Soil Moisture and Temperature Effects on Granule Dissolution and Urease Activity of Urea with and without Inhibitors—An Incubation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Handling
2.2. Tested Urea-Based Fertilizers
2.3. Urea Granule Dissolution
2.4. Urease Activity Assay
2.5. Statistical Analysis
3. Results
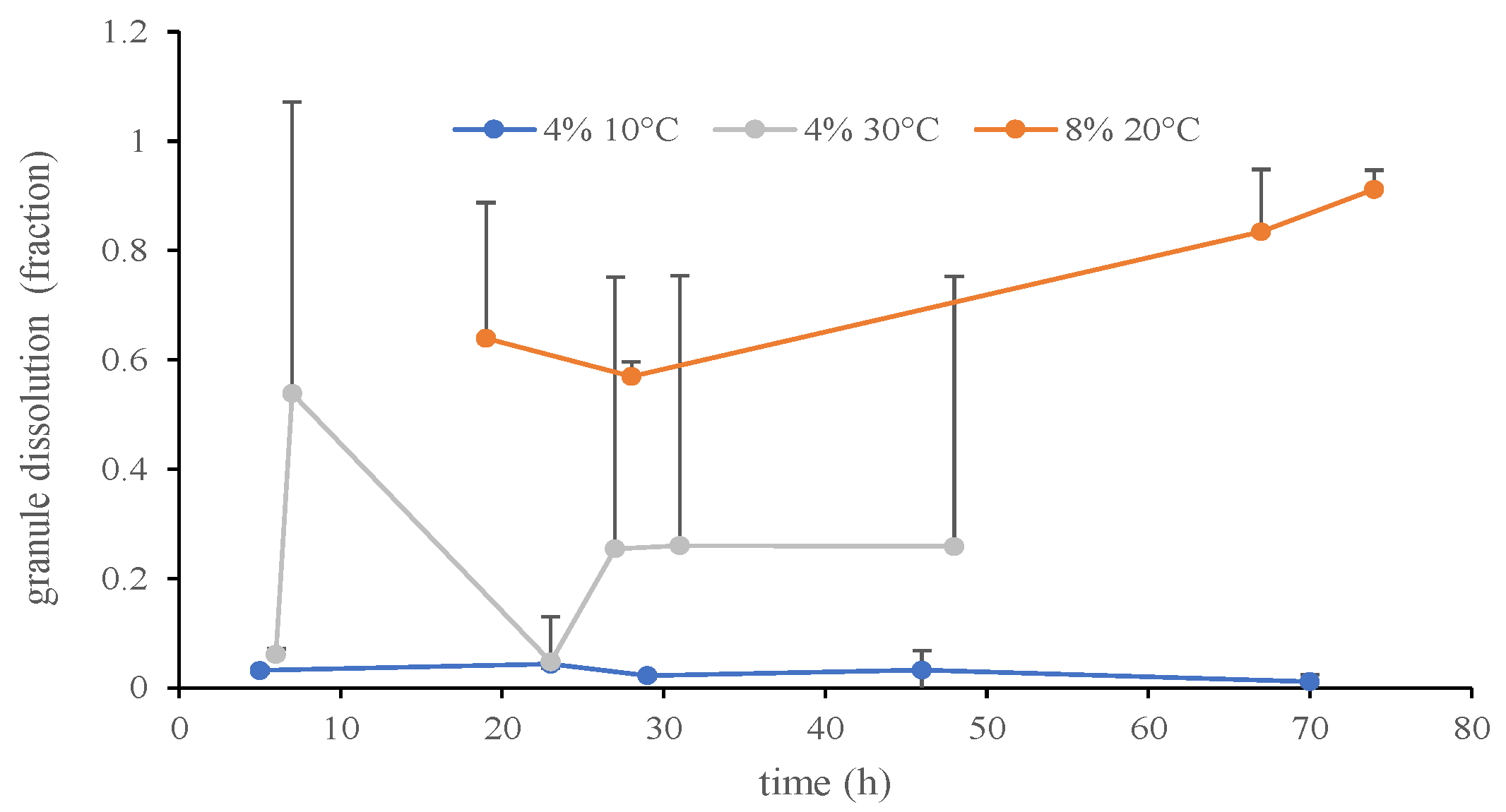
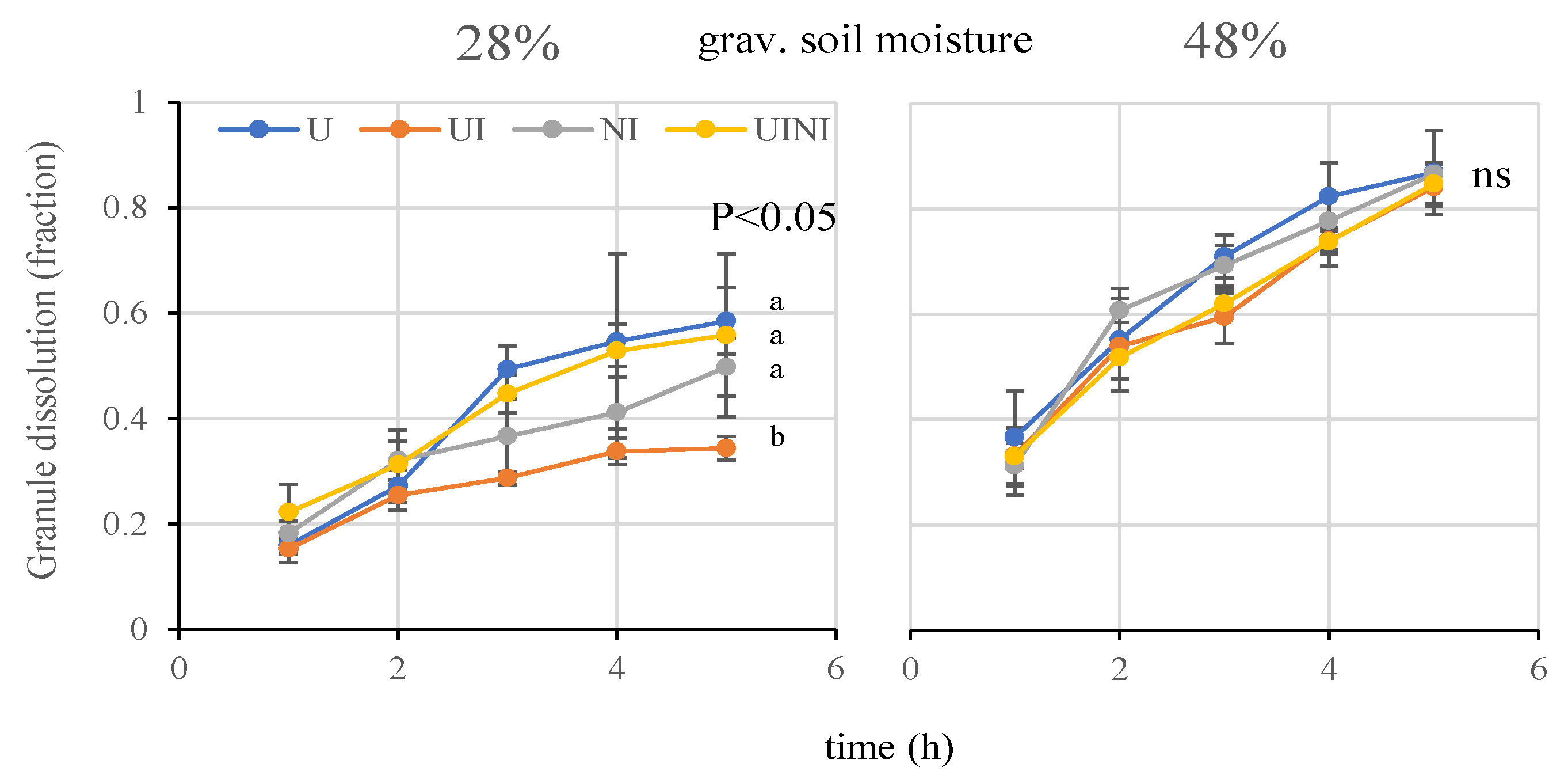
3.1. Urea Granule Dissolution
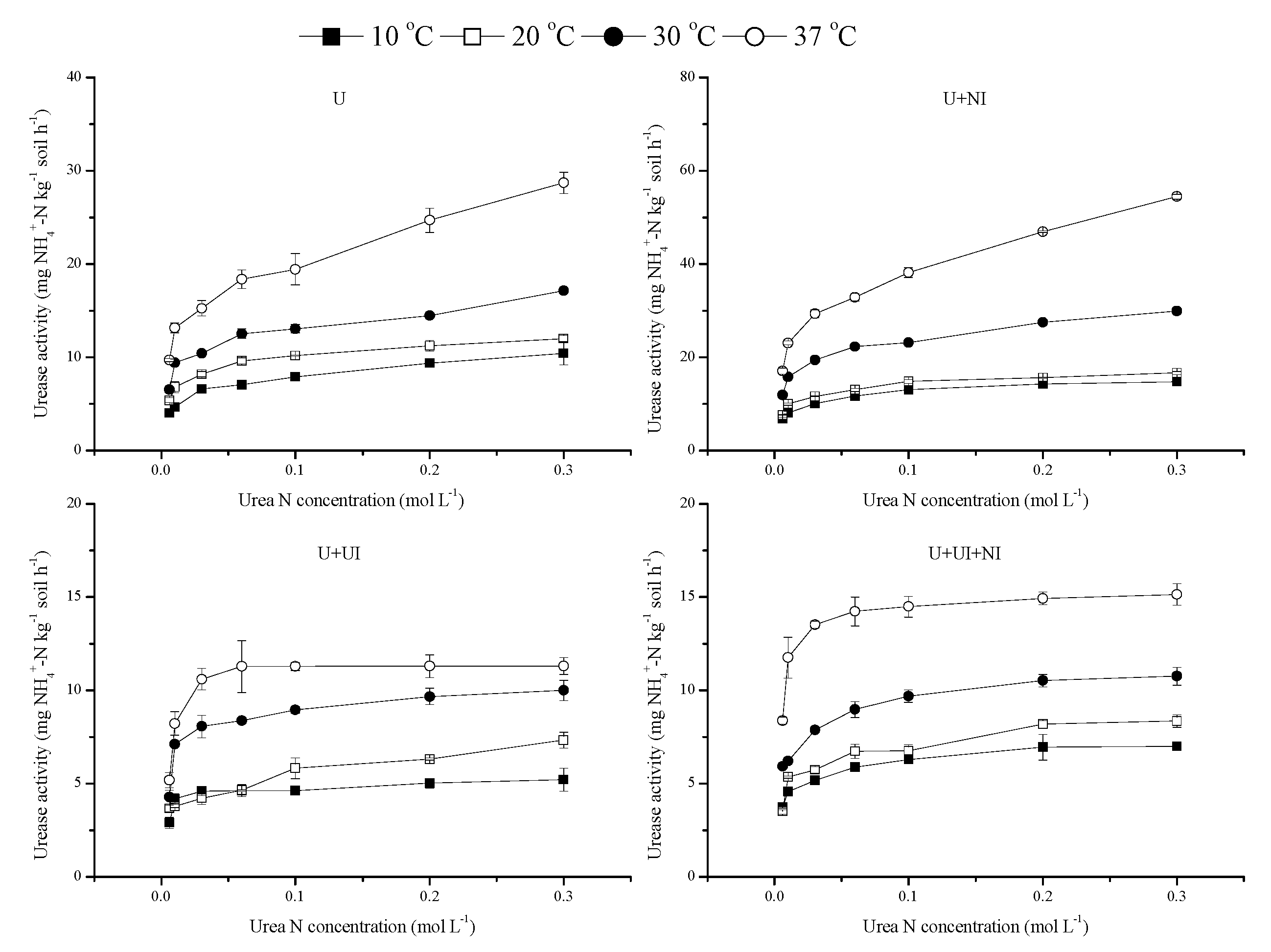
3.2. Urease Activity
4. Discussion
4.1. Urea Granule Dissolution
4.2. Urea Hydrolysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di, H.J.; Cameron, K.C. Nitrate Leaching Losses and Pasture Yields as Affected by Different Rates of Animal Urine Nitrogen Returns and Application of a Nitrification Inhibitor—A Lysimeter Study. Nutr. Cycl. Agroecosystems 2007, 79, 281–290. [Google Scholar] [CrossRef]
- Rawluk, C.D.L.; Grant, C.A.; Racz, G.J. Ammonia Volatilization from Soils Fertilized with Urea and Varying Rates of Urease Inhibitor NBPT. Can. J. Soil Sci. 2001, 81, 239–246. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; Misselbrook, T.; Camp, V.; Vallejo, A. Effect of Water Addition and the Urease Inhibitor NBPT on the Abatement of Ammonia Emission from Surface Applied Urea. Atmos. Environ. 2011, 45, 1517–1524. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.; Yin, J.; Chadwick, D.; Norse, D.; Lu, Y.; Liu, X.; Chen, X.; Zhang, F.; Powlson, D.; et al. Enhanced-Efficiency Fertilizers Are Not a Panacea for Resolving the Nitrogen Problem. Glob. Chang. Biol. 2018, 24, e511–e521. [Google Scholar] [CrossRef]
- Rachhpal-Singh; Nye, P.H. A Model of Ammonia Volatilization from Applied Urea. I. Development of the Model. J. Soil Sci. 1986, 37, 9–20. [Google Scholar] [CrossRef]
- Le Cadre, E.; Génermont, S.; Azam, F.; Recous, S. The SAHGA Model to Calculate the Spatial Ammoniacal Heterogeneity at the Soil Surface after Fertiliser Granule Application. Biol. Fertil. Soils 2004, 40, 178–180. [Google Scholar] [CrossRef]
- Cabrera, M.L.; Kissel, D.E.; Bock, B.R. Urea Hydrolysis in Soil: Effects of Urea Concentration and Soil pH. Soil Biol. Biochem. 1991, 23, 1121–1124. [Google Scholar] [CrossRef]
- Ransom, C.J.; Jolley, V.D.; Blair, T.A.; Sutton, L.E.; Hopkins, B.G. Nitrogen Release Rates from Slow- and Controlled-Release Fertilizers Influenced by Placement and Temperature. PLoS ONE 2020, 15, e0234544. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.L.; Kissel, D.E.; Craig, J.R.; Qafoku, N.P.; Vaio, N.; Rema, J.A.; Morris, L.A. Relative Humidity Controls Ammonia Loss from Urea Applied to Loblolly Pine. Soil Sci. Soc. Am. J. 2010, 74, 543–549. [Google Scholar] [CrossRef]
- Cartes, P.; Jara, A.A.; Demanet, R.; Mora, M. de la L. Urease Activity and Nitrogen Mineralization Kinetics as Affected by Temperature and Urea Input Rate in Southern Chilean Andisols. Rev. Cienc. Suelo Nutr. Veg. 2009, 9, 69–82. [Google Scholar] [CrossRef]
- Juan, Y.H.; Chen, Z.H.; Chen, L.J.; Wu, Z.J.; Wang, R.; Sun, W.T.; Zhang, Y.L. Kinetic and Thermodynamic Behaviors of Soil Urease as Affected by Urease Inhibitors. J. Soil Sci. Plant Nutr. 2010, 10, 1–11. [Google Scholar] [CrossRef]
- Moyo, C.C.; Kissel, D.E.; Cabrera, M.L. Temperature Effects on Soil Urease Activity. Soil Biol. Biochem. 1989, 21, 935–938. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of Water-Filled Pore Space on Carbon Dioxide and Nitrous Oxide Production in Tilled and Nontilled Soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-Term Assay of Soil Urease Activity Using Colorimetric Determination of Ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kissel, D.E.; Cabrera, M.L.; Craig, J.; Ariyama, J.; Vaio, N.; Rema, J. Predicting Urea Hydrolysis in a Loblolly Pine Forest Floor. Soil Sci. Soc. Am. J. 2014, 78, 2071–2077. [Google Scholar] [CrossRef]
- Amtul, Z.; Atta-ur-Rahman; Siddiqui, R.A.; Choudhary, M.I. Chemistry and Mechanism of Urease Inhibition. Curr. Med. Chem. 2002, 9, 1323–1348. [Google Scholar] [CrossRef]
- Longo, R.M.; de Melo, W.J. Urea Hydrolysis in Oxisols: Effects of Substrate Concentration, Temperature, pH, Incubation Time and Storage Conditions. Rev. Bras. Cienc. Solo 2005, 29, 651–657. [Google Scholar] [CrossRef]
- Xu, J.G.; Heeraman, D.A.; Wang, Y. Fertilizer and Temperature Effects on Urea Hydrolysis in Undisturbed Soil. Biol. Fertil. Soils 1993, 16, 63–65. [Google Scholar] [CrossRef]
- Tomar, J.S.; Mackenzie, A.F. Effects of Catechol and P-Benzoquinone on the Hydrolysis of Urea and Energy Barriers of Urease Activity in Soils. Can. J. Soil Sci. 1984, 64, 51–60. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; Misselbrook, T.H.; Arce, A.; Mingot, J.I.; Diez, J.A.; Vallejo, A. An Inhibitor of Urease Activity Effectively Reduces Ammonia Emissions from Soil Treated with Urea under Mediterranean Conditions. Agric. Ecosyst. Environ. 2008, 126, 243–249. [Google Scholar] [CrossRef]
- Wang, Z.P.; Van Cleemput, O.; Demeyer, P.; Baert, L. Effect of Urease Inhibitors on Urea Hydrolysis and Ammonia Volatilization. Biol. Fertil. Soils 1991, 11, 43–47. [Google Scholar] [CrossRef]
- Guthrie, T.F.; Bomke, A.A. Effects of Low Temperature and Nitrification Inhibitors on Urea Hydrolysis. Can. J. Soil Sci. 1981, 61, 529–532. [Google Scholar] [CrossRef]
- Yadvinder-Singh; Beauchamp, E.G. Nitrogen Transformations near Urea in Soil: Effects of Nitrification Inhibition, Nitrifier Activity and Liming. Fertil. Res. 1988, 18, 201–212. [Google Scholar] [CrossRef]
- Patra, D.D.; Kiran, U.; Chand, S.; Anwar, M. Use of Urea Coated with Natural Products to Inhibit Urea Hydrolysis and Nitrification in Soil. Biol. Fertil. Soils 2009, 45, 617–621. [Google Scholar] [CrossRef]
- Venkatesan, S.; Sudhahar, V.; Senthurpandian, V.K.; Murugesan, S. Urea Hydrolysis of Tea Soils as Influenced by Incubation Period, Soil PH, and Nitrification Inhibitor. Commun. Soil Sci. Plant Anal. 2007, 38, 2295–2307. [Google Scholar] [CrossRef]
- Ni, K.; Kage, H.; Pacholski, A. Effects of Novel Nitrification and Urease Inhibitors (DCD/TZ and 2-NPT) on N2O Emissions from Surface Applied Urea: An Incubation Study. Atmos. Environ. 2018, 175, 75–82. [Google Scholar] [CrossRef]
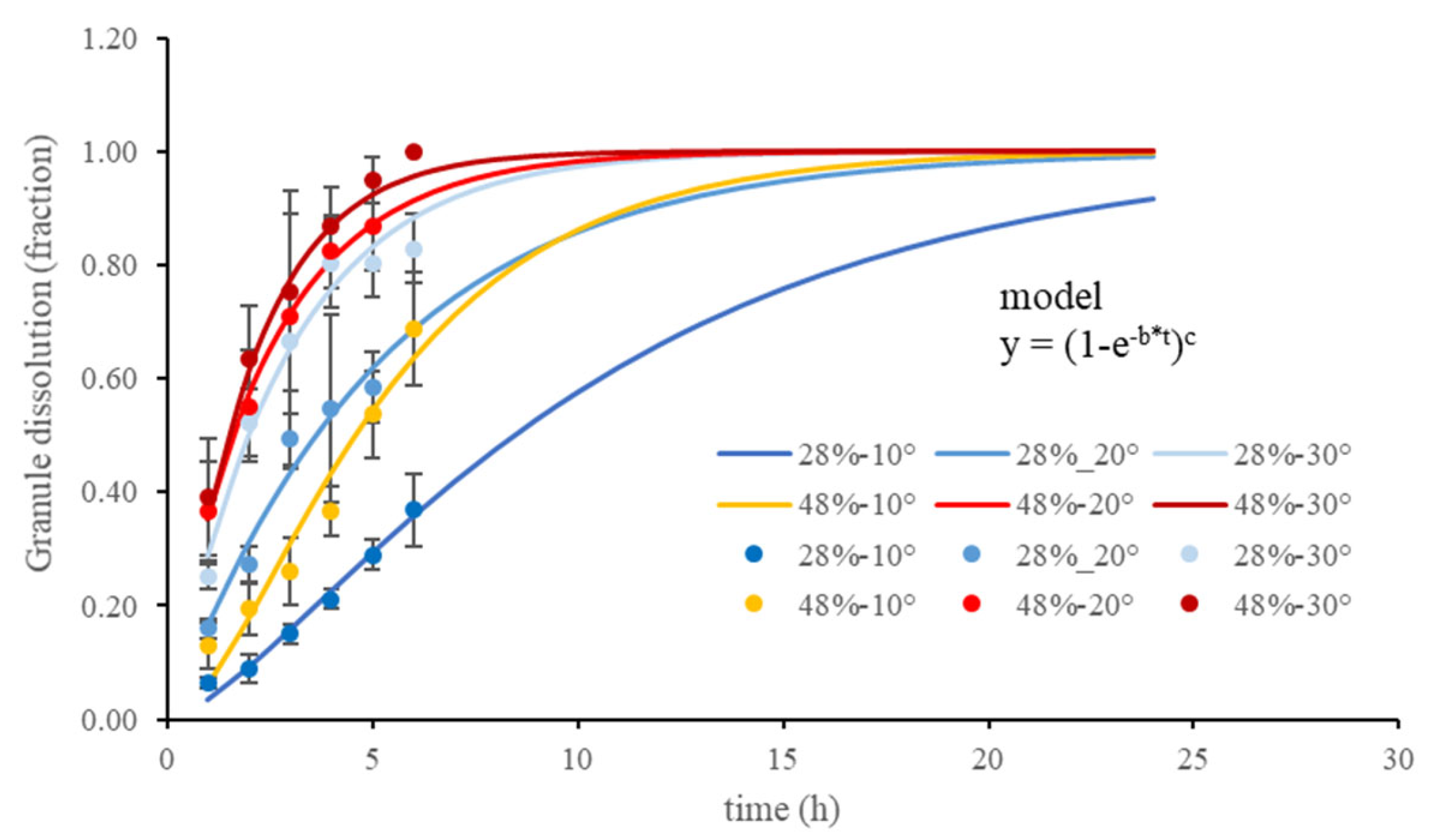

| Grav. Water Content (%) | Temperature (°C) | b (1/h) | i (Dimensionless) | Tmax (h) | Adj. R2 | p |
|---|---|---|---|---|---|---|
| 28 | 10 | 0.12 | 1.55 | 2.56 | 0.92 | <0.001 |
| 20 | 0.20 | 1.05 | 1.65 | 0.79 | <0.001 | |
| 30 | 0.36 | 1.05 | 1.06 | 0.78 | <0.001 | |
| 48 | 10 | 0.26 | 1.89 | 2.00 | 0.87 | <0.001 |
| 20 | 0.39 | 0.92 | 0.85 | 0.87 | <0.001 | |
| 30 | 0.55 | 1.12 | 0.71 | 0.84 | <0.001 |
| Treatment | Temperature (°C) | Vmax (mg N kg−1 h−1) | Km (mol L−1) | Vmax/Km | Adj. R2 |
|---|---|---|---|---|---|
| U | 10 | 9.60 ** | 0.0114 ** | 844 | 0.88 |
| 20 | 11.40 ** | 0.0077 ** | 1479 | 0.93 | |
| 30 | 15.30 ** | 0.0086 ** | 1772 | 0.84 | |
| 37 | 25.61 ** | 0.0137 ** | 1866 | 0.80 | |
| U + NI | 10 | 14.30 ** | 0.0083 ** | 1733 | 0.93 |
| 20 | 15.86 ** | 0.0071 ** | 2228 | 0.92 | |
| 30 | 27.72 ** | 0.0092 ** | 3028 | 0.89 | |
| 37 | 49.83 ** | 0.0167 ** | 2989 | 0.85 | |
| U + UI | 10 | 5.06 ** | 0.0034 ** | 1468 | 0.85 |
| 20 | 6.33 ** | 0.0071 ** | 894 | 0.57 | |
| 30 | 9.78 ** | 0.0059 ** | 1662 | 0.90 | |
| 37 | 10.94 ** | 0.0044 ** | 2502 | 0.73 | |
| U + UI + NI | 10 | 7.92 ** | 0.0071 ** | 1117 | 0.85 |
| 20 | 6.77 ** | 0.0055 ** | 1238 | 0.91 | |
| 30 | 10.11 ** | 0.0055 ** | 1848 | 0.89 | |
| 37 | 15.42 ** | 0.0040 ** | 3872 | 0.93 |
| Fertilizer | Estimates | Adj. R2 | RMSE (mg N kg−1 h−1) | rRMSE | ||
|---|---|---|---|---|---|---|
| Vmax (mg N kg−1 h−1) | Km (mol N L−1) | a | ||||
| U | 5.54 ** | 0.0109 ** | 0.0391 ** | 0.887 | 1.91 | 0.15 |
| U + NI | 6.63 ** | 0.0125 ** | 0.0522 ** | 0.913 | 3.46 | 0.19 |
| U + UI | 3.65 ** | 0.005 ** | 0.0304 ** | 0.905 | 0.76 | 0.13 |
| U + UI + NI | 3.95 ** | 0.0047 ** | 0.0353 ** | 0.832 | 1.39 | 0.19 |
| Parameter | Variable | Estimate | p |
|---|---|---|---|
| D0 | Intercept | 3.82 ** | <0.001 |
| D1 | Temperature | 1.03 ** | <0.001 |
| D2 | Urea concentration | 7.87 ** | <0.001 |
| D3 | UI | 0.60 ** | <0.001 |
| D4 | NI | 1.65 ** | <0.001 |
| D5 | UI* NI | 0.75 ** | <0.001 |
| Adj. R2 = 0.86 | RMSE = 3.22 | rRMSE = 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, K.; Pacholski, A.S. Soil Moisture and Temperature Effects on Granule Dissolution and Urease Activity of Urea with and without Inhibitors—An Incubation Study. Agriculture 2022, 12, 2037. https://doi.org/10.3390/agriculture12122037
Ni K, Pacholski AS. Soil Moisture and Temperature Effects on Granule Dissolution and Urease Activity of Urea with and without Inhibitors—An Incubation Study. Agriculture. 2022; 12(12):2037. https://doi.org/10.3390/agriculture12122037
Chicago/Turabian StyleNi, Kang, and Andreas Siegfried Pacholski. 2022. "Soil Moisture and Temperature Effects on Granule Dissolution and Urease Activity of Urea with and without Inhibitors—An Incubation Study" Agriculture 12, no. 12: 2037. https://doi.org/10.3390/agriculture12122037
APA StyleNi, K., & Pacholski, A. S. (2022). Soil Moisture and Temperature Effects on Granule Dissolution and Urease Activity of Urea with and without Inhibitors—An Incubation Study. Agriculture, 12(12), 2037. https://doi.org/10.3390/agriculture12122037







