1H NMR Reveals Dynamic Changes of Primary Metabolites in Purple Passion Fruit (Passiflora edulis Sims) Juice during Maturation and Ripening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents and Chemicals
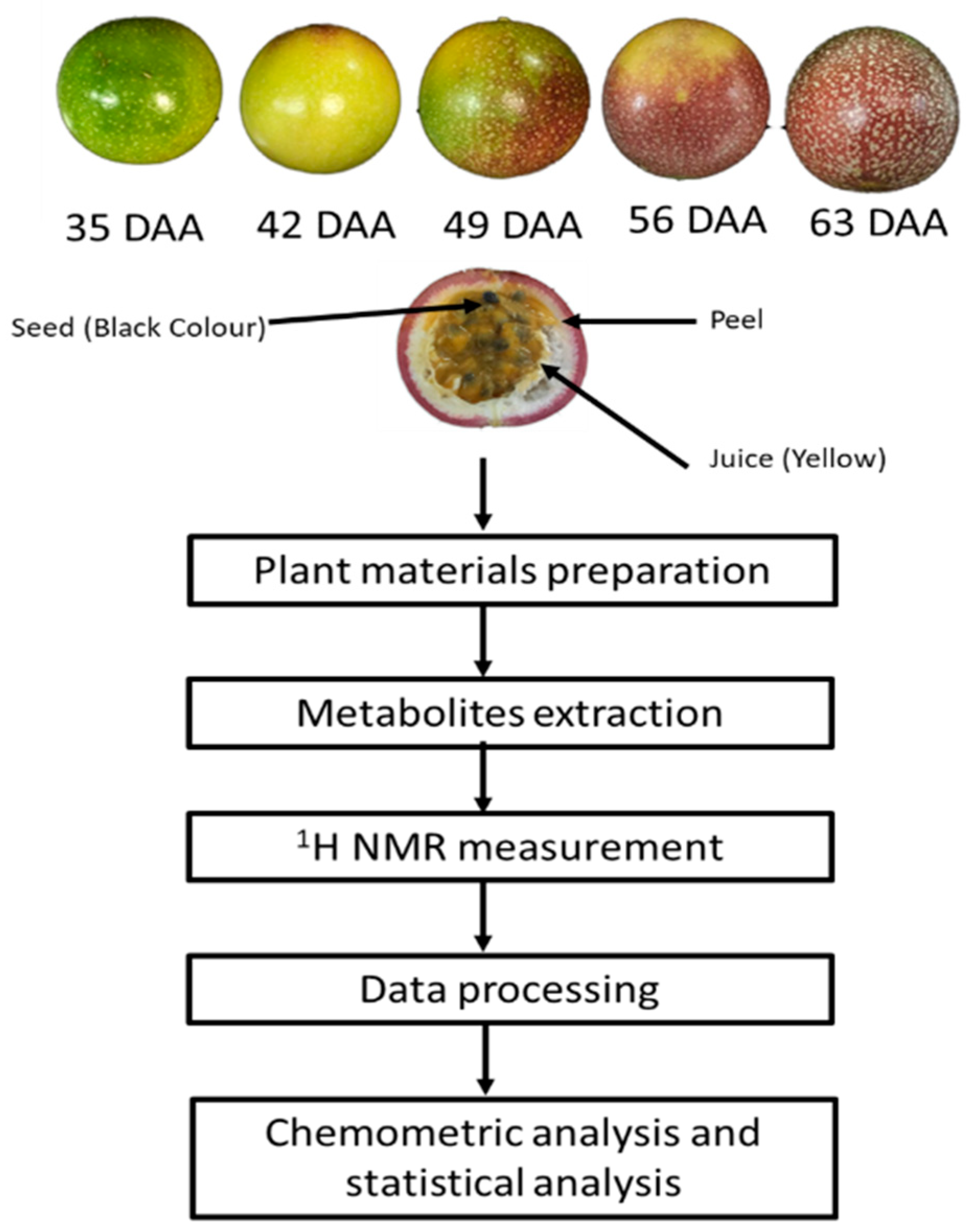
2.2. Fruit Harvesting Stage and Experimental Design
2.3. Preparation of 1H NMR Extraction
2.4. 1H NMR Spectroscopy Measurement
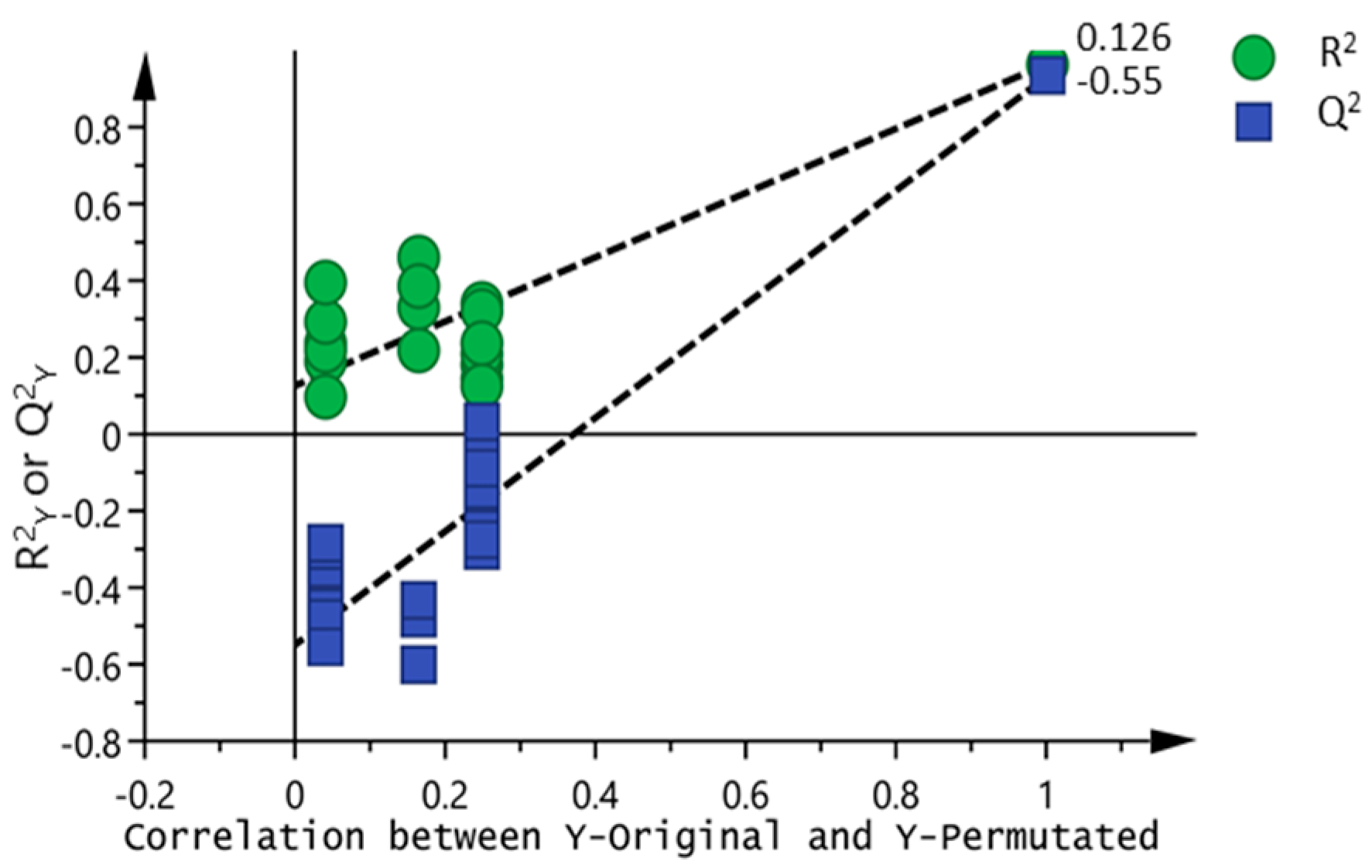
2.5. 1H NMR Spectra Multivariate Statistical Analysis
2.6. Construction of Metabolic Pathway
3. Results
3.1. Metabolic Assignments for Passion Fruit Juice
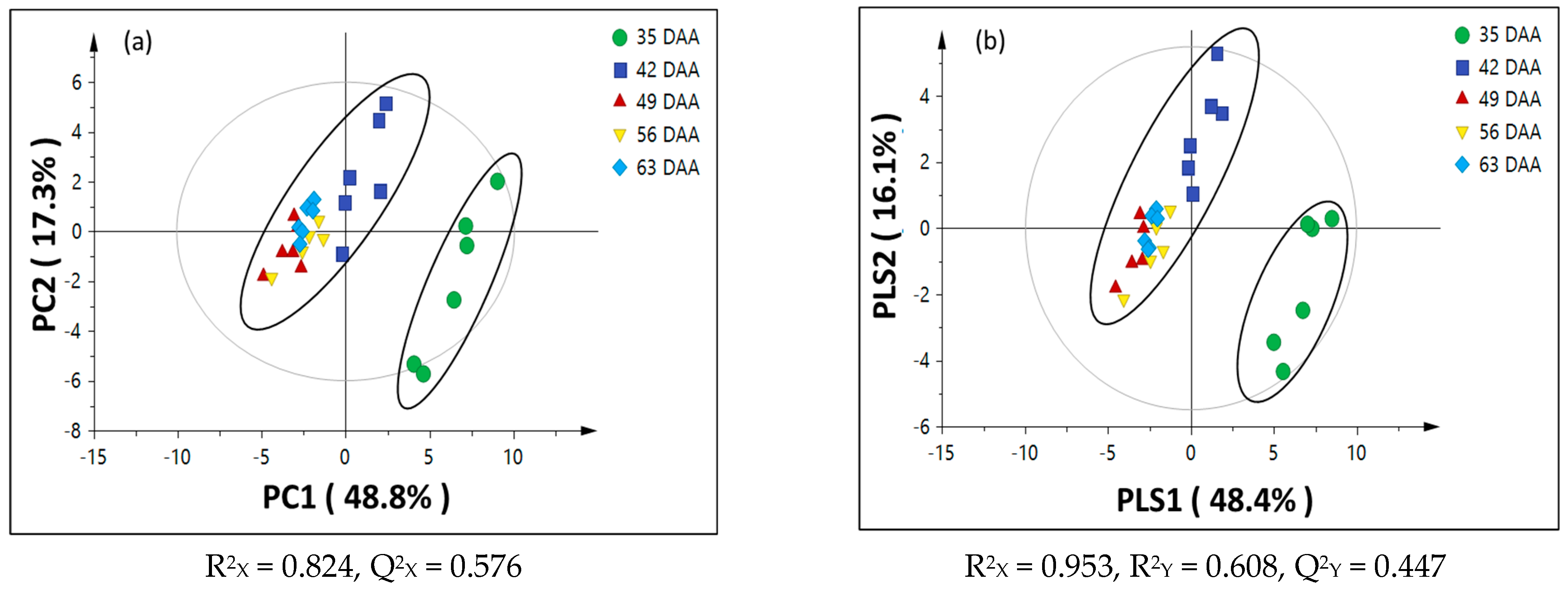
3.2. Chemometric Analysis of Passion Fruit Metabolite Data
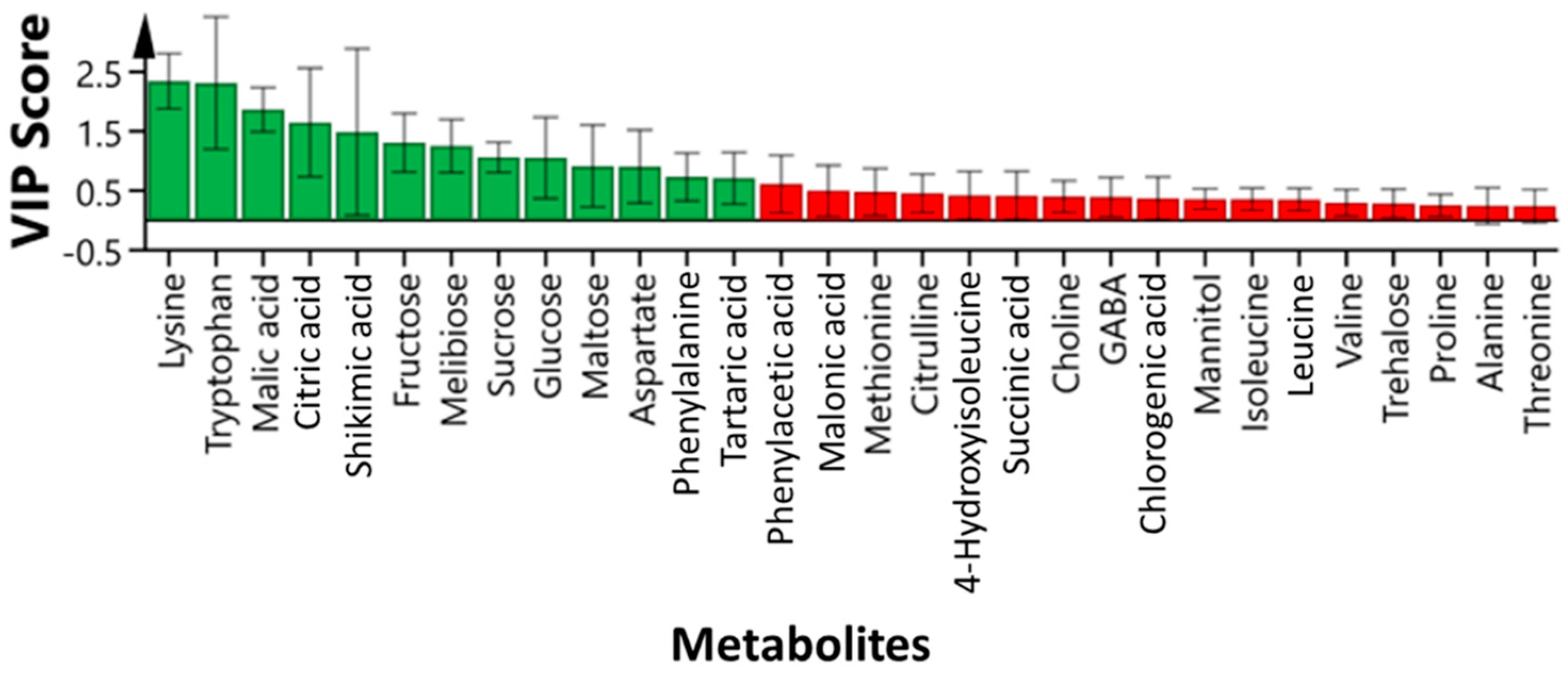
3.3. Identification of Highly Influenced Primary Metabolites in Passion Fruit Using 1H NMR
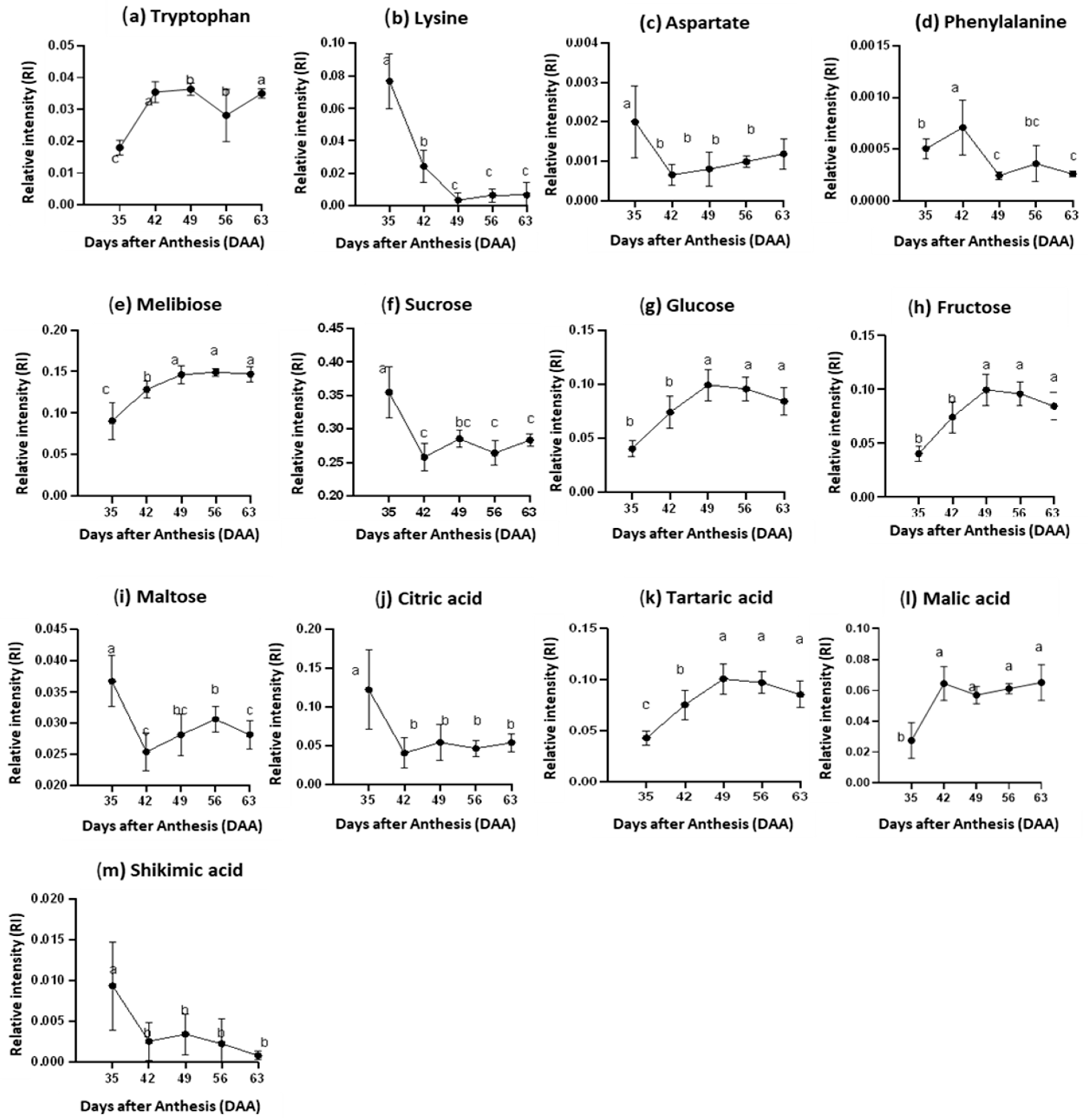
3.4. Changes of Highly Influential Metabolites in Juice
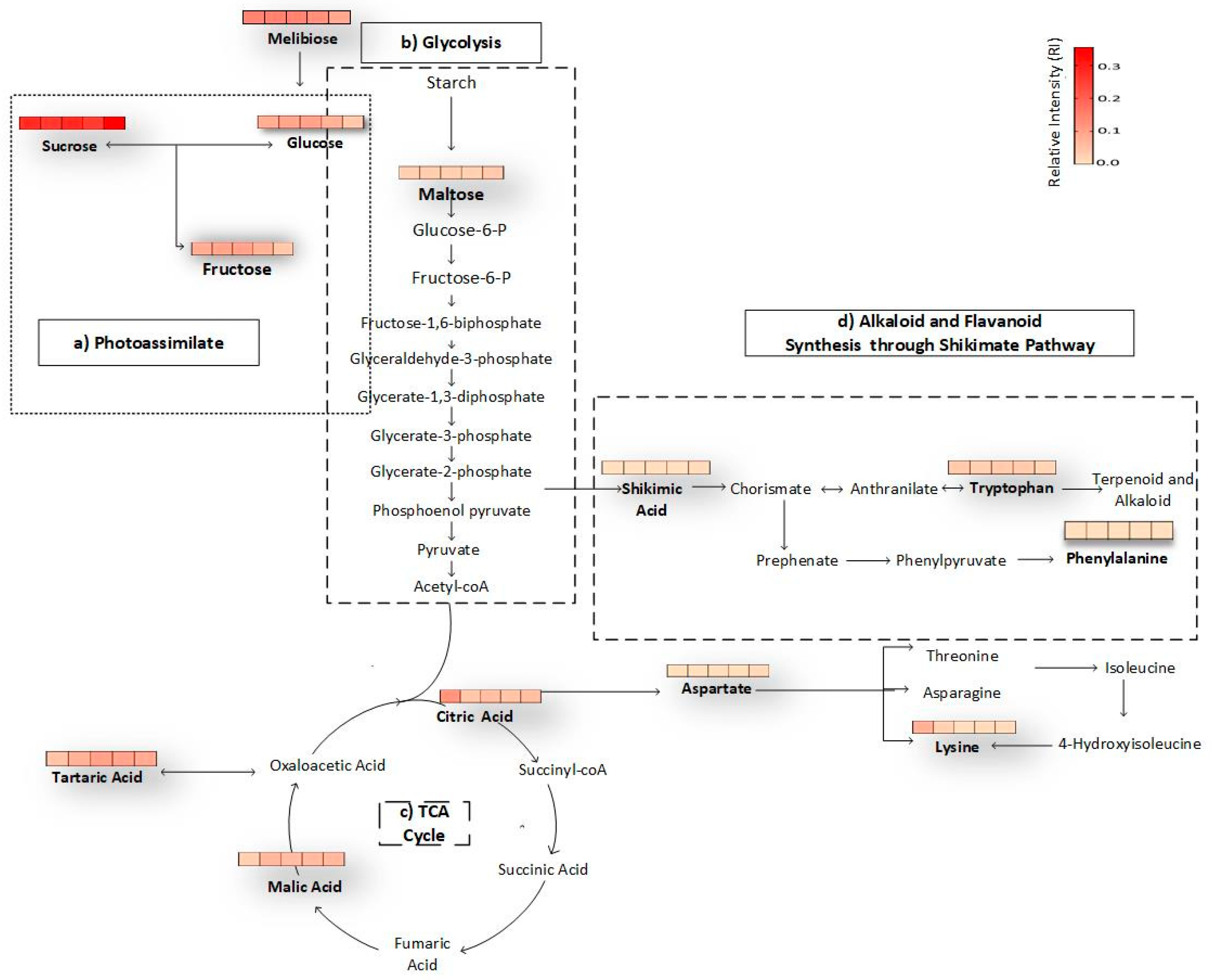
3.5. Biochemical Pathways of Passion Fruit during Maturation and Ripening Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Zhao, Z.; Fang, J.; Wang, M. Passiflora edulis: An insight into current research on phytochemistry and pharmacology. Front. Pharmacol. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Syazwani, S. Physicochemical quality, antioxidant compounds and activity of MD-2 pineapple fruit at five ripening stages. Int. Food Res. J. 2016, 23, 549–555. [Google Scholar]
- Shahidah, M.N.; Ding, P. Trends and advances in edible biopolymer coating for tropical fruit: A Review. Food Res. Int. 2020, 134, 109208. [Google Scholar] [CrossRef]
- Shahidah, M.N.; Siti, N.F.M.H.; Ding, P. Effects of temperature and storage duration on keeping quality of Malaysian grown passion fruit (Passiflora edulis Sims f. flavicarpa Deg.). Acta Hortic. 2021, 1327, 557–562. [Google Scholar] [CrossRef]
- Mediani, A.; Abas, F.; Maulidiani, M.; Khatib, A.; Tan, C.; Ismail, I.S.; Shaari, K.; Ismail, A. Characterization of metabolite profile in Phyllanthus niruri and correlation with bioactivity elucidated by nuclear magnetic resonance-based metabolomics. Molecules 2017, 22, 902. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, L.; Li, Y.; Li, K.; Liu, X.; Zhou, J.; Yang, C.; Liu, X.; Fang, C.; Luo, J. Integrative metabolomic and transcriptomic analyses reveal metabolic changes and its molecular basis in rice mutants of the strigolactone pathway. Metabolites 2020, 10, 425. [Google Scholar] [CrossRef]
- Muriel, Q.; Angosto, T.; Yuste-Lisbona, F.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Hanganu, A.; Chira, N.A. When detection of dairy food fraud fails: An alternative approach through proton nuclear magnetic resonance spectroscopy. J. Dairy Sci. 2021, 104, 8454–8466. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Rosas-Bautista, A.; Rico-Arzate, E.; Cruz-Narvaez, Y.; Zepeda-Vallejo, L.G.; Lalaleo, L.; Hi-dalgo-Martínez, D.; Becerra-Martínez, E. Study of nutritional quality of pomegranate (Punica Granatum L.) juice using 1H NMR-based metabolomic approach: A comparison between conventionally and organically grown fruits. LWT 2020, 134, 110222. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Kadum, H.; Zarei, M.; Meor Hussin, A.S. Effects of metabolite changes during lacto-fermentation on the biological activity and consumer acceptability for dragon fruit juice. LWT 2020, 121, 108992. [Google Scholar] [CrossRef]
- Zainal, B.; Ding, P.; Ismail, I.S.; Saari, N. 1H NMR metabolomics profiling unveils the compositional changes of hydro-cooled rockmelon (Cucumis melo L. reticulatus cv glamour) during storage related to in vitro antioxidant activity. Sci. Hortic. 2019, 246, 618–633. [Google Scholar] [CrossRef]
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Giusti, A.M.; Mannina, L. New hybrid tomato cultivars: An NMR-based chemical characterization. Appl. Sci. 2020, 10, 1887. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Park, S.; Hyun, S.; Yang, S.; Lee, J.; Auh, J.; Kim, J.; Cho, S.; Marriott, P.; Choi, H. Biochemical monitoring of black raspberry (Rubus coreanus Miquel) fruits according to maturation stage by 1H NMR using multiple solvent systems. Food Res. Int. 2011, 44, 1977–1987. [Google Scholar] [CrossRef]
- Shahidah, M.N.; Phebe, D.; Choon, S. Role of assisted pollination in fruit shape of purple passion fruit (Passiflora edulis Sims.). Int. J. Agric. For. Plant. 2018, 8, 128–131. [Google Scholar]
- Shahidah, M.; Ding, P. Size and area of Passiflora edulis Sims. mesocarp cells during fruit growth and development. Trans. Malays. Soc. Plant Physiol. 2021, 28, 58–62. [Google Scholar]
- Zolkeflee, N.K.; Isamail, N.; Maulidiani, M.; Abdul Hamid, N.; Ramli, N.; Azlan, A.; Abas, F. Metabolite variations and antioxidant activity of Muntingia calabura leaves in response to different drying methods and ethanol ratios elucidated by NMR-based metabolomics. Phytochem. Anal. 2020, 32, 69–83. [Google Scholar] [CrossRef]
- Kiralj, R.; Ferreira, M.M. Basic validation procedures for regression models in QSAR and QSPR studies: Theory and application. J. Braz. Chem. Soc. 2009, 20, 770–787. [Google Scholar] [CrossRef] [Green Version]
- Min, H.; Mi, K.; Ho, C.; Hwa, B.; Lee, J.; Kong, G.; Jin, S.; Kim, S.; Wook, S.; Chul, B. Mass spectrometry based metabolomic approaches in urinary biomarker study of women’s cancers. Clin. Chim. Acta 2009, 400, 63–69. [Google Scholar] [CrossRef]
- Oh, J.; Yoon, D.; Han, J.; Choi, H.; Sung, G. 1H NMR based metabolite profiling for optimizing the ethanol extraction of Wolfiporia cocos. Saudi J. Biol. Sci. 2018, 25, 1128–1134. [Google Scholar] [CrossRef]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Trygg, J.; Wikström, C.; Wold, S. Multivariate analysis. In Multi-and Megavariate Data Analysis: Part II: Advanced Applications and Method Extensions; Umetric Academy: Malmö, Sweden, 2006; pp. 24–56. [Google Scholar]
- Villa-Ruano, N.; Velásquez-Valle, R.; Zepeda-Vallejo, L.; Pérez-Hernández, N.; Velázquez-Ponce, M.; Arcos-Adame, V.; Becerra-Martínez, E. 1H NMR-based metabolomic profiling for identification of metabolites in Capsicum annuum cv. mirasol infected by beet mild curly top virus (BMCTV). Food Res. Int. 2018, 106, 870–877. [Google Scholar] [CrossRef]
- Aizat, W.; Dias, D.; Stangoulis, J.C.; Able, J.; Roessner, U.; Able, A. Metabolomics of capsicum ripening reveals modification of the ethylene related-pathway and carbon metabolism. Postharvest Biol. Technol. 2014, 89, 19–31. [Google Scholar] [CrossRef]
- Li, Y.; He, Q.; Du, S.; Guo, S.; Geng, Z.; Deng, Z. Study of methanol extracts from different parts of Peganum harmala L. using 1H-NMR plant metabolomics. J. Anal. Methods Chem. 2018, 2018, 6532789. [Google Scholar] [CrossRef] [Green Version]
- Awin, T.; Mediani, A.; Maulidiani; Leong, S.; Muhd Faudzi, S.M.; Shaari, K.; Abas, F. Phytochemical and bioactivity alterations of Curcuma species harvested at different growth stages by NMR-based metabolomics. J. Food Compos. Anal. 2019, 77, 66–76. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, F.; Li, Z.; Qin, X.; Zhang, L. Comparison of fruits of Forsythia suspensa at two different maturation stages by NMR-based metabolomics. Molecules 2015, 20, 10065–10081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobolev, A.; Brosio, E.; Gianferri, R.; Segre, A. Metabolic profile of lettuce leaves by high-field NMR spectra. Magn. Reson. Chem. 2005, 43, 625–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Huang, J.; Chen, F. NMR studies of a series of shikimic acid derivatives. J. Chin. Chem. Soc. 2007, 54, 1313–1320. [Google Scholar] [CrossRef]
- Mendoza, D.; Pablo, J.; Cuaspud, O.; Esturau-Escofet, N.; Hernández-espino, C.; Rodríguez, E.; Miguel, D.; Arias, M. 1H-NMR-based metabolomic of plant cell suspension cultures of Thevetia peruviana treated with salicylic acid and methyl jasmonate. Ind. Crop. Prod. 2019, 135, 217–229. [Google Scholar] [CrossRef]
- Fisher, G.; Almanza-Merchán, J.; Ramírez, F. Source-sink relationships in fruit species: A review. Rev. Colomb. Cienc. Hortícolas 2012, 6, 238–253. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, P.; Ma, F.; Dandekar, A.; Cheng, L. Sugar metabolism and accumulation in the fruit of transgenic apple trees with decreased sorbitol synthesis. Hortic. Res. 2018, 5, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Lo, S.; Ho, T.H. Source–sink communication: Regulated by hormone, nutrient, and stress. Trends Plant Sci. 2015, 20, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Moing, A.; Renaud, C.; Gaudillère, M.; Raymond, P.; Roudeillac, P.; Denoyes-Rothan, B. Biochemical changes during fruit development of four strawberry cultivars. J. Am. Soc. Hortic. Sci. 2001, 126, 394–403. [Google Scholar] [CrossRef]
- Falchi, R.; Bonghi, C.; Drincovich, M.; Famiani, F.; Lara, M.; Walker, R.; Vizzotto, G. Sugar Metabolism in stone fruit: Source-sink relationships and environmental and agronomical effects. Front. Plant Sci. 2020, 11, 573982. [Google Scholar] [CrossRef]
- Aluko, O.; Li, C.; Wang, Q.; Liu, H. Sucrose utilization for improved crop yields: A review article. Int. J. Mol. Sci. 2021, 22, 4704. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Liu, H.; Liu, J.; Jiao, Z. Profiles of sugar and organic acid of fruit juices: A comparative study and implication for authentication. J. Food Qual. 2020, 2020, 7236534. [Google Scholar] [CrossRef]
- Li, M.; Feng, F.; Cheng, L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE 2012, 7, e33055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takehiko, S.; Ryohei, N.; Vladimir, S.; Avi, S.; Eduardo, B. Vacuolar citrate/H+ symporter of citrus juice cells. Planta 2014, 224, 472–480. [Google Scholar] [CrossRef]
- Sadka, A.; Shlizerman, L.; Kamara, I.; Blumwald, E. Primary metabolism in citrus fruit as affected by its unique structure. Front. Plant Sci. 2019, 10, 1167. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, O.; Aizat, W.; Baharum, S.; Azizan, K.; Noor, N. Metabolomics analysis of developing Garcinia mangostana seed reveals modulated levels of sugars, organic acids and phenylpropanoid compounds. Sci. Hortic. 2018, 233, 323–330. [Google Scholar] [CrossRef]
- Ramaiya, S.D.; Bujang, J.S.; Zakaria, M.H.; King, W.S.; Shaffiq Sahrir, M.A. Sugars, ascorbic acid, total phenolic content and total antioxidant activity in passion fruit (Passiflora) cultivars. J. Sci. Food Agric. 2013, 93, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, P. Flavour Characterization of Selected Acidulants in Model and Food System. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 1994. [Google Scholar]
- Ikegaya, A.; Toyoizumi, T.; Ohba, S.; Nakajima, T.; Kawata, T.; Ito, S.; Arai, E. Effects of distribution of sugars and organic acids on the taste of strawberries. Food Sci. Nutr. 2019, 7, 2419–2426. [Google Scholar] [CrossRef]
- Pareek, S. Nutritional and biochemical composition of lychee (Litchi chinensis Sonn.) cultivars. In Nutritional Composition of Fruit Cultivars; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 395–418. [Google Scholar]
- Bacher, A.; Eisenreich, W. Metabolic studies using the retrobiosynthesis concept–Theory, Technology, and Examples. In Comprehensive Natural Products II: Chemistry and Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 675–694. [Google Scholar]
- Erb, M.; Kliebenstein, D. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Radwanski, E.; Last, R. Tryptophan biosynthesis and metabolism: Biochemical and molecular genetics. Plant Cell 1995, 7, 921–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Chen, C.; Fu, X. Study on the bioaccessibility of phenolic compounds and bioactivities of passion fruit juices from different regions in vitro digestion. J. Food Process. Preserv. 2021, 45, e15056. [Google Scholar] [CrossRef]
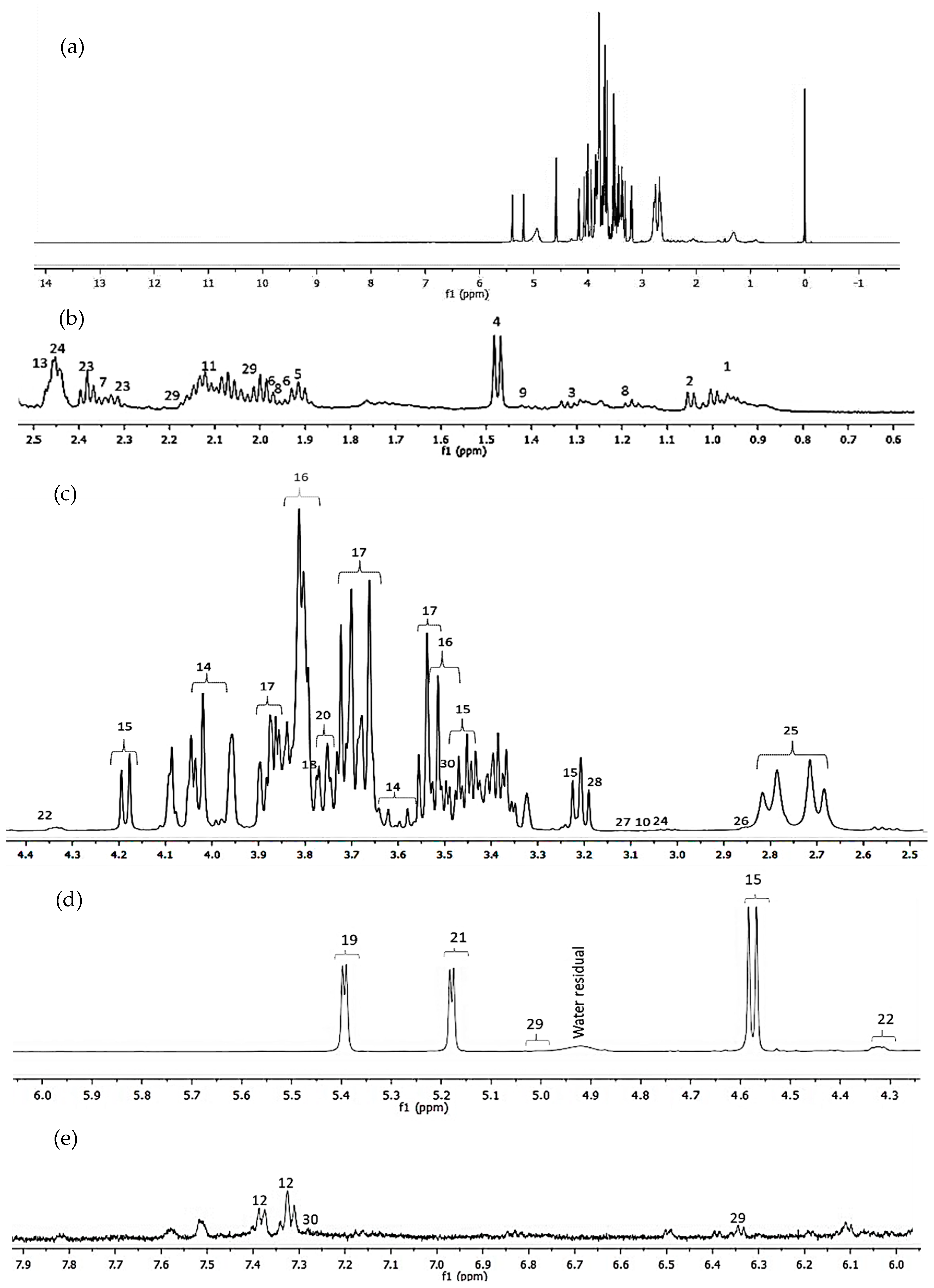

| Assigned No. | Metabolite Compound | Chemical Shift (ppm) | References |
|---|---|---|---|
| Amino Acid | |||
| 1 | Leucine | 0.94 (t, J = 6.5 Hz) | [13] |
| 2 | Valine | 1.05 (d, J = 7.0 Hz), 0.99 (d, J = 7.0 Hz) | [16] |
| 3 | Threonine | 1.33 (d, J = 6.6 Hz) | [19] |
| 4 | Alanine | 1.48 (d, J = 7.3 Hz) | [16] |
| 5 | 4-Aminobutyrate (GABA) | 1.90 (m) | [19] |
| 6 | Proline | 1.98 (m), 2.02 (m) | [19] |
| 7 | 4-Hydroxyisoleucine | 2.16 (m) | [23] |
| 8 | Citrulline | 1.15 (m) | [13] |
| 9 | Isoleucine | 0.85–0.96 (m), 1.42–1.46 (m) | [19] |
| 10 | Lysine | 3.03 (t, J = 7.5 Hz) | [23] |
| 11 | Methionine | 2.12 (s) | [24] |
| 12 | Phenylalanine | 7.32 d (J = 7.5 Hz), 7.36–7.38 (m) | [25] |
| 13 | Aspartate | 2.57 dd (J = 7.4, 18.9 Hz) | [19] |
| 14 | Tryptophan | 3.57 (dd, J = 13.4, 5.0 Hz) | [11] |
| Sugar | |||
| 15 | Glucose | 3.21 (dd, J = 10.5, 6.3), 3.46 (m), 4.59 (d, J = 7.9 Hz) | [11] |
| 16 | Sucrose | 3.59 (m), 3.78 (t, 9.5), 3.87 (dd, J = 8.1, 3.9 Hz), 4.17 (d, J = 8.7 Hz) | [11] |
| 17 | Fructose | 3.54–3.59 (m), 3.65–3.70 (m), 3.73–3.82 (m), 4.01 (dd, J = 12.7, 1.3 Hz) | [11] |
| 18 | Mannitol | 3.89 (m) | [13] |
| 19 | Maltose | 5.39 (d, J = 3.8 Hz) | [13] |
| 20 | Melibiose | 3.76 (d, J = 3.3 Hz), 3.82 (d, J = 5.0 Hz) | [6] |
| 21 | Trehalose | 5.18 (d, J = 3.8 Hz) | [19] |
| Organic Acid | |||
| 22 | Tartaric acid | 4.34 (s) | [26] |
| 23 | Succinic acid | 2.38 (s), 2.43 (s) | [19] |
| 24 | Malic acid | 2.50 (dd, J = 15.5, 8.6 Hz), 3.0 (m) | [13] |
| 25 | Citric acid | 2.72 (d, J = 15.1 Hz) | [13] |
| 26 | Shikimic acid | 2.80 (d, J = 15.5 Hz) | [27] |
| 27 | Malonic acid | 3.10 (s) | [19] |
| Secondary Metabolites | |||
| 28 | Choline | 3.18 (s) | [16] |
| 29 | Chlorogenic acid | 2.08 (m), 2.20 (m), 4.04 (m), 6.39 (d, J = 16.0 Hz) | [26] |
| 30 | Phenylacetic acid | 7.30 (m), 3.50 (s) | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md Nor, S.; Ding, P.; Abas, F.; Mediani, A. 1H NMR Reveals Dynamic Changes of Primary Metabolites in Purple Passion Fruit (Passiflora edulis Sims) Juice during Maturation and Ripening. Agriculture 2022, 12, 156. https://doi.org/10.3390/agriculture12020156
Md Nor S, Ding P, Abas F, Mediani A. 1H NMR Reveals Dynamic Changes of Primary Metabolites in Purple Passion Fruit (Passiflora edulis Sims) Juice during Maturation and Ripening. Agriculture. 2022; 12(2):156. https://doi.org/10.3390/agriculture12020156
Chicago/Turabian StyleMd Nor, Shahidah, Phebe Ding, Faridah Abas, and Ahmed Mediani. 2022. "1H NMR Reveals Dynamic Changes of Primary Metabolites in Purple Passion Fruit (Passiflora edulis Sims) Juice during Maturation and Ripening" Agriculture 12, no. 2: 156. https://doi.org/10.3390/agriculture12020156
APA StyleMd Nor, S., Ding, P., Abas, F., & Mediani, A. (2022). 1H NMR Reveals Dynamic Changes of Primary Metabolites in Purple Passion Fruit (Passiflora edulis Sims) Juice during Maturation and Ripening. Agriculture, 12(2), 156. https://doi.org/10.3390/agriculture12020156






