Scion–Rootstock Relationship: Molecular Mechanism and Quality Fruit Production
Abstract
:1. Introduction
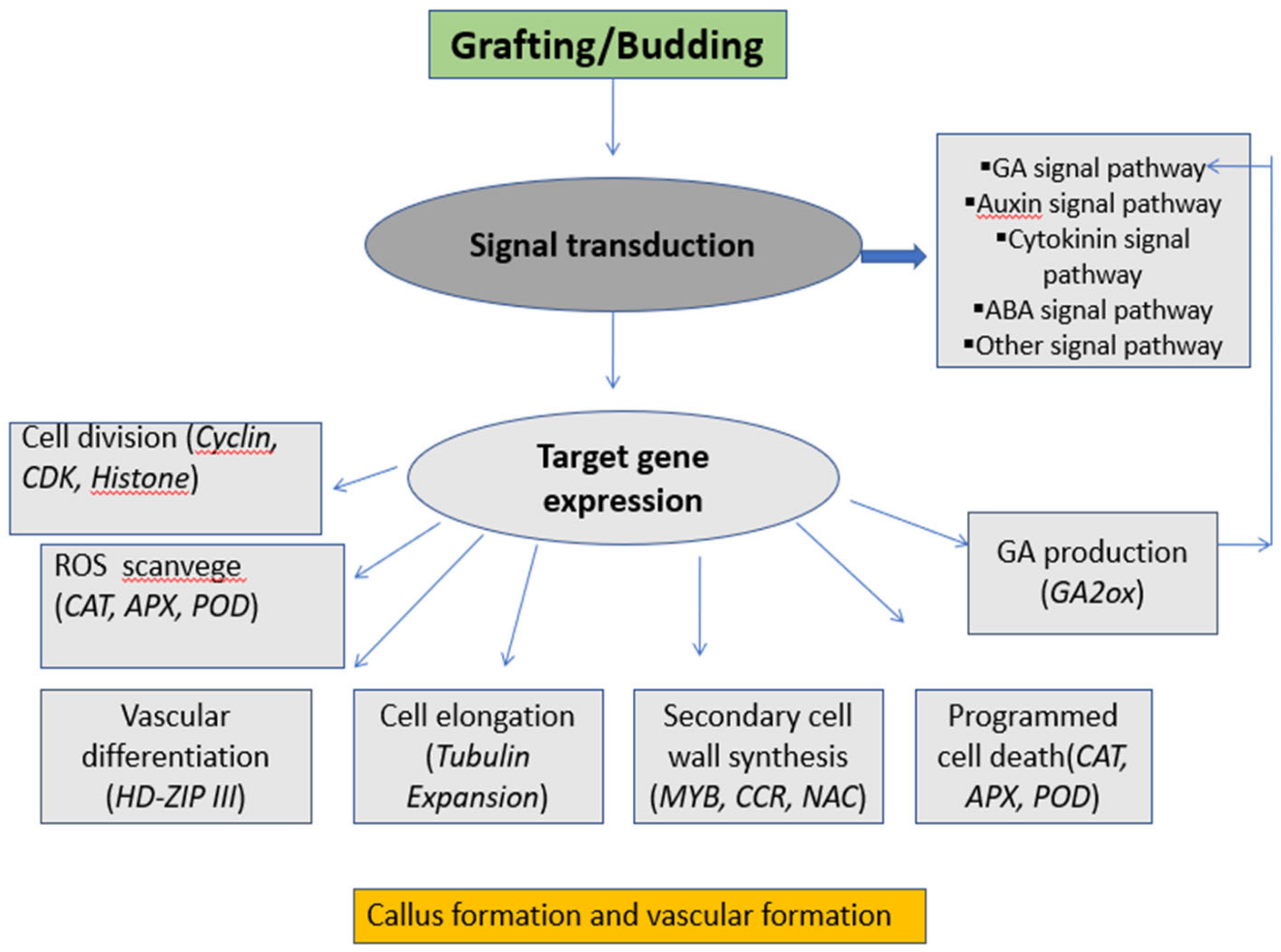
2. Molecular Mechanism between Scion and Rootstock
DNA, RNA, and Protein Transfer during Rootstock–Scion Interaction
3. Role of Hormones during Rootstock–Scion Interaction
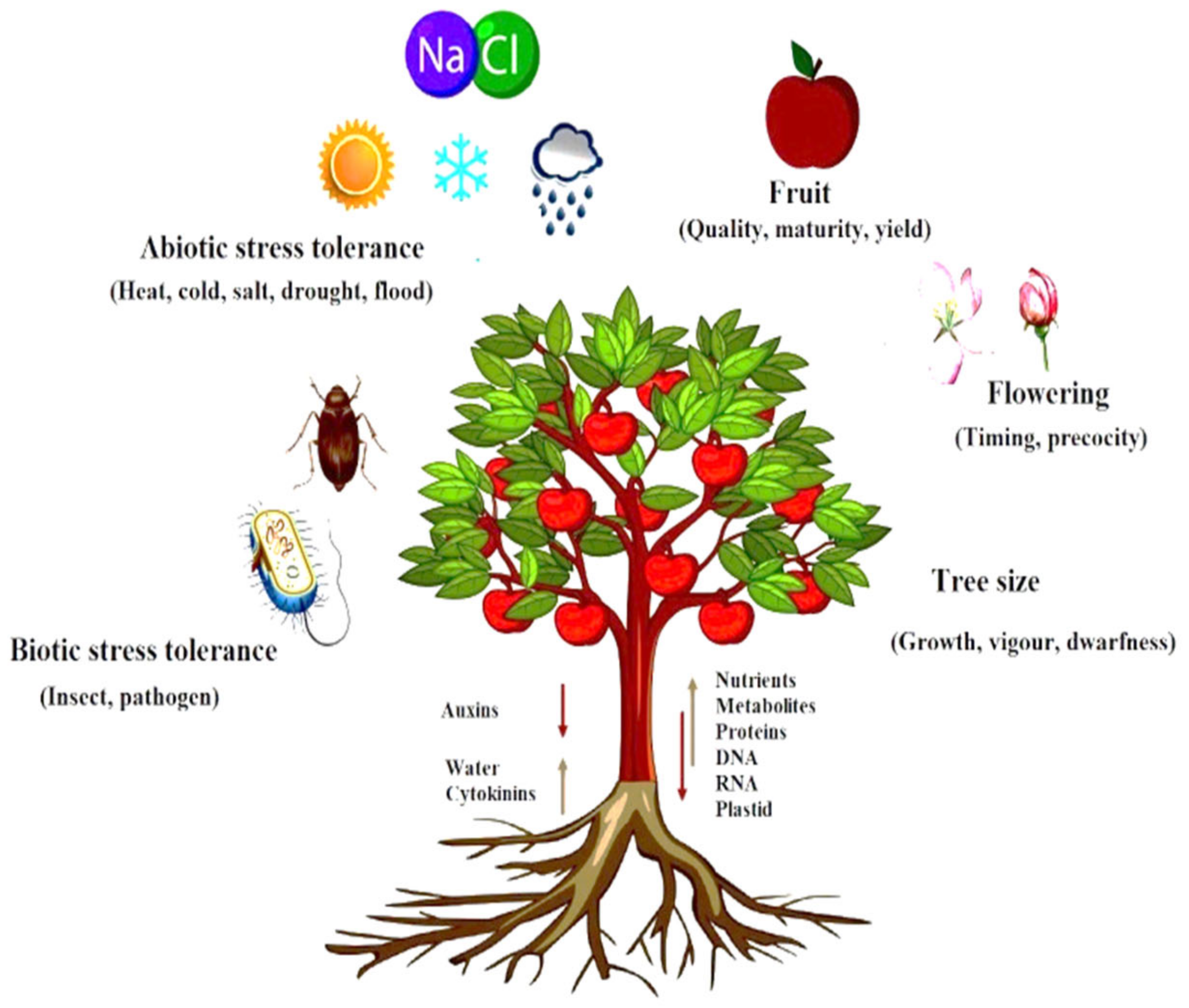
4. Effect of Rootstock on Scion
4.1. Effect on Tree Vigor
4.2. Effects of Rootstock on Biotic Resistance
4.3. Effect of Rootstock on Abiotic Resistance
4.4. Effect of Rootstock on Tree Flowering and Fruiting
4.5. Effect of Rootstock on Fruit Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baron, D.; Amaro, A.C.E.; Pina, A.; Ferreira, G. An overview of grafting re-establishment in woody fruit species. Sci. Hortic. 2019, 243, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Faria-Silva, L.; Gallon, C.Z.; Silva, D.M. Photosynthetic performance is determined by scion/rootstock combination in mango seedling propagation. Sci. Hortic. 2020, 265, 109247. [Google Scholar] [CrossRef]
- Dubey, A.K.; Sharma, R.M. Effect of rootstocks on tree growth, yield, quality, and leaf mineral composition of lemon (Citrus limon (L.) Burm.). Sci. Hortic. 2016, 200, 131–136. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, L.; Wu, R. Plant grafting: How genetic exchange promotes vascular reconnection. New Phytol. 2017, 214, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, C.; Else, M. Understanding how rootstocks dwarf fruit trees. Compact Fruit Tree 2001, 34, 46–49. [Google Scholar]
- Pina, A.; Irisarri, P.; Errea, P.; Zhebentyayeva, T. Mapping quantitative trait loci associated with graft (in) compatibility in apriot (Prunus armeniaca L.). Front. Plant Sci. 2021, 12, 622906. [Google Scholar] [CrossRef]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef]
- Gaffney, T.; Friedrich, L.; Vernooij, B.; Negrotto, D.; Nye, G.; Uknes, S.; Ward, E.; Kessmann, H.; Ryals, J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science 1993, 261, 754–756. [Google Scholar] [CrossRef]
- Paultre, D.S.G.; Gustin, M.P.; Molnar, A.; Oparka, K.J. Lost in transit: Long-distance tracking and phloem unloading of protein signals in Arabidopsishomografts. Plant Cell. 2016, 28, 2016–2025. [Google Scholar] [CrossRef] [Green Version]
- Wetmore, R.H.; Rier, J.P. Experimental induction of vascular tissues in callus of angiosperms. Am. J. Bot. 1963, 50, 418–430. [Google Scholar] [CrossRef]
- Miyashima, S.; Koi, S.; Hashimoto, T.; Nakajima, K. Non-cellautonomous microRNA165 acts in a dose-dependent manner to regulate multiple differentiation status in the Arabidopsis root. Development 2011, 138, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, C.W.; Molnar, A.; Bassett, A.; Baulcombe, D.C. Mobile 24nt small RNAs direct transcriptional gene silencing in the root meristems of Arabidopsis thaliana. Curr. Bio. 2011, 21, 1678–1683. [Google Scholar] [CrossRef] [Green Version]
- Stegemann, S.; Bock, R. Exchange of genetic material between cells in plant tissue grafts. Science 2009, 324, 649–651. [Google Scholar] [CrossRef]
- Stegemann, S.; Keuthe, M.; Greiner, S.; Bock, R. Horizontal transfer of chloroplast genomes between plant species. Proc. Natl. Acad. Sci. USA 2012, 109, 2434–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Liu, W.; Wang, T.; Zhang, J.; Li, X.; Zhang, W. Systemic Long-Distance Signaling and Communication between Rootstock and Scion in Grafted Vegetables. Front. Plant Sci. 2020, 11, 460. [Google Scholar] [CrossRef]
- Rasool, A.; Mansoor, S.; Bhat, K.M.; Hassan, G.I.; Baba, T.R.; Alyemeni, M.N.; Ahmad, P. Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Front. Plant Sci. 2020, 11, 1778. [Google Scholar] [CrossRef]
- Kapazoglou, A.; Tani, E.; Avramidou, E.V.; Abraham, E.M.; Gerakari, M.; Megariti, S.; Doupis, G.; Doulis, A.G. Epigenetic changes and transcriptional reprogramming upon woody plant grafting for crop sustainability in a changing environment. Front. Plant Sci. 2021, 11, 613004. [Google Scholar] [CrossRef] [PubMed]
- Taller, J.; Hirata, Y.; Yagishita, N.; Kita, M.; Ogata, S. Graft- induced genetic changes and the inheritance of several characteristics in pepper (Capsicum annuum L.). Theor. Appl. Genet. 1998, 97, 705–713. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, A.; Song, T.; Jin, Y.; Xu, X.; Gao, Y.; Qi, H. Transcriptome analysis reveals the effects of grafting on sugar and α-linolenic acid metabolisms in fruits of cucumber with two different rootstocks. Plant Physiol. Biochem. 2018, 130, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zheng, B. Molecular responses during plant grafting and its regulation by auxins, cytokinins, and gibberellins. Biomoleculess 2019, 9, 397. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, I.; Stegemann, S.; Golczyk, H.; Karcher, D.; Bock, R. Horizontal genome transfer as an asexual path to the formation of new species. Nature 2014, 511, 232–235. [Google Scholar] [CrossRef]
- Molnar, A.; Melnyk, C.W.; Bassett, A.; Hardcastle, T.J.; Dunn, R.; Baulcombe, D.C. Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 2010, 328, 872–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitwood, D.H.; Timmermans, M.C. Small RNAs are on the move. Nature 2010, 467, 415. [Google Scholar] [CrossRef] [PubMed]
- Sima, X.; Jiang, B.; Fang, J.; He, Y.; Fang, Z.; Km, S.K.; Ren, W.; Qiu, L.; Chen, X.; Zheng, B. Identification by deep sequencing and profiling of conserved and novel hickory microRNAs involved in the graft process. Plant Biotechnol. Rep. 2015, 9, 115–124. [Google Scholar] [CrossRef]
- Kanehira, A.; Yamada, K.; Iwaya, T.; Tsuwamoto, R.; Kasai, A.; Nakazono, M.; Harada, T. Apple phloem cells contain some mRNAs transported over long distances. Tree Genet. Genomes 2010, 6, 635–642. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, L.; Jittayasothorn, Y.; Kang, Y.; Jiao, C.; Fei, Z.; Zhong, G.Y. Messenger RNA exchange between scions and rootstocks in grafted grapevines. BMC Plant Biol. 2015, 15, 251. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zheng, Y.; Ham, B.K.; Chen, J.; Yoshida, A.; Kochian, L.V.; Fei, Z.; Lucas, W.J. Vascular-mediated signalling involved in early phosphate stress response in plants. Nat. Plants 2016, 2, 16033. [Google Scholar] [CrossRef]
- Xoconostle-Cazares, B.; Xiang, Y.; Ruiz-Medrano, R.; Wang, H.L.; Monzer, J.; Yoo, B.C.; McFarland, K.C.; Franceschi, V.R.; Lucas, W.J. Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science 1999, 283, 94–98. [Google Scholar] [CrossRef]
- Wu, Y.; Cosgrove, D.J. Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J. Exp. Bot. 2000, 51, 1543–1553. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Choi, D.; Kende, H. Expansins: Ever-expanding numbers and functions. Curr. Opin. Plant Biol. 2001, 4, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhang, W.; Huang, J.; Zhao, L.; Ma, C.; Hao, L.; Yuan, H.; Harada, T.; Li, T. KNOTTED1 mRNA undergoes long-distance transport and interacts with movement protein binding protein 2C in pear (Pyrus betulaefolia). Plant Cell Tissue Organ Cult. 2015, 121, 109–119. [Google Scholar] [CrossRef]
- Malz, S.; Sauter, M. Expression of two PIP genes in rapidly growing internodes of rice is not primarily controlled by meristem activity or cell expansion. Plant Mol. Biol. 1999, 40, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.; Maurel, C. The role of aquaporins in root water uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, B.; Liu, L.; Huang, J.; Cheng, X.; Zhu, Y.; Xu, H. Analysis on physiological and biochemical traits of survival of Carya cathayensis grafted seedling. J. Fujian Coll. For. 2002, 22, 320–324. [Google Scholar]
- Zheng, B.S.; Chu, H.L.; Jin, S.H.; Huang, Y.J.; Wang, Z.J.; Chen, M.; Huang, J.Q. cDNA-AFLP analysis of gene expression in hickory (Carya cathayensis) during graft process. Tree Physiol. 2010, 30, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Yuan, H.; Tong, Y.; Zhao, L.; Qiu, L.; Guo, W.; Shen, C.; Liu, H.; Yan, D.; Zheng, B. Comparative proteomic analysis of the graft unions in hickory (Carya cathayensis) provides insights into response mechanisms to grafting process. Front. Plant Sci. 2017, 8, 676. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.S.; Gao, L.X.; Yuan, H.W.; Xu, D.B.; Liang, Z.; Tao, S.C.; Edqvist, J. Auxin enhances grafting success in Carya cathayensis (Chinese hickory). Planta 2018, 247, 761–772. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Sun, H.; Tian, J.; Zhang, G.; Gong, G.; Ren, Y.; Zhang, J.; Li, M.; Zhang, H.; Li, H.; et al. Grafting delays watermelon fruit ripening by altering gene expression of ABA centric phytohormone signaling. Front. Plant Sci. 2021, 12, 624319. [Google Scholar] [CrossRef]
- Loupit, G.; Cookson, S.J. Identifying Molecular Markers of Successful Graft Union Formation and Compatibility. Front. Plant Sci. 2020, 11, 610352. [Google Scholar] [CrossRef]
- Spiegelman, Z.; Ham, B.K.; Zhang, Z.; Toal, T.W.; Brady, S.M.; Zheng, Y.; Fei, Z.; Lucas, W.J.; Wolf, S.A. Tomato phloem-mobile protein regulates the shoot-to-root ratio by mediating the auxin response in distant organs. Plant J. 2015, 83, 853–863. [Google Scholar] [CrossRef]
- Yuan, H.; Zhao, L.; Chen, J.; Yang, Y.; Xu, D.; Tao, S.; Zheng, B. Identification and expression profiling of the Aux/IAA gene family in Chinese hickory (Carya cathayensis Sarg.) during the grafting process. Plant Physiol. Biochem. 2018, 127, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, Z.; Yin, H.; Yan, B.; Ren, Z.; Xu, J.; Mu, C.; Zhang, Y.; Wang, M.; Liu, H. Auxin redistribution and shifts in PIN gene expression during Arabidopsis grafting. Russ. J. Plant Physiol. 2014, 61, 688–696. [Google Scholar] [CrossRef]
- Ruzicka, K.; Ursache, R.; Hejatko, J.; Helariutta, Y. Xylem development-from the cradle to the grave. New Phytol. 2015, 207, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Huh, S.U.; Kojima, M.; Sakakibara, H.; Paek, K.H.; Hwang, I. The cytokinin-activated transcriptionfactor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in Arabidopsis. Dev. Cell 2010, 19, 284–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, L.; Jiang, B.; Fang, J.; Shen, Y.; Fang, Z.; Rm, S.K.; Zheng, B. Analysis of transcriptome in hickory (Carya cathayensis), and uncover the dynamics in the hormonal signaling pathway during graft process. BMC Genom. 2016, 17, 935. [Google Scholar] [CrossRef] [Green Version]
- Corso, M.; Vannozzi, A.; Ziliotto, F.; Zouine, M.; Maza, E.; Nicolato, T.; Bonghi, C. Grapevine rootstocks differentially affect the rate of ripening and modulate auxin-related genes in cabernet sauvignon berries. Front. Plant Sci. 2016, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Melnyk, C.W.; Gabel, A.; Hardcastle, T.J.; Robinson, S.; Miyashima, S.; Grosse, I.; Meyerowitz, E.M. Transcriptome dynamics at Arabidopsis graft junctions reveal an intertissue recognition mechanism that activates vascular regeneration. Proc. Natl. Acad. Sci. USA 2018, 115, 2447–2456. [Google Scholar] [CrossRef] [Green Version]
- Dubrovsky, J.G.; Sauer, M.; Napsucialy-Mendivil, S.; Ivanchenko, M.G.; Friml, J.; Shishkova, S.; Benková, E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl. Acad. Sci. USA 2008, 105, 8790–8794. [Google Scholar] [CrossRef] [Green Version]
- Marhavy, P.; Vanstraelen, M.; De Rybel, B.; Zhaojun, D.; Bennett, M.J.; Beeckman, T.; Benkova, E. Auxin reflux between the endodermis and pericycle promotes lateral root initiation. EMBO J. 2013, 32, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Ohashi-Ito, K.; Bergmann, D.C. Regulation of the Arabidopsis root vascular initial population by LONESOME HIGHWAY. Development 2007, 134, 2959–2968. [Google Scholar] [CrossRef] [Green Version]
- Kubo, M.; Udagawa, M.; Nishikubo, N.; Horiguchi, G.; Yamaguchi, M.; Ito, J.; Demura, T. Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 2005, 19, 1855–1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dettmer, J.; Elo, A.; Helariutta, Y. Hormone interactions during vascular development. Plant Mol. Biol. 2009, 69, 347. [Google Scholar] [CrossRef]
- Mo, Z.; He, H.; Su, W.; Peng, F. Analysis of differentially accumulated proteins associated with graft union formation in pecan (Carya illinoensis). Sci. Hortic. 2017, 224, 126–134. [Google Scholar] [CrossRef]
- Ni, J.; Gao, C.; Chen, M.S.; Pan, B.Z.; Ye, K.; Xu, Z.F. Gibberellin promotes shoot branching in the perennial woody plant Jatropha curcas. Plant Cell Physiol. 2015, 56, 1655–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, C.; Guo, Z.H.; Hao, P.P.; Wang, G.M.; Jin, Z.M.; Zhang, S.L. Multiple regulatory roles of AP2/ERF transcription factor in angiosperm. Bot. Stud. 2017, 58, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, K.; Sugawara, E.; Aoki, R.; Takuma, K.; Terao-Morita, M.; Satoh, S.; Asahina, M. Differential cellular control by cotyledon-derived phytohormones involved in graft reunion of Arabidopsis hypocotyls. Plant Cell Physiol. 2016, 57, 2620–2631. [Google Scholar] [CrossRef] [Green Version]
- Hjellstrom, M.; Olsson, A.S.; Engstrom, P.; Soderman, E.M. Constitutive expression of the water deficit inducible homeobox gene ATHB7 in transgenic Arabidopsis causes a suppression of stem elongation growth. Plant Cell Environ. 2003, 26, 1127–1136. [Google Scholar] [CrossRef]
- Goswami, A.K.; Singh, S.K.; Srivastav, M.; Nagaraja, A.; Prakash, J.; Kumar, C. Important rootstock in different fruit crops. Biotech Article 2017, 4, 31–36. [Google Scholar]
- Tietel, Z.; Srivastava, S.; Fait, A.; Tel-Zur, N.; Carmi, N.; Raveh, E. Impact of scion/rootstock reciprocal effects on metabolomics of fruit juice and phloem sap in grafted Citrus reticulata. PLoS ONE 2020, 15, e0227192. [Google Scholar] [CrossRef]
- JukicSpika, M.; Dumicic, G.; BrkicBubola, K.; Soldo, B.; Goreta-Ban, S.; VuletinSelak, G.; Ljubenkov, I.; Mandusic, M.; Zanic, K. Modification of the sensory profile and volatile aroma compounds of tomato fruits by the scion-rootstock interactive effect. Front. Plant Sci. 2021, 11, 616431. [Google Scholar] [CrossRef]
- Chai, X.; Wang, X.; Li, H.; Xu, X.; Wu, T.; Zhang, X.; Han, Z. Apple scion cultivars regulate the rhizosphere microbiota of scion/rootstock combinations. Appl. Soil Ecol. 2022, 170, 104305. [Google Scholar] [CrossRef]
- Jayswal, D.K.; Lal, N. Rootstock and scion relationship in fruit crops. Agrials 2020, 2, 10. [Google Scholar]
- Murti, G.S.R.; Upreti, K.K. Endogenous hormones and phenols in rootstock seedlings of mango cultivars and their relationship with seedling vigour. Eur. J. Hortic. Sci. 2003, 68, 2–7. [Google Scholar]
- Reddy, Y.T.N.; Kurian, R.M.; Ranachander, P.R.; Singh, G.; Kohali, R.R. Long-term effects of rootstocks on growth and fruit yield patterns of ‘Alphonso’ mango (Mangifera indica L.). Sci. Hortic. 2003, 97, 95–108. [Google Scholar] [CrossRef]
- Ezzahouani, A.; Williams, L.E. Influence of rootstock on leaf water potential, yield, and berry composition of Ruby Seedless grapevines. Amer. J. Enol. Viticult. 1995, 46, 559–563. [Google Scholar]
- Nimbolkar, P.K.; Awachare, C.; Reddy, Y.T.N.; Chander, S.; Hussain, F. Role of rootstocks in fruit production—A review. J. Agric. Eng. 2016, 3, 183–188. [Google Scholar]
- Sharma, Y.K.; Goswami, A.M.; Sharma, R.R. Effect of dwarfing aneuploid guava rootstock in high density orcharding. Indian J. Hortic. 1992, 49, 31–36. [Google Scholar]
- Hayat, F.; Li, J.; Liu, W.; Li, C.; Song, W.; Iqbal, S.; Khan, U.; UmerJaved, H.; Ahsan Altaf, M.; Tu, P. Influence of citrus rootstocks on scion growth, hormone levels, and metabolites profile of shatangju mandarin (Citrus reticulata Blanco). Horticulturae 2022, 8, 608. [Google Scholar] [CrossRef]
- Khan, M.N.; Hayat, F.; Asim, M.; Iqbal, S.; Ashraf, T.; Asghar, S. Influence of citrus rootstocks on growth performance and leaf mineral nutrition of Salustiana sweet orange [Citrus sinensis (L.). obsek]. J. Pure Appl. Agric 2020, 5, 2617–8680. [Google Scholar]
- Lavagi-Craddock, I.; Dang, T.; Comstock, S.; Osman, F.; Bodaghi, S.; Vidalakis, G. Transcriptome analysis of citrus dwarfing viroid induced dwarfing phenotype of sweet orange on trifoliate orange rootstock. Microorganisms 2022, 10, 1144. [Google Scholar] [CrossRef]
- Girardi, E.A.; Sola, J.G.P.; Scapin, M.d.S.; Moreira, A.S.; Bassanezi, R.B.; Ayres, A.J.; Peña, L. The perfect match: Adjusting high tree density to rootstock vigor for improving cropping and land use efficiency of sweet orange. Agronomy 2021, 11, 2569. [Google Scholar] [CrossRef]
- Hervalejo, A.; Arjona-Lopez, J.M.; Romero-Rodríguez, E.; Arenas-Arenas, F.J. Suitability of two dwarfing citrus rootstocks for ‘Salustiana’ orange trees grown under super-high-density conditions with mechanical harvesting. N. Z. J. Crop Hortic. Sci. 2022, 1–12. [Google Scholar] [CrossRef]
- Continella, A.; Pannitteri, C.; La Malfa, S.; Legua, P.; Distefano, G.; Nicolosi, E.; Gentile, A. Influence of different rootstocks on yield precocity and fruit quality of ‘Tarocco Scire’ pigmented sweet orange. Sci. Hortic. 2018, 230, 62–67. [Google Scholar] [CrossRef]
- Hayat, F.; Li, J.; Iqbal, S.; Peng, Y.; Hong, L.; Balal, R.M.; Khan, M.N.; Nawaz, M.A.; Khan, U.; Farhan, M.A.; et al. A mini review of citrus rootstocks and their role in high-density orchards. Plants 2022, 11, 2876. [Google Scholar] [CrossRef] [PubMed]
- Pereira Costa, D.; SanchesStuchi, E.; Girardi, E.A.; Moreira, A.S.; da Silva Gesteira, A.; Coelho Filho, M.A.; da Silva Ledo, C.A.; da Silva, A.L.V.; de Leao, H.C.; Sampaio Passos, O. Less is more: A hard way to get potential dwarfing hybrid rootstocks for Valencia sweet orange. Agriculture 2021, 11, 354. [Google Scholar] [CrossRef]
- Plavcova, L.; Meszaros, M.; Silhan, K.; Jupa, R. Relationships between trunk radial growth and fruit yield in apple and pear trees on size-controlling rootstocks. Ann. Bot. 2022, 130, 477–489. [Google Scholar] [CrossRef]
- Smolka, A.; Li, X.Y.; Heikelt, C.; Welander, M.; Zhu, L.H. Effects of transgenic rootstocks on growth and development of non-transgenic scion cultivars in apple. Transgenic Res. 2010, 19, 933–948. [Google Scholar] [CrossRef]
- Prassinos, C.; Ko, J.H.; Lang, G.; Iezzoni, A.F.; Han, K.H. Rootstock-induced dwarfing in cherries is caused by differential cessation of terminal meristem growth and is triggered by rootstock-specific gene regulation. Tree Physiol. 2009, 29, 927–936. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Zhang, X.; Wu, T.; Xu, X.; Han, Z.; Wang, Y. Methylation effect on IPT5b gene expression determines cytokinin biosynthesis in apple rootstock. Biochem. Biophys. Res. Commun. 2017, 482, 604–609. [Google Scholar] [CrossRef]
- Habibi, F.; Liu, T.; Folta, K.; Sarkhosh, A. Physiological, biochemical, and molecular aspects of grafting in fruit trees. Hortic. Res. 2022, 9, 1–18. [Google Scholar] [CrossRef]
- Hayat, F.; Iqbal, S.; Coulibaly, D.; Razzaq, M.K.; Nawaz, M.A. An insight into dwarfing mechanism: Contribution of scion-rootstock. Fruit Res. 2021, 1, 3. [Google Scholar] [CrossRef]
- Mayer, N.A.; Ueno, B.; Reighard, G.L. Selection of Prunus mume as rootstocks for peaches on PTSL site. Acta Hortic. 2015, 1084, 89–96. [Google Scholar] [CrossRef]
- Roberto, S.; Stuchi, E.S. Dwarfing-canopy and rootstock cultivars for fruit trees. Rev. Bras. 2019, 41, e-997. [Google Scholar] [CrossRef]
- Campbell, J. Pear Rootstocks. NSW 2003, 12. [Google Scholar]
- Lliso, I.; Forner, J.B.; Talón, M. The dwarfing mechanism of citrus rootstocks F&A 418 and 23 is related to competition between vegetative and reproductive growth. Tree Physiol. 2004, 24, 225–232. [Google Scholar]
- Tworkoski, T.; Fazio, G. Hormone and growth interactions of scions and size-controlling rootstocks of young apple trees. Plant Growth Regul. 2016, 78, 105–119. [Google Scholar] [CrossRef]
- Fazio, G. Genetics, breeding, and genomics of apple rootstocks. In The Apple Genome; Springer: Cham, Switzerland, 2021; pp. 105–130. [Google Scholar]
- Sathisha, J.; Ramteke, S.D.; Karibasappa, G.S. Physiological and biochemical characterization of guava rootstocks. S. Afr. J Enol. Vitic. 2007, 28, 163–168. [Google Scholar]
- Mestre, P.F.; Asins, M.J.; Pina, J.A.; Carbonell, E.A.; Navarro, L. Molecular markers flanking Citrus tristeza virus resistance gene from Poncirus trifoliata (L.) Raf. Theor. Appl. Genet. 1997, 94, 458–464. [Google Scholar] [CrossRef]
- Freitas, V.M.; Correa, V.R.; Motta, F.C.; Sousa, M.G.; Gomes, A.C.M.M.; Carneiro, M.D.G. Resistant accessions of wild Psidium spp. to Meloidogyne enterolobii and histological characterization of resistance. Plant Pathol. J. 2014, 63, 738–746. [Google Scholar] [CrossRef]
- Costa, S.R.; Santos, C.A.F.; Castro, J.M.C. Assessing Psidium guajava, P. guineense hybrid tolerance to Meloidogyne enterologii. Acta Hortic. 2012, 959, 59–66. [Google Scholar] [CrossRef]
- Ribeiro, I.J.A.; Rossetto, C.J.; Donadio, L.C.; Sabino, J.C.; Martins, A.I.M.; Gallo, P.B. Mango Wilt. XIV Selection of mango (Mangifera indica L.) rootstocks resistant to the mango wilt Fungus Ceratocystis fimbriata Ell &Halst. Acta Hortic. 2000, 513, 69–79. [Google Scholar]
- Vos, J.E.; Schoeman, M.H. In-vitro selection and commercial release of guava wilt resistant rootstock. Acta Hortic. 2000, 513, 69–79. [Google Scholar] [CrossRef]
- Milan, A.R. Breeding of Psidium species for root knot nematode resistance in Malaysia. Acta Hortic. 2005, 735, 61–69. [Google Scholar] [CrossRef]
- Kellam, M.K.; Coffey, M.D. Quantitative comparison of the resistance to Phytophthora root rot in three avocado rootstocks. J. Phytopathol. 1985, 75, 230–234. [Google Scholar] [CrossRef]
- Sharma, D.R.; Kumar, R.; Rattanpal, H.S. Reaction of citrus germplasm against Citrus psylla and whitefly. Haryana J. Hortic. Sci. 2005, 34, 274. [Google Scholar]
- Ferris, H.; Zheng, L.; Walker, M.A. Soil temperature effects on the interaction of grape rootstocks and plant-parasitic nematodes. J. Nematol. 2013, 45, 49. [Google Scholar] [PubMed]
- Sarip, J.; Nur Sulastri, J.; Sanimah, S.; Salehudin, M.R.; Razali, M.; Noor Faimah, G.; Noraisah, R.; Puteri, D.E.Z.; Fauzam, C.H.; Zulfa, M.R. Viorica a promising rootstock in producing highly tolerance grafted papaya against papaya dieback disease. Trans. Malaysian Soc. Plant Physiol. 2018, 25, 56–60. [Google Scholar]
- Janick, J. Genetics and breeding of tree fruits and nuts. In Proceedings of the XXVI International Horticultural Congress, Toronto, ON, Canada, 11–17 August 2002. [Google Scholar]
- Chitarra, W.; Perrone, I.; Avanzato, C.G.; Minio, A.; Boccacci, P.; Santini, D.; Gambino, G. Grapevine grafting: Scion transcript profiling and defense-related metabolites induced by rootstocks. Front. Plant Sci. 2017, 8, 654. [Google Scholar] [CrossRef]
- Santhi, V.P.; Nireshkumar, N.; Vasugi, C.; Parthiban, S.; Masilamani, P. Role of rootstocks to mitigate biotic and abiotic stresses in tropical and subtropical fruit crops: A. IJCS 2020, 8, 499–510. [Google Scholar] [CrossRef]
- Duran, V.H.; Raya, A.M.; Aguilar, J. Salt tolerance of mango rootstocks (Magnifera indica L. cv. Osteen). Span. J. Agric. Res. 2003, 1, 68–78. [Google Scholar]
- Khalid, M.F.; Morillon, R.; Anjum, M.A.; Ejaz, S.; Rao, M.J.; Ahmad, S.; Hussain, S. Volkamer lemon tetraploid rootstock transmits the salt tolerance when grafted with diploid kinnow mandarin by strong antioxidant defense mechanism and efficient osmotic adjustment. J. Plant Growth Regul. 2022, 41, 1125–1137. [Google Scholar] [CrossRef]
- Valizadeh, V.; Kaji, B.; Abbasifar, A.; Bagheri, H.; Zandievakili, G.; Daryabeigi, A. First report: Grafting of three iranian commercial pomegranate cultivars on drought tolerant rootstocks. Int. J. Hortic. Sci. 2020, 7, 69–79. [Google Scholar]
- Serra, A.; Strever, P.; Myburgh, A.; Deloire, A. The interaction between rootstocks and cultivars (Vitis vinifera L.) to enhance drought tolerance in grapevine. Aust. J. Grape Wine Res. 2014, 20, 1–14. [Google Scholar] [CrossRef]
- Rickes, L.N.; Klumb, E.K.; Benitez, L.C.; Braga, E.J.B.; Bianchi, V.J. Differential expression of the genes involved in responses to water-deficit stress in peach trees cv. Chimarrita grafted onto two different rootstocks. Bragantia 2019, 78, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.Q.; Liu, J.H. Genetic transformation and genes for resistance to abiotic and biotic stresses in citrus and its related genera. Plant Cell Tissue Organ Cul. 2013, 113, 137–147. [Google Scholar] [CrossRef]
- Shen, Q.X.; Chen, C.N.; Brands, A.; Pan, S.M.; Ho, T.H.D. The stress- and abscisic acid-induced barley gene HVA22: Developmental regulation and homologues in diverse organisms. Plant Mol. Biol. 2001, 45, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Lazare, S.; Yasuor, H.; Yermiyahu, U.; Kuhalskaya, A.; Brotman, Y.; Ben-Gal, A.; Dag, A. It takes two: Reciprocal scion-rootstock relationships enable salt tolerance in ‘Hass’ avocado. Plant Sci. 2021, 312, 111048. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Srivastav, M.; Sharma, Y.K.; Pandey, R.N.; Deshmukh, P.S. Dry mass production and distribution of nutrients in two mango rootstocks as affected by salinity. Indian J. Hortic. 2007, 64, 670–672. [Google Scholar]
- Gunjate, R.T. Advances in mango culture in India. Acta Hortic. 2009, 820, 69–78. [Google Scholar] [CrossRef]
- Souza, L.D.P.; Nobre, R.G.; Silva, E.M.D.; Lima, G.S.D.; Pinheiro, F.W.; Almeida, L.L.D.S. Formation of ‘Crioula’guava rootstock under saline water irrigation and nitrogen doses. Rev. Bras. Eng. Agric. Ambient. 2016, 20, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Jiang, H.; Li, W. Effects of low temperature stress on the cold resistance of rootstock and branch of wine grapes. Int. J. Fruit Sci. 2012, 29, 1040–1046. [Google Scholar]
- Tramontini, S.; Vitali, M.; Centioni, L.; Schubert, A.; Lovisolo, C. Rootstock control of scion response to water stress in grapevine. Environ. Exp. Bot. 2013, 93, 20–26. [Google Scholar] [CrossRef]
- Guo, S.H.; Niu, Y.J.; Zhai, H.; Han, N.; Du, Y.P. Effects of alkaline stress on organic acid metabolism in roots of grape hybrid rootstocks. Sci. Hortic. 2018, 227, 255–260. [Google Scholar] [CrossRef]
- Garcla-Legaz, M.F.; Ortiz, J.M.; Garcla-Lidun, A.; Cerd, A. Effect of salinity on growth, ion content and CO2 assimilation rate in lemon varieties on different rootstocks. Physiol. Plant. 1993, 89, 427–432. [Google Scholar] [CrossRef]
- Flachowsky, H.; Peil, A.; Sopanen, T.; Elo, A.; Hanke, V. Overexpression of BpMADS4 from silver birch (Betula pendula Roth.) induces early-flowering in apple (Malus× domestica Borkh.). Plant Breed. 2007, 126, 137–145. [Google Scholar] [CrossRef]
- Nishikawa, F.; Endo, T.; Shimada, T.; Fujii, H.; Shimizu, T.; Kobayashi, Y.; Omura, M. Transcriptional changes in CiFT-introduced transgenic trifoliate orange (Poncirus trifoliata L. Raf.). Tree Physiol. 2010, 30, 431–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knabel, M.; Friend, A.P.; Palmer, J.W.; Diack, R.; Wiedow, C.; Alspach, P.; Deng, C.; Gardiner, S.E.; Tustin, D.S.; Schaffer, R.; et al. Genetic control of pear rootstock–induced dwarfing and precocity is linked to a chromosomal region syntenic to the apple Dw1 loci. BMC Plant Biol. 2015, 15, 230. [Google Scholar] [CrossRef] [Green Version]
- Davies, F.T.; Geneve, R.L.; Kester, D.E. Hartmann and Kester’s Plant Propagation: Principles and Practice; Prentice Hall: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Bennici, S.; Las Casas, G.; Distefano, G.; Gentile, A.; Lana, G.; Di Guardo, M.; Continella, A. Rootstock affects floral induction in citrus engaging the expression of the flowering locus t (Cift). Agriculture 2021, 11, 140. [Google Scholar] [CrossRef]
- Baumes, R.; Wirth, J.; Bureau, S.; Gunata, Y.; Razungles, A. Bio-generation of C13- norisoprenoid compounds: Experiments supportive for an apo-carotenoid pathway in grapevines. Anal. Chim. Acta 2002, 458, 3–14. [Google Scholar] [CrossRef]
- Bindon, K. Influence of Partial Root Zone Drying on Aspects of Grape and Wine Quality. Ph.D. Thesis, School of Agriculture and Wine, University of Adelaide, Adelaide, SA, Australia, 2004; pp. 182–204. [Google Scholar]
- Stern, R.A.; Doron, I. Performance of ‘Coscia’ pear (Pyrus communis L.) on nine rootstocks in the north of Israel. Sci. Hortic. 2009, 119, 252–256. [Google Scholar] [CrossRef]
- Cano, A.; Bermejo, A. Influence of rootstock and cultivar on bioactive compounds in citrus peels. J. Sci. Food Agric. 2011, 91, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.M.; Pech, J.M.; Taylor, J.; Clingeleffer, P.; Walker, R.R.; Nicholas, P.R. Effects of irrigation and rootstock on Vitis vinifera (L.) cv. S hiraz berry composition and shrivel, and wine composition and wine score. Aust. J. Grape Wine Res. 2016, 22, 124–136. [Google Scholar] [CrossRef]
- Fallahi, E. Influence of rootstock and irrigation methods on water use, mineral nutrition, growth, fruit yield, and quality in ‘Gala’ apple. Hort Tech. 2012, 22, 731–737. [Google Scholar] [CrossRef]
- Noguera-Artiaga, L.; Perez-Lopez, D.; Burgos-Hernandez, A.; Wojdilo, A.; Carbonell-Barrachina, Á.A. Phenolic and triterpenoid composition and inhibition of α-amylase of pistachio kernels (Pistacia vera L.) as affected by rootstock and irrigation treatment. Food Chem. 2018, 261, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, G.; Tietel, Z.; Porat, R. Effects of rootstock/scion combinations on the flavor of citrus fruit. J. Agric. Food Chem. 2013, 61, 11286–11294. [Google Scholar] [CrossRef]
- Seker, M.; Ekinci, N.; Gür, E. Effects of different rootstocks on aroma volatile constituents in the fruits of peach (Prunus persica L. Batsch cv. ‘Cresthaven’). N. Z. J. Crop Hortic. Sci. 2017, 45, 1–13. [Google Scholar] [CrossRef]
- Aguilar-Hernández, M.G.; Sanchez-Bravo, P.; Hernandez, F.; Carbonell-Barrachina, A.A.; Pastor-Perez, J.J.; Legua, P. Determination of the volatile profile of lemon peel oils as affected by rootstock. Foods 2020, 9, 241. [Google Scholar] [CrossRef]

| Protein | Response | Crop | Reference |
|---|---|---|---|
| CmPP16 | Favoring the process of molecular transport and reduce the degradation of mRNAs. | Cucurbita maxima | [28] |
| PbPTB3 | Play role in long-distance movement of mRNAs across the graft junction by binding of PbPTB3 to PbWoxT1 mRNA. | Pyrus betlaefolia | [31] |
| Cyclophilin, SICyp1 | Play role in increased auxin response and promoting the growth of roots. | Tomato | [40] |
| PIP1B | Enhanced water levels and cell elongation, leading to better callus formation and successful grafting. | Carya cathayensis | [35] |
| DEPs | At graft unions, 341 and 369 DEPs were found to be upregulated. | Carya cathayensis | [41] |
| PIN | Reunion of vascular tissues is favored by the auxin movement from top to downward direction mediated by PIN proteins. | Arabidopsis | [42] |
| Phytohormones | Genes | Response | Reference |
|---|---|---|---|
| Auxin | Aux/IAA | Control graft union healing and graft compatibility. | [45] |
| GH3 | Showed positive responses to grafting. | [46] | |
| VviGH3-21 | Role in the grafted plant growth. | [46] | |
| PIN1 and ABCB1 | Carriers in auxin transport, regulate the graft development. | [47] | |
| CcPIN1b and CcLAX3) | Carriers for PAT and favor the process of grafting. | [37] | |
| ARF | The process of grafting further regulates various biochemical pathways promoting vascular connection between the scion and the stock. | [35] | |
| MrPIN1, MrSHR | Downregulation in the gene expression of MrPIN1 and MrSHR in roots of the grafted plants. | [48] | |
| MrPIN3 | Upregulation and enhanced distribution of auxins, which further induces the division of pericycle cell. | [49] | |
| HCA2 | Grafting site which is important for the reconnection of phloem. | [47] | |
| Type-B ARRs | Induction of cell division and callus formation. | [50] | |
| LHW | Growth and development of stele cells as well as in formation of protoxylem. | [50] | |
| VND7 | Expresses during protoxylem formation. | [51] | |
| VND6 | Expresses during metaxylem formation. | [51] | |
| CRE1/WOL/AHK4 | Regulation of proliferation and specification of vascular cells. | [52] | |
| GA | GA20OX | Upregulation of gene and involved in GA-biosynthesis. | [53] |
| JcGA20ox1 | Enhanced stem elongation and increased outgrowth of lateral buds. | [54] | |
| Ethylene | APETALA2 | TFs exist in all plant species and are activated in response to multiple stresses or developmental pathways. | [55] |
| ANAC071 | Reduced the formation of vascular tissues at the graft junction. | [56] | |
| ABA | ATHB7 | This gene is specifically expressed in differentiating xylem and is also induced by drought stress. | [57] |
| Fruit Crop | Rootstock | Effect | Reference |
|---|---|---|---|
| Peach | Okinawa and Capdebosq | Vigor | [82] |
| Nano | Semi- dwarf | [83] | |
| Mango | Olour and Vellaicolamban | Dwarfing | [64] |
| Pear | Quince C | Dwarfing | [84] |
| Pyrodwarf | Dwarfing | [58] | |
| Citrus | Poncirus trifoliate | Dwarfing | [83] |
| Flying Dragon | Dwarfing | [83] | |
| Troyer citrange | Dwarfing | [58] | |
| Fand A 418 | Dwarfing | [85] | |
| Guava | Pusa srijan (Aneuploid 82) | Dwarfing | [58] |
| Apple | M-2, MM-104 | Vigorous | [58] |
| B-9 | Semi-dwarfing | [58] | |
| M-9 | Dwarfing | [86] |
| Crop | Rootstock | Resistant/Tolerant Trait | Reference |
|---|---|---|---|
| Mango | Carabao, Pico Manga d Agua | Resistant to wilt | [92] |
| Guava | Psidium friedrichsthalianum, P. cattleianum var. lucidum | Resistance to root-knot nematode | [90] |
| Psidium guineense | Resistance to root-knot nematode | [91] | |
| Dimple, Jonelle, GU 8 | GWD resistance Nematode tolerance | [93] | |
| GU8 P. longipes and P. arayan | Root knot nematode resistant root stock | [94] | |
| Avocado | Duke 7 and G6 | Resistance to P. cinnamomi | [95] |
| Citrus | Star ruby and ruby red | Resistant against citrus psylla | [96] |
| Crop | Rootstock | Resistant/Tolerant Trait | Reference |
|---|---|---|---|
| Mango | Kurukan | Tolerant to salinity | [110] |
| 13/3 | Tolerant to salinity | [111] | |
| Guava | Crioula | Tolerant to salinity | [112] |
| Grape | Beta | Cold hardiness | [113] |
| 140Ru | Tolerance to water deficit condition | [114] | |
| A15 and A17 | Tolerance to alkalinity | [115] | |
| Loquat | Anger | Tolerance to saline conditions | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shivran, M.; Sharma, N.; Dubey, A.K.; Singh, S.K.; Sharma, N.; Sharma, R.M.; Singh, N.; Singh, R. Scion–Rootstock Relationship: Molecular Mechanism and Quality Fruit Production. Agriculture 2022, 12, 2036. https://doi.org/10.3390/agriculture12122036
Shivran M, Sharma N, Dubey AK, Singh SK, Sharma N, Sharma RM, Singh N, Singh R. Scion–Rootstock Relationship: Molecular Mechanism and Quality Fruit Production. Agriculture. 2022; 12(12):2036. https://doi.org/10.3390/agriculture12122036
Chicago/Turabian StyleShivran, Mukesh, Nimisha Sharma, Anil Kumar Dubey, Sanjay Kumar Singh, Neha Sharma, Radha Mohan Sharma, Narendra Singh, and Rakesh Singh. 2022. "Scion–Rootstock Relationship: Molecular Mechanism and Quality Fruit Production" Agriculture 12, no. 12: 2036. https://doi.org/10.3390/agriculture12122036
APA StyleShivran, M., Sharma, N., Dubey, A. K., Singh, S. K., Sharma, N., Sharma, R. M., Singh, N., & Singh, R. (2022). Scion–Rootstock Relationship: Molecular Mechanism and Quality Fruit Production. Agriculture, 12(12), 2036. https://doi.org/10.3390/agriculture12122036








