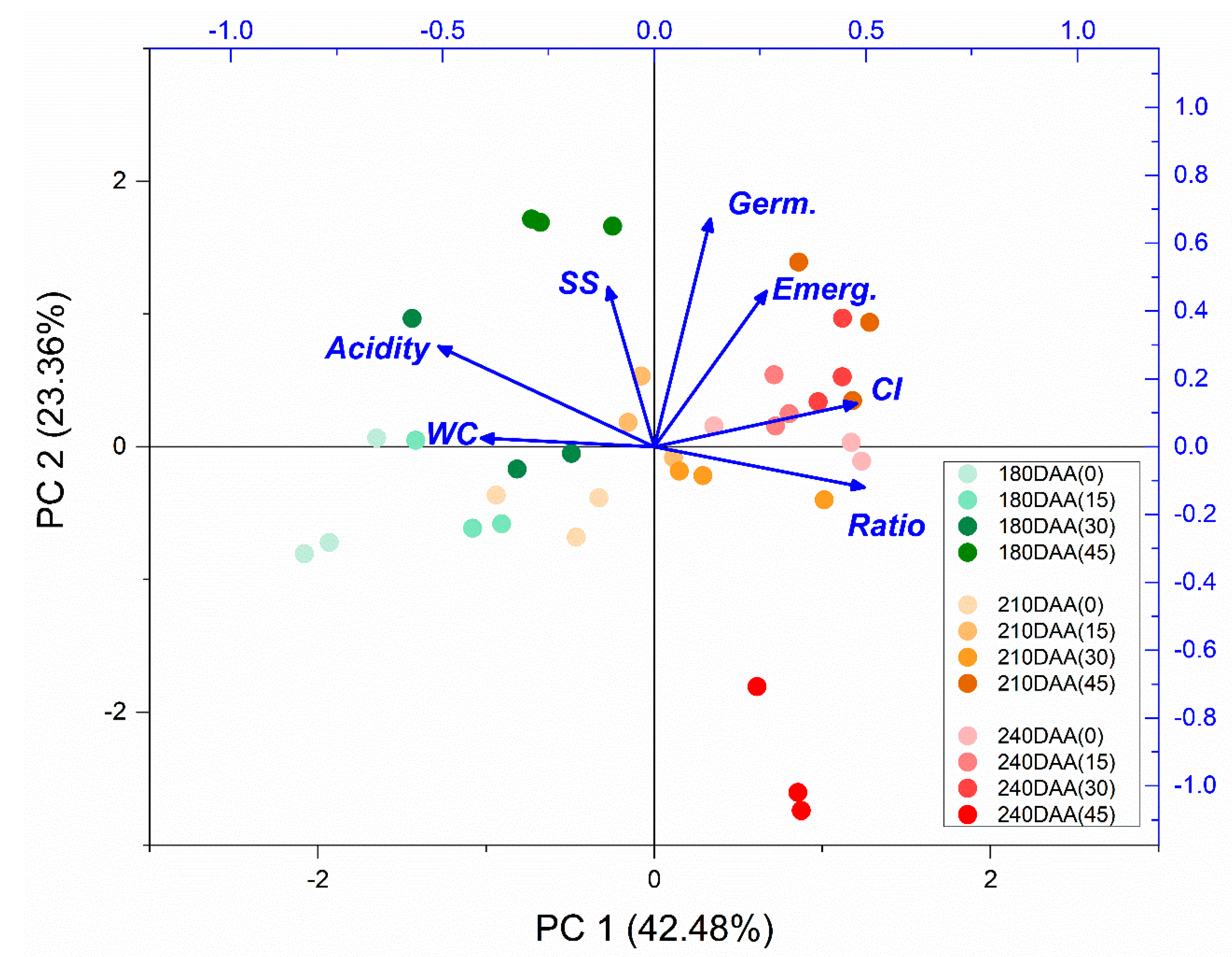
3.3. Principal Component Analysis (PCA)
4. Discussion
4.1. Physicochemical Evaluations of Swingle Citrumelo Fruits and Juice
4.2. Evaluation of Swingle Citrumelo Seeds
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peske, S.T.; Villela, F.A.; Meneghello, G.E. Sementes: Fundamentos Científicos e Tecnológicos, 2nd ed.; UFPel: Pelotas, Brazil, 2012; 573p. [Google Scholar]
- Gomes-Junior, F.G.; Arruda, N.; Marcos-Filho, J. Swingle citrumelo seed vigor and storability associated with fruit maturity classes based on RGB parameters. Sci. Agric. 2017, 74, 357–363. [Google Scholar] [CrossRef]
- Gonçalves, B.H.L.; Liberato, E.M.S.; Leonel, S.; Modesto, J.H.; Souza, J.M.A. Efeito do uso de reguladores vegetais na germinação das sementes de citrumelo Swingle. Sci. Plena 2013, 9, 1–7. [Google Scholar]
- Gimenes-Fernandes, N.; Bassanezi, R.B. Integrated management of citrus diseases: Sudden death. Fitopatol. Bras. 2003, 28, 66–72. [Google Scholar]
- Rodrigues, F.A.; Freitas, G.F.; Moreira, R.A.; Pasqual, M. Characterization of fruits and seeds germination of rootstock trifoliata Flying Fragon and citrumelo Swingle. Rev. Bras. Frutic. 2010, 32, 1180–1188. [Google Scholar] [CrossRef]
- Conceição, P.M.; Azevedo, F.A.; Souza, A.J.B.; Próspero, A.G.; Morelli, M.; Forti, V.A. Ideal harvesting point of ‘Limeira-IAC382’ trifoliate orange fruits for seed extraction. Rev. Bras. Frutic. 2020, 42, 1–9. [Google Scholar] [CrossRef]
- Justino, E.V.; Boiteux, L.S.; Fonseca, M.E.N.; Filho, J.G.S.; Nascimento, W.M. Determinação da maturidade fisiológica de sementes de pimenta dedo de moça Capsicum baccatum var. pendulum. Hortic. Bras. 2015, 33, 324–331. [Google Scholar] [CrossRef][Green Version]
- Pereira, F.E.C.B.; Salvadora, B.T.; Silva, M.I.L.; Grangeiro, L.C.; Benedito, C.P. Qualidade fisiológica de sementes de pimenta em função da idade e do tempo de repouso pós-colheita dos frutos. Rev. Ciênc. Agron. 2014, 45, 737–744. [Google Scholar] [CrossRef][Green Version]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes, G.; Leonardo, J.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Jimenez-Cuesta, M.; Cayuela, J.C.; Martinrez-Javega, J.M. Teoria y Practica de la Desverdizacion de los Citricos; Ministerio de Agricultura, Pesca y Alimentación–INIA: Madrid, Spain, 1983; p. 22.
- Association of Official Analytical Chemistry (AOAC). Official Methods of Analysis, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000; p. 1928. [Google Scholar]
- do Brasil, F.; Brasília, D.F.; Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Regras Para Análise de Sementes; MAPA/ACS: Brasília, Brazil, 2009; p. 399.
- Silva, F.A.S.; Azevedo, C.A.V. The Assistat Software Version 7.7 and its use in the analysis of experimental data. Afr. J. Agric. Res. 2016, 11, 3733–3740. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Principal component analysis and redundancy analysis. In Analysing Ecological Data; Springer: New York, NY, USA, 2007. [Google Scholar]
- Balzarini, M.; Di Rienzo, J.; Tablada, M.; Gonzales, L.; Bruno, C.; Córdoba, M.; Robledo, W.; Casanoves, F. Estadística y Biometría, 2nd ed.; Universidad Nacional de Cordoba: Córdoba, Argentina, 2017. [Google Scholar]
- Kluge, R.; Jomori, M.L.L.; Sasaki, F.F.C.; Berno, N.D.; Gimenes, L.C. Desverdecimento e armazenamento refrigerado de tangor ‘Murcott’ em função de concentração e tempo de exposição ao etileno. Semin. Ciênc. Agrár. 2014, 35, 825–834. [Google Scholar] [CrossRef][Green Version]
- Sartori, I.A.; Koller, O.C.; Schwarz, S.F.; Bender, R.J.; Schäfer, G. Maturação de frutos de seis cultivares de laranjas-doces na depressão central do Rio Grande do Sul. Rev. Bras. Frutic. 2002, 24, 364–369. [Google Scholar] [CrossRef]
- Chitarra, M.I.F.; Chitarra, A.B. Pós-Colheita de Frutas e Hortaliças: Fisiologia e Manuseio, 2nd ed.; UFLA: Lavras, Brazil, 2005; p. 786. [Google Scholar]
- Agostini, J.S.; Scalon, S.Q.; Lescan, C.H.; Silva, K.E.; Garcete, G.J. Nota Científica: Conservação pós-colheita de Laranjas Champagne (Citrus reticulata × Citrus sinensis). Braz. J. Food Technol. 2014, 17, 177–184. [Google Scholar] [CrossRef]
- Koblitz, M.G.B. Matérias-Primas Alimentícias: Composição e Controle de Qualidade; Guanabara Koogan: Rio de Janeiro, Brazil, 2014. [Google Scholar]
- Almeida, M.B. Determinação do Estádio Ótimo de Maturação a Colheita do Limão “Siciliano”, Produzidos no Estado do Ceará. Master’s Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2014. [Google Scholar]
- Adams, C.R.; Klein, C. Uso de Biofilmes na Conservação Pós-Colheita de Lima-da-Pérsia (Citrus Limettioides Tanaka); Unoesc Ciência—ACET: Santa Catarina, Mexico, 2018; Volume 9, pp. 85–92. [Google Scholar]
- Carvalho, J.A.; Pinho, E.V.R.; Oliveira, J.A.; Guimarães, R.M.; Bonome, L.T. Qualidade de sementes de limão Cravo (Citrus limonia Osbeck) durante o armazenamento. Rev. Bras. Sementes 2002, 24, 286–298. [Google Scholar] [CrossRef][Green Version]
- Struving, T.B.; Machado, D.L.M.; Santos, D.; Siqueira, D.L.; Lucena, C.C.; Matarazzo, P.H.M. Qualidade fisiológica de sementes de citros durante o armazenamento em ambiente refrigerado. Ciênc. Rural. 2013, 43, 1777–1782. [Google Scholar] [CrossRef]
- Silva, T.T.A.; Guimarães, R.M.; von Pinho, E.V.R.; Abreu, L.A.S. Storage of ‘Swingle’ citrumelo seeds in different maturation stages subjected to fungicide treatment. Rev. Bras. Sementes 2011, 33, 768–776. [Google Scholar] [CrossRef]
- Ricci, N.; Pacheco, A.C.; Conde, A.S.; Custódio, C.C. Qualidade de sementes de pimenta jalapenho em função da maturação e tempo de permanência nos frutos. Pesqui. Agropecu. Trop. 2013, 43, 123–129. [Google Scholar] [CrossRef][Green Version]
- Carvalho, N.M.; Nakagawa, J. Sementes: Ciência, Tecnologia e Produção, 4th ed.; Funep: Jaboticabal, Brazil, 2000. [Google Scholar]
- Pinheiro, D.T.; Oliveira, R.M.; Silveira, A.S.; Léon, M.J.Z.; Brum, L.B.T.L.; Dias, D.F. Antioxidant enzyme activity and physiological potential of Capsicum baccatum var. baccatum seeds as a function of post-harvest storage of fruit. J. Seed Sci. 2020, 42, 1–13. [Google Scholar] [CrossRef]
- Morelli, M.; Azevedo, F.A.; Conceição, P.M.; Souza, A.J.B. Maturation and physiological quality of IAC-863 Rangpur lime seeds. Comun. Sci. 2019, 10, 454–460. [Google Scholar] [CrossRef]
- Oliveira, R.P.; Scivittaro, W.B. Formação do porta-enxerto Trifoliata: Época de semeadura e tegumento na emergência de plântulas. Ciênc. Rural. 2007, 37, 281–283. [Google Scholar] [CrossRef]
- Cardoso, V.J.M. Germinação. In Fisiologia Vegetal, 2nd ed.; Kerbauy, G.B., Ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2012; pp. 384–408. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Carvalho, S.A.; Silva, L.F.C. Monitoring the viability of citrus rootstocks seeds stored under refrigeration. Rev. Bras. Frutic. 2013, 35, 238–245. [Google Scholar] [CrossRef]
- Saipari, E.; Goswami, A.M.; Dadlani, M. Effect of seed drying on germination behavior in citrus. Sci. Hortic. 1998, 73, 185–190. [Google Scholar] [CrossRef]
- Roberts, E.H. Predicting the storage life of seeds. Proceedings 1973, 1, 499–514. [Google Scholar]
- Usberti, R.; Roberts, E.H.; Ellis, R.H. Prediction of cottonseed longevity. Pesq. Agropec. Bras. 2006, 41, 1435–1441. [Google Scholar] [CrossRef]
- Reuther, W. Climate and citrus behavior. In The Citrus Industry; University of California Press: Berkeley, CA, USA, 1973; Volume 3, pp. 280–337. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelli, M.; Azevedo, F.A.d.; de Souza, A.J.B.; Martinelli, R.; da Conceição, P.M. Physiological Quality of Swingle Citrumelo Seed after Refrigerated Storage of Fruits and Seeds. Agriculture 2021, 11, 1243. https://doi.org/10.3390/agriculture11121243
Morelli M, Azevedo FAd, de Souza AJB, Martinelli R, da Conceição PM. Physiological Quality of Swingle Citrumelo Seed after Refrigerated Storage of Fruits and Seeds. Agriculture. 2021; 11(12):1243. https://doi.org/10.3390/agriculture11121243
Chicago/Turabian StyleMorelli, Marilia, Fernando Alves de Azevedo, Ana Julia Borim de Souza, Rodrigo Martinelli, and Patrícia Marluci da Conceição. 2021. "Physiological Quality of Swingle Citrumelo Seed after Refrigerated Storage of Fruits and Seeds" Agriculture 11, no. 12: 1243. https://doi.org/10.3390/agriculture11121243
APA StyleMorelli, M., Azevedo, F. A. d., de Souza, A. J. B., Martinelli, R., & da Conceição, P. M. (2021). Physiological Quality of Swingle Citrumelo Seed after Refrigerated Storage of Fruits and Seeds. Agriculture, 11(12), 1243. https://doi.org/10.3390/agriculture11121243





