A Comparative Study on the Structural Properties and Lipid Profile of Mushroom (Pleurotus ostreatus) Powder Obtained by Different Drying Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chemicals
2.3. Drying Process
2.3.1. Freeze Drying (FD)
2.3.2. Hot Air Drying (HAD)
2.3.3. Microwave Drying (MWD)
2.3.4. Sun Drying (SD)
2.4. Preparation of Dried Mushroom Powder
2.5. Powder Morphology
2.5.1. X-ray Microtomography Analysis
2.5.2. Scanning Electron Microscopy
2.6. Thin Layer Chromatography
2.7. In Vitro Digestion Methods
2.8. Gas Chromatographic Analysis
2.9. Statistical Analysis
3. Results and Discussion
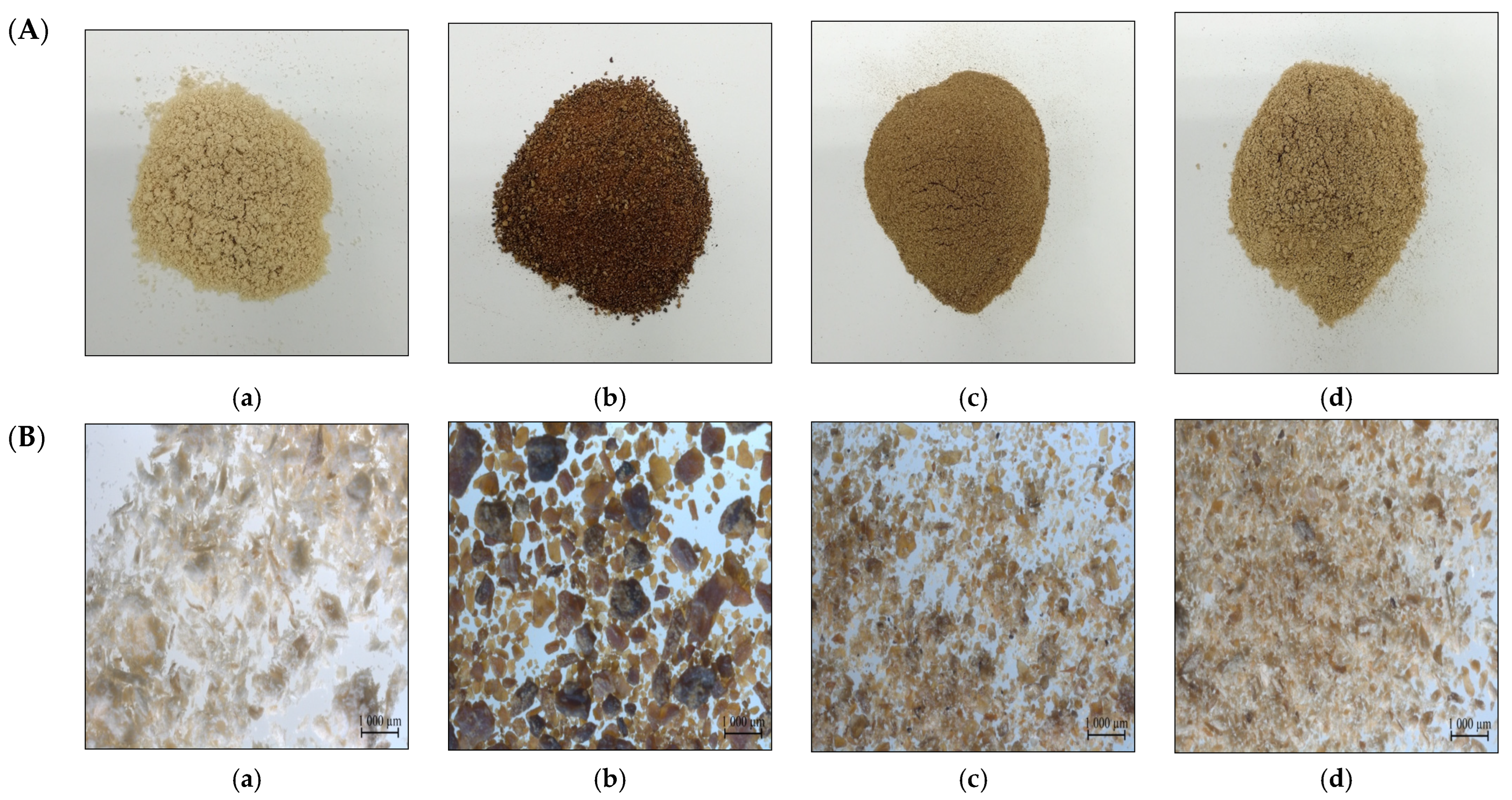
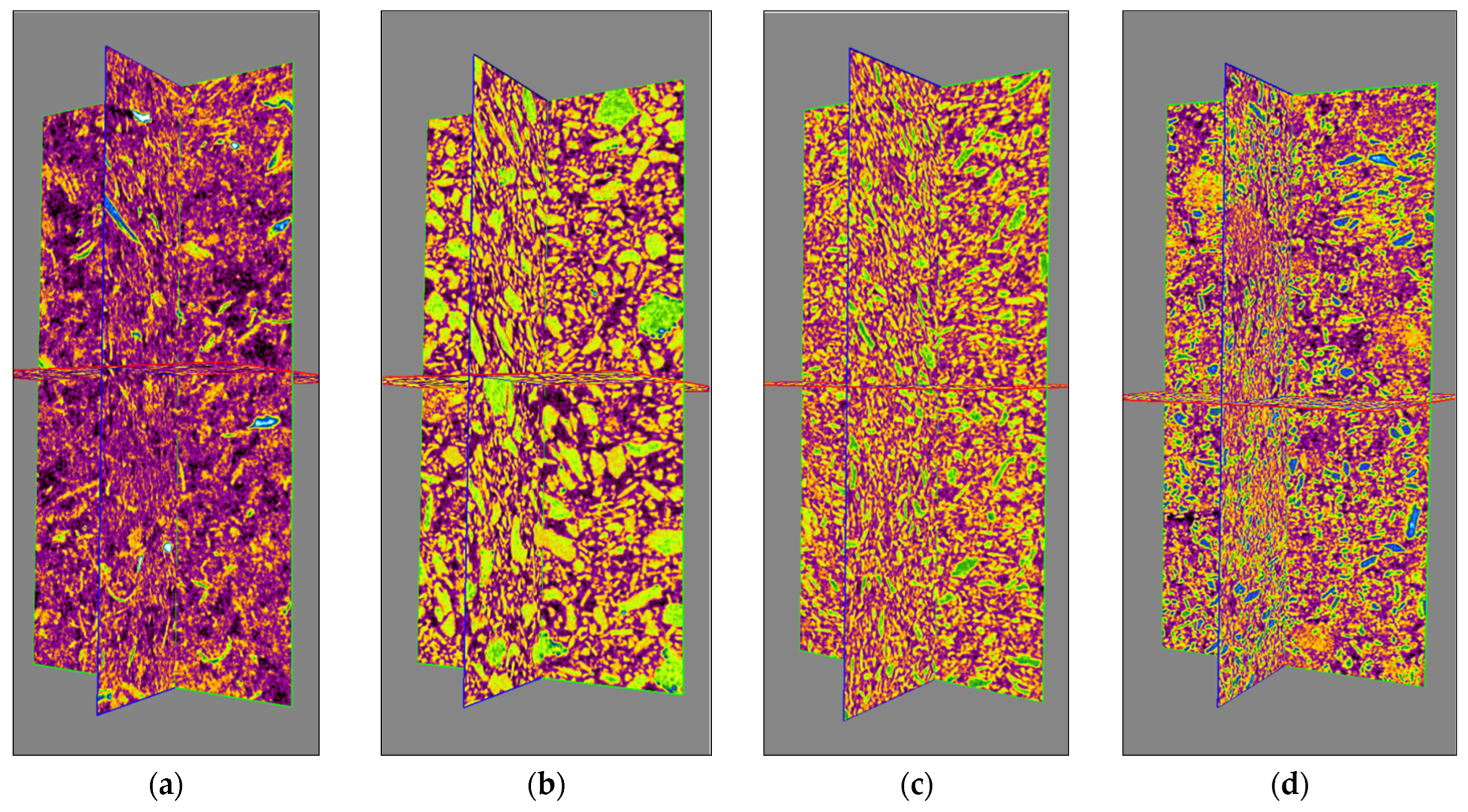
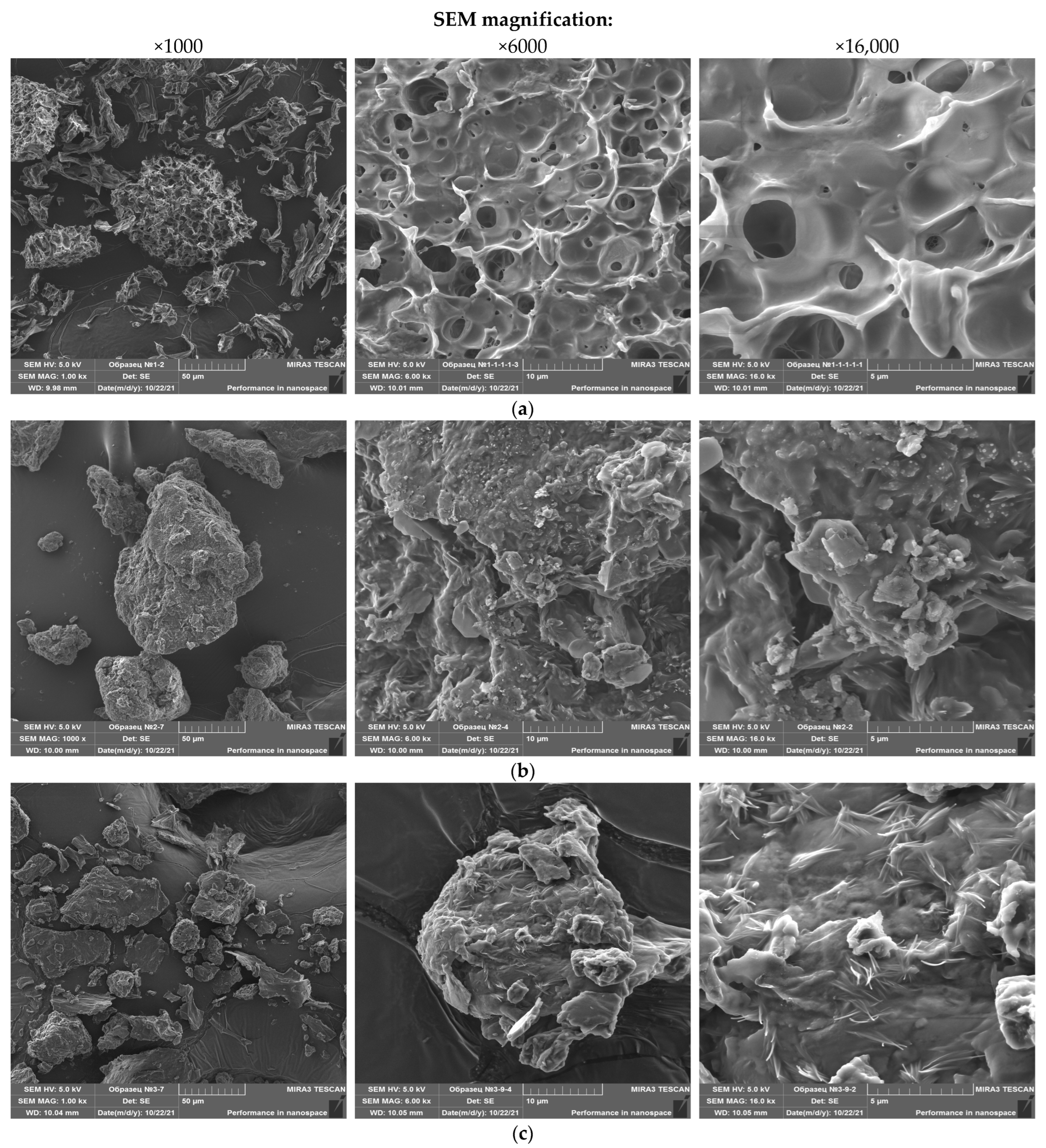
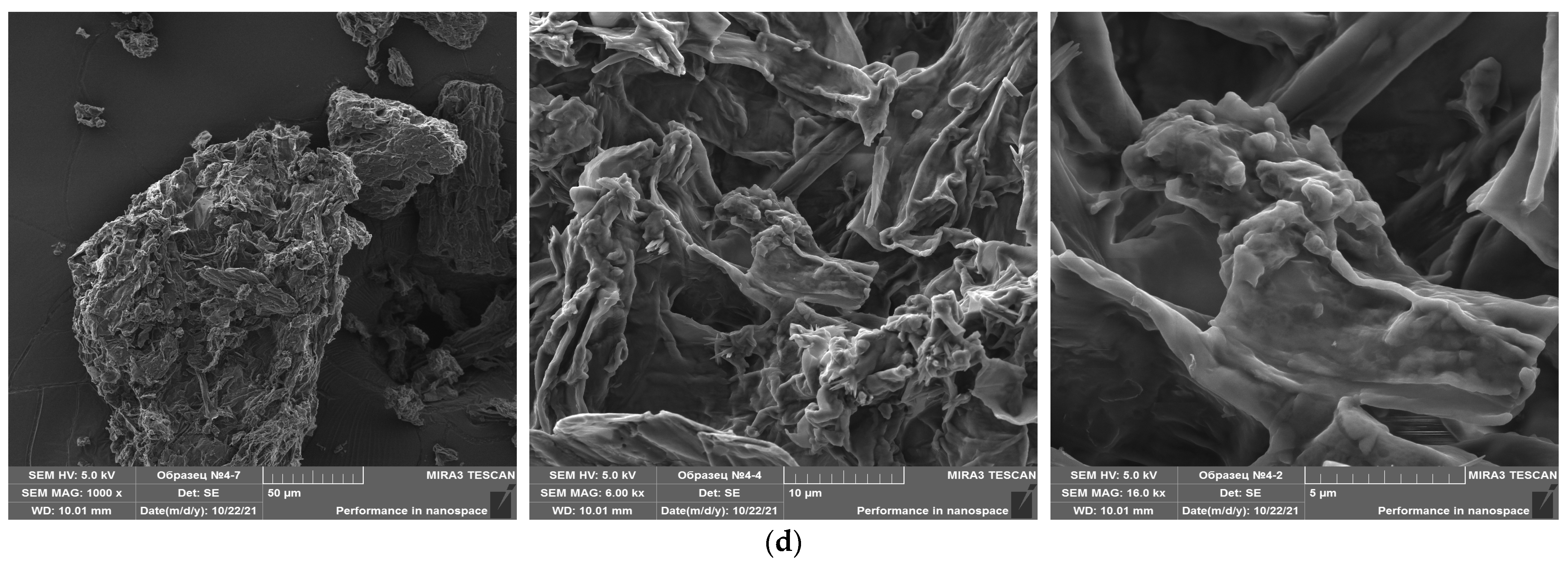
3.1. Particle Morphology and Microstructural Properties
3.1.1. Particle Size Analysis
3.1.2. X-ray Microtomography Diffraction Analysis
3.1.3. SEM of Mushroom Powder
3.2. Thin Layer Chromatography
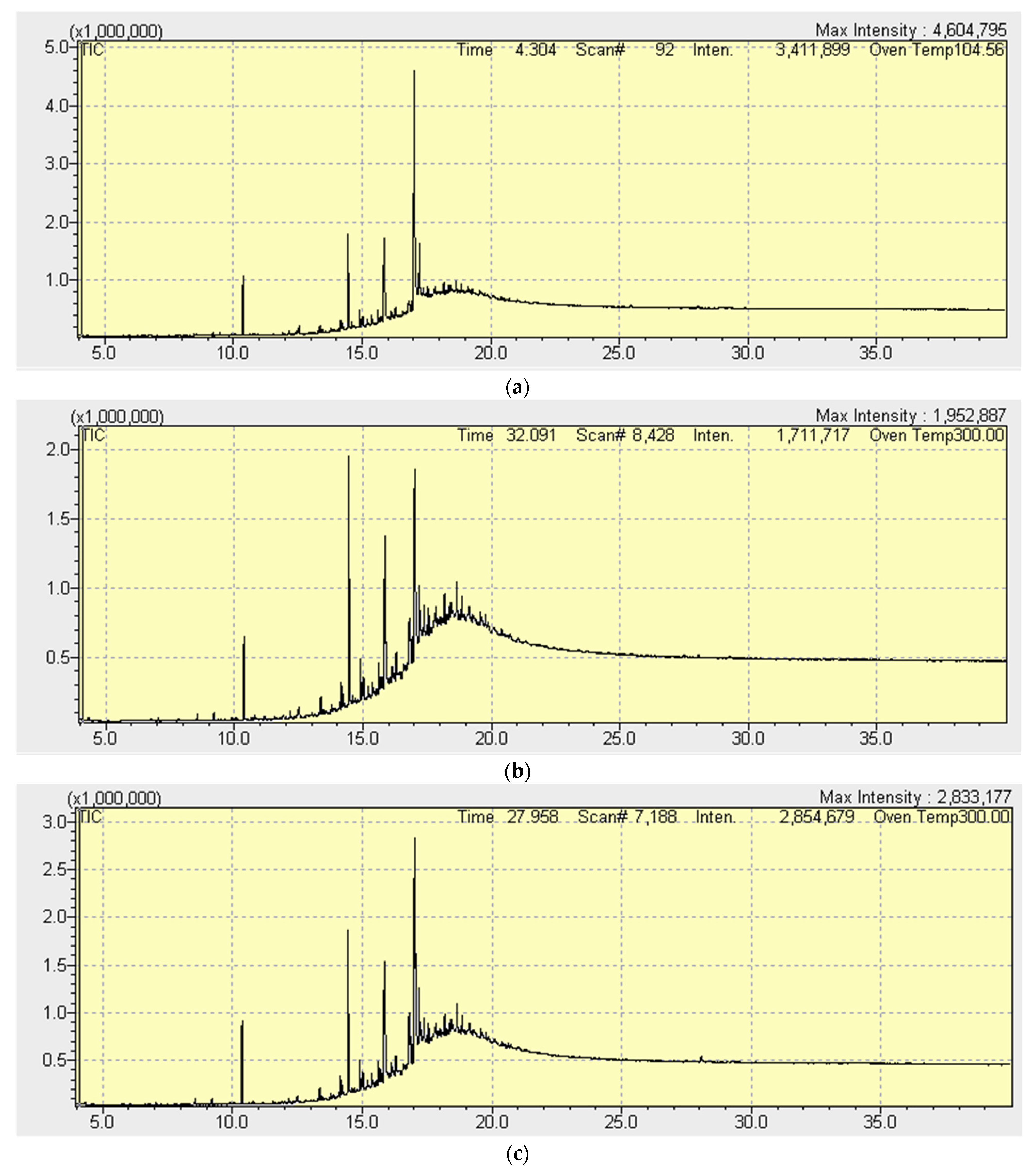
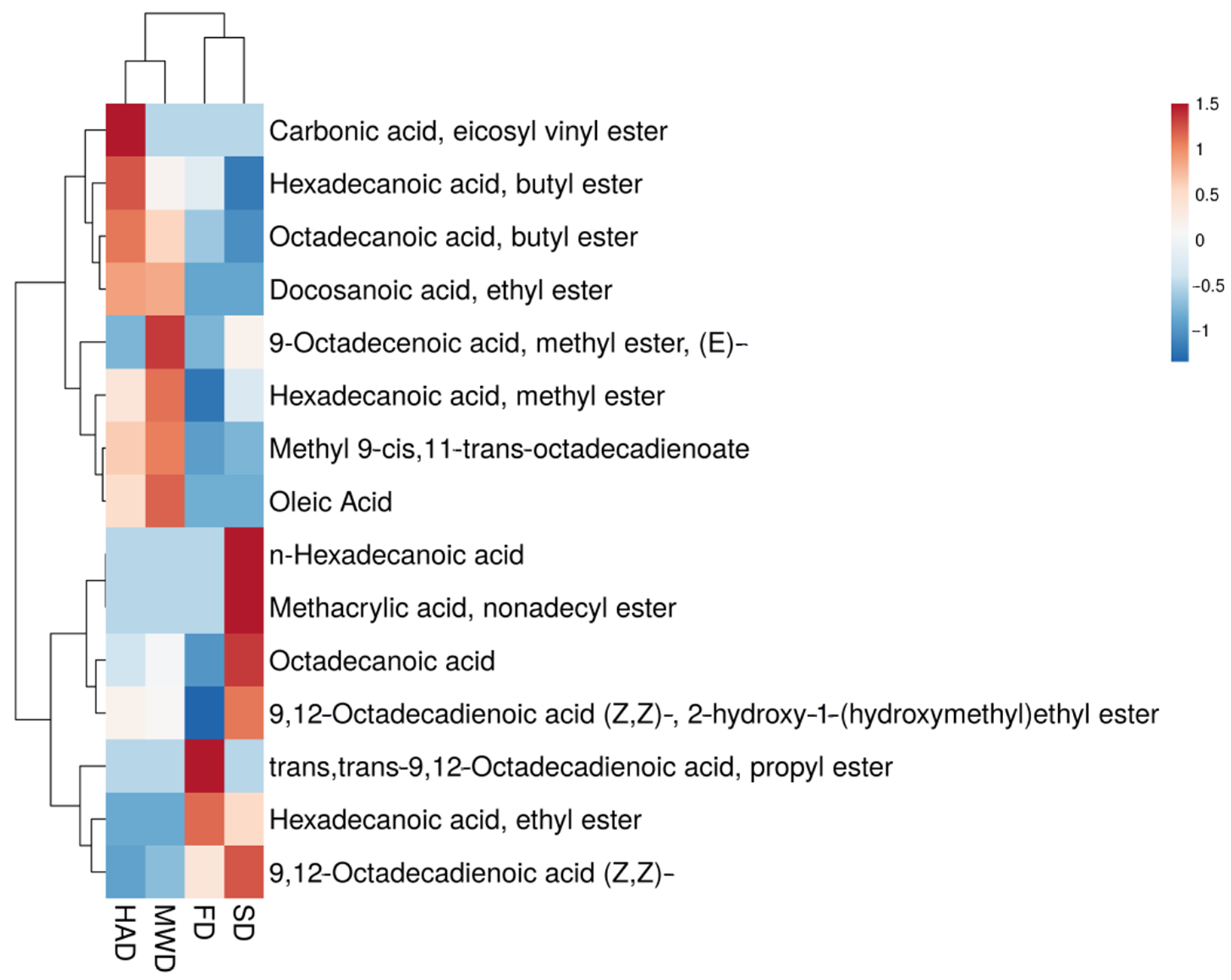
3.3. Gas Chromatographic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, S.A.; Pahmeyer, M.J.; Mehdizadeh, M.; Nagdalian, A.A.; Oboturova, N.P.; Taha, A. Consumer Behavior and Industry Implications. In The Age of Clean Label Foods; Springer: Berlin/Heidelberg, Germany, 2022; pp. 209–247. [Google Scholar] [CrossRef]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef] [PubMed]
- Vardanega, R.; Muzio, A.F.; Silva, E.K.; Prata, A.S.; Meireles, M.A.A. Obtaining functional powder tea from Brazilian ginseng roots: Effects of freeze and spray drying processes on chemical and nutritional quality, morphological and redispersion properties. Food Res. Int. 2018, 116, 932–941. [Google Scholar] [CrossRef]
- Baltacıoğlu, C.; Baltacıoğlu, H.; Seyhan, R.; Uğur, Ö.; Avcu, O. Investigation of the effect of oyster mushroom (Pleurotus ostreatus) powder on biscuit production and effect on quality criteria by Fourier-transform infrared spectroscopy. J. Food Process. Preserv. 2020, 45, e15174. [Google Scholar] [CrossRef]
- Parvin, R.; Farzana, T.; Mohajan, S.; Rahman, H.; Rahman, S.S. Quality improvement of noodles with mushroom fortified and its comparison with local branded noodles. NFS J. 2020, 20, 37–42. [Google Scholar] [CrossRef]
- Majeed, M.; Khan, M.U.; Owaid, M.N.; Shariati, M.A.; Igor, P.; Ntsefong, G.N. Development of oyster mushroom powder and its effects on physicochemical and rheological properties of bakery products. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1221–1227. [Google Scholar] [CrossRef]
- Salehi, F. Characterization of different mushrooms powder and its application in bakery products: A review. Int. J. Food Prop. 2019, 22, 1375–1385. [Google Scholar] [CrossRef]
- Kurt, A.; Gençcelep, H. Enrichment of meat emulsion with mushroom (Agaricus bisporus) powder: Impact on rheological and structural characteristics. J. Food Eng. 2018, 237, 128–136. [Google Scholar] [CrossRef]
- El Attar, A.; Ahmed, N.E.H.; El Soda, M. The effect of Oyster mushroom (Pleurotus ostreatus) as functional food on yoghurt quality. Life Sci. J. 2021, 18, 7–19. [Google Scholar] [CrossRef]
- Wang, L.; Brennan, M.A.; Brennan, C.S. Improving antioxidant capacity of foods: Adding mushroom powder to pasta. Pathology 2020, 289–296. [Google Scholar] [CrossRef]
- Losoya-Sifuentes, C.; Simões, L.S.; Cruz, M.; Rodriguez-Jasso, R.M.; Loredo-Treviño, A.; Teixeira, J.A.; Nobre, C.; Belmares, R. Development and Characterization of Pleurotus ostreatus Mushroom—Wheat Bread. Starch-Stärke 2021, 74, 2100126. [Google Scholar] [CrossRef]
- Lesa, K.N.; Khandaker, M.U.; Iqbal, F.M.R.; Sharma, R.; Islam, F.; Mitra, S.; Bin Emran, T. Nutritional Value, Medicinal Importance, and Health-Promoting Effects of Dietary Mushroom (Pleurotus ostreatus). J. Food Qual. 2022, 2022, 2454180. [Google Scholar] [CrossRef]
- Majesty, D.; Ijeoma, E.; Winner, K.; Prince, O. Nutritional, anti-nutritional and biochemical studies on the oyster mushroom, Pleurotus ostreatus. EC Nutr. 2019, 14, 36–59. [Google Scholar]
- Selvamani, S.; El-Enshasy, H.A.; Dailin, D.J.; Malek, R.A.; Hanapi, S.Z.; Ambehabati, K.K.; Sukmawati, D.; Leng, O.M.; Moloi, N. Antioxidant Compounds of the Edible Mushroom Pleurotus ostreatus. Int. J. Biotechnol. Wellness Ind. 2018, 7, 1–14. [Google Scholar] [CrossRef]
- Goswami, B.; Majumdar, S.; Das, A.; Barui, A.; Bhowal, J. Evaluation of bioactive properties of Pleurotus ostreatus mushroom protein hydrolysate of different degree of hydrolysis. LWT 2021, 149, 111768. [Google Scholar] [CrossRef]
- Dimitrijevic, M.V.; Mitic, V.D.; Nikolic, J.S.; Djordjevic, A.S.; Mutic, J.J.; Jovanovic, V.P.S.; Stojanovic, G.S. First Report about Mineral Content, Fatty Acids Composition and Biological Activities of Four Wild Edible Mushrooms. Chem. Biodivers. 2018, 16, e1800492. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, W.J.A.B.N.; Handunnetti, S.M.; Wanigatunge, C.A.; Fernando, G.H.; Abeytunga, D.T.U.; Suresh, T.S. Anti-Inflammatory Activity of Pleurotus ostreatus, a Culinary Medicinal Mushroom, in Wistar Rats. Evid.-Based Complement. Altern. Med. 2020, 2020, 6845383. [Google Scholar] [CrossRef]
- dos Santos, L.F.; Zanatta, A.L.; Soccol, V.T.; Torres, M.F.; Bonatto, S.J.R.; Rubel, R.; Soccol, C.R. Hypolipidemic and antiatherosclerotic potential of Pleurotus ostreatus, cultived by submerged fermentation in the high-fat diet fed rats. Biotechnol. Bioprocess Eng. 2013, 18, 201–208. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G. Blood glucose lowering and effect of oyster ( Pleurotus ostreatus )- and shiitake ( Lentinus subnudus )-supplemented diet on key enzymes linked diabetes and hypertension in streptozotocin-induced diabetic in rats. Food Front. 2021, 3, 161–171. [Google Scholar] [CrossRef]
- Dicks, L.; Jakobs, L.; Sari, M.; Hambitzer, R.; Ludwig, N.; Simon, M.-C.; Stehle, P.; Stoffel-Wagner, B.; Helfrich, H.-P.; Ahlborn, J.; et al. Fortifying a meal with oyster mushroom powder beneficially affects postprandial glucagon-like peptide-1, non-esterified free fatty acids and hunger sensation in adults with impaired glucose tolerance: A double-blind randomized controlled crossover trial. Eur. J. Nutr. 2021, 61, 687–701. [Google Scholar] [CrossRef]
- Schill, S.; Stessl, B.; Meier, N.; Tichy, A.; Wagner, M.; Ludewig, M. Microbiological Safety and Sensory Quality of Cultivated Mushrooms (Pleurotus eryngii, Pleurotus ostreatus and Lentinula edodes) at Retail Level and Post-Retail Storage. Foods 2021, 10, 816. [Google Scholar] [CrossRef]
- Lee, M.-J.; Seog, E.-J.; Lee, J.-H. Physicochemical Properties of Chaga (Inonotus obliquus) Mushroom Powder as Influenced by Drying Methods. Prev. Nutr. Food Sci. 2007, 12, 40–45. [Google Scholar] [CrossRef]
- Marçal, S.; Sousa, A.S.; Taofiq, O.; Antunes, F.; Morais, A.M.; Freitas, A.C.; Barros, L.; Ferreira, I.C.; Pintado, M. Impact of postharvest preservation methods on nutritional value and bioactive properties of mushrooms. Trends Food Sci. Technol. 2021, 110, 418–431. [Google Scholar] [CrossRef]
- Piskov, S.; Timchenko, L.; Rzhepakovsky, I.; Avanesyan, S.; Bondareva, N.; Sizonenko, M.; Areshidze, D. Effect of pre-treatment conditions on the antiatherogenic potential of freeze-dried oyster mushrooms. Foods Raw Mater. 2019, 7, 375–386. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, L.; Wang, L.; Liu, H. Microstructure-modified products from stone-milled wheat bran powder improve gly-cemic response and sustain colonic fermentation. Int. J. Biol. Macromol. 2020, 153, 1193–1201. [Google Scholar] [CrossRef]
- Yun, P.; Devahastin, S.; Chiewchan, N. Physical properties, microstructure and digestion behavior of amylose-lipid powder complexes prepared using conventional and spray-drying based methods. Food Biosci. 2020, 37, 100724. [Google Scholar] [CrossRef]
- Pascual-Pineda, L.A.; Hernández-Marañon, A.; Castillo-Morales, M.; Uzárraga-Salazar, R.; Rascón-Díaz, M.P.; Flores-Andrade, E. Effect of water activity on the stability of freeze-dried oyster mushroom (Pleurotus ostreatus) powder. Dry. Technol. 2020, 1–14. [Google Scholar] [CrossRef]
- Rahmah, N.L.; Sukardi; Wulantiasari, W.; Wijayanti, N. Physicochemical properties of white oyster mushroom (Pleurotus ostreatus) flavouring powder. IOP Conf. Ser. Earth Environ. Sci. 2020, 443, 012009. [Google Scholar] [CrossRef]
- Piskov, S.; Timchenko, L.; Grimm, W.-D.; Rzhepakovsky, I.; Avanesyan, S.; Sizonenko, M.; Kurchenko, V. Effects of Various Drying Methods on Some Physico-Chemical Properties and the Antioxidant Profile and ACE Inhibition Activity of Oyster Mushrooms (Pleurotus ostreatus). Foods 2020, 9, 160. [Google Scholar] [CrossRef]
- Mutukwa, I.B.; Hall, C.A.; Cihacek, L.; Lee, C.W.; Iii, C.A.H. Evaluation of drying method and pretreatment effects on the nutritional and antioxidant properties of oyster mushroom (Pleurotus ostreatus). J. Food Process. Preserv. 2019, 43, e13910. [Google Scholar] [CrossRef]
- Ucar, T.M.; Karadag, A. The effects of vacuum and freeze-drying on the physicochemical properties and in vitro digestibility of phenolics in oyster mushroom (Pleurotus ostreatus). J. Food Meas. Charact. 2019, 13, 2298–2309. [Google Scholar] [CrossRef]
- Pellegrino, R.M.; Ianni, F.; Blasi, F.; Angelini, P.; Emiliani, C.; Venanzoni, R.; Cossignani, L. Lipidomic profiling of Pleurotus ostreatus by LC/MS Q-TOF analysis. Food Res. Int. 2022, 156, 111335. [Google Scholar] [CrossRef] [PubMed]
- Sande, D.; de Oliveira, G.P.; e Moura, M.A.F.; Martins, B.D.A.; Lima, M.T.N.S.; Takahashi, J.A. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef] [PubMed]
- Keomixay, P.; Nhu, T.T.H.; Van Tai, N.; Thuy, N.M. Effects of drying and grinding in production of healthy vegetarian soup mix. Int. J. Adv. Agric. Sci. Technol. 2019, 6, 29–39. [Google Scholar]
- Ofodile, L.N.; Nicholas-Okpara, N.V.A.; Ani, E.; Ikegwu, E.M.; Saanu, A.; Ezenwa, P.C.; Osorinde, R.T. Production and nutritional composition of juice powder from oyster mushroom Pleurotus ostreatus (Jacq.) Kummer. Funct. Foods Health Dis. 2020, 10, 482. [Google Scholar] [CrossRef]
- Majid, I.; Nanda, V. Effect of sprouting on the physical properties, morphology and flowability of onion powder. J. Food Meas. Charact. 2017, 11, 2033–2042. [Google Scholar] [CrossRef]
- Nagdalian, A.; Rzhepakovsky, I.; Siddiqui, S.; Piskov, S.; Oboturova, N.; Timchenko, L.; Lodygin, A.; Blinov, A.; Ibrahim, S. Analysis of the content of mechanically separated poultry meat in sausage using computing microtomography. J. Food Compos. Anal. 2021, 100, 103918. [Google Scholar] [CrossRef]
- McDougall, G.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red wine – Their stability under simulated gastrointestinal digestion. Phytochemistry 2005, 66, 2540–2548. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Aparna, G.G.; Chauhan, A.K.; Singh, M.; Singh, A. Effect of Dehydration Techniques on Nutritional Quality, Functional Property, and Sensory Acceptability of Dried Onion Powder. J. Food Agric. Res. 2021, 1, 30–46. [Google Scholar]
- Oyinloye, T.M.; Yoon, W.B. Effect of Freeze-Drying on Quality and Grinding Process of Food Produce: A Review. Processes 2020, 8, 354. [Google Scholar] [CrossRef]
- Kotwaliwale, N.; Bakane, P.; Verma, A. Changes in textural and optical properties of oyster mushroom during hot air drying. J. Food Eng. 2007, 78, 1207–1211. [Google Scholar] [CrossRef]
- Piskov, S.I.; Timchenko, L.D.; Rzhepakovsky, I.V.; Avanesyan, S.S.; Sizonenko, M.N.; Areshidze, D.A.; Kovalev, D.A. The influence of the drying method for food properties and hypolidemic potential of oyster mushrooms (Pleurotus ostreatus). Vopr. Pitan. Probl. Nutr. 2018, 87, 65–76. [Google Scholar] [CrossRef]
- Kapoor, R.; Feng, H. Characterization of physicochemical, packing and microstructural properties of beet, blueberry, carrot and cranberry powders: The effect of drying methods. Powder Technol. 2021, 395, 290–300. [Google Scholar] [CrossRef]
- Nejatdarabi, S.; Mohebbi, M. Effect of foam-mat drying condition on physical properties and rehydration behavior of mushroom powder. Iran. Food Sci. Technol. Res. J. 2021, 17, 1–16. [Google Scholar] [CrossRef]
- Forero, D.P.; Carriazo, J.G.; Osorio, C. Effect of different drying methods on morphological, thermal, and biofunctional properties of lulo (Solanum quitoense Lam.) fruit powders. Dry. Technol. 2016, 34, 1085–1094. [Google Scholar] [CrossRef]
- Hitayezu, E.; Kang, Y.-H. Effect of particle size on the physicochemical and morphological properties of Hypsizygus marmoreus mushroom powder and its hot-water extracts. Korean J. Food Preserv. 2021, 28, 504–549. [Google Scholar] [CrossRef]
- Luo, Z.; Zhou, L.; Zhu, Y.; Zhou, C. Effects of different drying methods on the physicochemical property and edible quality of fermented Pyracantha fortuneana fruit powder. Int. J. Food Sci. Technol. 2020, 56, 773–784. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, X.; Islam, N.; Zheng, H.; Li, J.; Liu, F.; Cao, Y.; Dai, Y. Comparison of hydrability, antioxidants, microstructure, and sensory quality of barley grass powder using ultra-micro-crushing combined with hot air and freeze drying. Food Sci. Nutr. 2021, 9, 1870–1880. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Physicochemical properties of whole fruit plum powders ob-tained using different drying technologies. Food Chem. 2016, 207, 223–232. [Google Scholar] [CrossRef]
- Ouaabou, R.; Ennahli, S.; Di Lorenzo, C.; Hanine, H.; Bajoub, A.; Lahlali, R.; Idlimam, A.; Oubahou, A.A.; Mesnaoui, M. Hygroscopic Properties of Sweet Cherry Powder: Thermodynamic Properties and Microstructural Changes. J. Food Qual. 2021, 2021, 3925572. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, A.; Zhang, X.; Liu, H.; Guo, D.; Zhu, Y. Effects of ultrafine grinding on physicochemical, functional and surface properties of ginger stem powders. J. Sci. Food Agric. 2020, 100, 5558–5568. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Zhang, Z.; Pan, H.; Fan, L. Effect of Physical Modification of Mushroom (A. chaxingu) Powders on their Physical and Chemical Properties. Food Sci. Technol. Res. 2014, 20, 731–738. [Google Scholar] [CrossRef]
- Burgain, J.; Petit, J.; Scher, J.; Rasch, R.; Bhandari, B.; Gaiani, C. Surface chemistry and microscopy of food powders. Prog. Surf. Sci. 2017, 92, 409–429. [Google Scholar] [CrossRef]
- Sadowska, A.; Rakowska, R.; Świderski, F.; Kulik, K.; Hallmann, E. Properties and microstructure of blackcurrant powders prepared using a new method of fluidized-bed jet milling and drying versus other drying methods. CyTA J. Food 2019, 17, 439–446. [Google Scholar] [CrossRef]
- Taskin, O.; Izli, G.; Izli, N. Physicochemical and Morphological Properties of European Cranberrybush Powder Manufactured by Freeze Drying. Int. J. Fruit Sci. 2021, 21, 1008–1017. [Google Scholar] [CrossRef]
- Özbek, H.N. Radio frequency-assisted hot air drying of carrots for the production of carrot powder: Kinetics and product quality. LWT 2021, 152, 112332. [Google Scholar] [CrossRef]
- Isik, N.I.E.; Izlin, N. Effect of different drying methods on drying characteristics, colour and microstructure properties of mushroom. J. Food Nutr. Res. 2014, 53, 105–116. [Google Scholar]
- Kong, D.; Wang, Y.; Li, M.; Liu, X.; Huang, M.; Li, X. Analysis of drying kinetics, energy and microstructural properties of turnips using a solar drying system. Sol. Energy 2021, 230, 721–731. [Google Scholar] [CrossRef]
- Forouzanfar, A.; Hojjati, M.; Noshad, M.; Szumny, A. Influence of UV-B Pretreatments on Kinetics of Convective Hot Air Drying and Physical Parameters of Mushrooms (Agaricus bisporus). Agriculture 2020, 10, 371. [Google Scholar] [CrossRef]
- Sadowska, A.; Świderski, F.; Hallmann, E. Bioactive, Physicochemical and Sensory Properties as Well as Microstructure of Organic Strawberry Powders Obtained by Various Drying Methods. Appl. Sci. 2020, 10, 4706. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S. Microstructure and its relationship with quality and storage stability of dried foods. In Food Microstructure and Its Relationship with Quality and Stability; Woodhead Publishing: Cambridge, UK, 2018; pp. 139–159. [Google Scholar] [CrossRef]
- Maray, A.R.M.; Mostafa, M.K.; El-Fakhrany, A.E.-D.M.A. Effect of pretreatments and drying methods on physico-chemical, sensory characteristics and nutritional value of oyster mushroom. J. Food Process. Preserv. 2017, 42, e13352. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Phoukham, K.; Van Ngo, T. Formulation and quality evaluation of pearl oyster mushroom soup powder supplement with some kinds of legumes and vegetables. Acta Sci. Pol. Technol. Aliment. 2015, 19, 435–443. [Google Scholar] [CrossRef]
- Purbowati, P.; Wening, D.K.; Afiatna, P.; Maryanto, S.; Nasifah, I. Instant Porridge with Red Beans (Phaseolus vulgaris L) and Oyster Mushrooms (Pleurotus ostreatus) as A Complementary Feeding. E3S Web Conf. 2021, 317, 04028. [Google Scholar] [CrossRef]
- Arora, B.; Kamal, S.; Sharma, V.P. Nutritional and quality characteristics of instant noodles supplemented with oyster mushroom (P. ostreatus). J. Food Process. Preserv. 2017, 42, e13521. [Google Scholar] [CrossRef]
- Proserpio, C.; Lavelli, V.; Laureati, M.; Pagliarini, E. Effect of Pleurotus ostreatus powder addition in vegetable soup on ß-glucan content, sensory perception, and acceptability. Food Sci. Nutr. 2019, 7, 730–737. [Google Scholar] [CrossRef]
- Srivastava, A.; Attri, B.; Verma, S. Development and evaluation of instant soup premix using oyster mushroom powder. Mushroom Res. 2019, 28, 65–69. [Google Scholar] [CrossRef]
- Jin, B.; Zhou, X.; Li, B.; Lai, W.; Li, X. Influence of In vitro Digestion on Antioxidative Activity of Coconut Meat Protein Hydrolysates. Trop. J. Pharm. Res. 2015, 14, 441. [Google Scholar] [CrossRef][Green Version]
- Adeoye-Isijola, M.O.; Olajuyigbe, O.O.; Jonathan, S.G.; Coopoosamy, R.M. Bioactive compounds in ethanol extract of Len-tinus squarrosulus Mont-a Nigerian medicinal macrofungus. Afr. J. Tradit. Complement. Altern. Med. 2018, 15, 42–50. [Google Scholar] [CrossRef]
- Fayssal, S.A.; El Sebaaly, Z.; Alsanad, M.A.; Najjar, R.; Böhme, M.; Yordanova, M.H.; Sassine, Y.N. Combined effect of olive pruning residues and spent coffee grounds on Pleurotus ostreatus production, composition, and nutritional value. PLoS ONE 2021, 16, e0255794. [Google Scholar] [CrossRef]
- Dong, W.; Hu, R.; Chu, Z.; Zhao, J.; Tan, L. Effect of different drying techniques on bioactive components, fatty acid composition, and volatile profile of robusta coffee beans. Food Chem. 2017, 234, 121–130. [Google Scholar] [CrossRef]
- Tu, X.; Tang, L.; Xie, G.; Deng, K.; Xie, L. Chemical Composition of Aromas and Lipophilic Extracts from Black Morel (Morchella importuna) Grown in China. Mycobiology 2020, 49, 78–85. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Ye, M.; Zhang, Y.; Wu, F.; Fu, M.; Sun, F.; Liu, X. Effect of seed size and drying temperature on the hot air-drying kinetics and quality of Chinese hickory (Caryacathayensis) storage. J. Food Process. Preserv. 2021, 45, e15488. [Google Scholar] [CrossRef]
- Bhasin, A.; Sharma, S.; Kapoor, S.; Garg, R. Fatty acid composition, mineral content and storage stability of UV treated Button mushroom powder. Think India J. 2019, 22, 1472–1484. [Google Scholar]
- Uribe, E.; Lemus-Mondaca, R.; Vega-Gálvez, A.; Zamorano, M.; Quispe-Fuentes, I.; Pasten, A.; Di Scala, K. Influence of process temperature on drying kinetics, physicochemical properties and antioxidant capacity of the olive-waste cake. Food Chem. 2014, 147, 170–176. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Ghaly, M.F.; Fahmi, S.M. Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J. Basic Microbiol. 2021, 61, 557–568. [Google Scholar] [CrossRef]
- Akpuaka, A.; Ekwenchi, M.M.; Dashak, D.A.; Dildar, A. Biological activities of characterized isolates of n-hexane extract of Azadirachta indica A. Juss (Neem) leaves. Nat. Sci. 2013, 11, 141–147. [Google Scholar]
- Chenniappan, J.; Sankaranarayanan, A.; Arjunan, S. Evaluation of Antimicrobial Activity of Cissus quadrangularis L. stem extracts against Avian Pathogens and Determination of its Bioactive Constituents using GC-MS. J. Sci. Res. 2020, 64, 90–96. [Google Scholar] [CrossRef]
- Musa, A.M.; Ibrahim, M.A.; Aliyu, A.B.; Abdullahi, M.S.; Tajuddeen, N.; Ibrahim, H.; Oyewale, A.O. Chemical composition and antimicrobial activity of hexane leaf extract of Anisopus mannii (Asclepiadaceae). J. Intercult. Ethnopharmacol. 2015, 4, 129–133. [Google Scholar] [CrossRef]
- Kumari, R.; Mishra, R.C.; Yadav, S.; Yadav, J.P. Exploring Molecular Docking Studies of Alanine Racemase Inhibitors from Elettaria cardamomum. Curr. Enzym. Inhib. 2019, 15, 91–102. [Google Scholar] [CrossRef]
- Radke, E.G.; Braun, J.M.; Meeker, J.D.; Cooper, G.S. Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ. Int. 2018, 121, 764–793. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Silano, V.; Baviera, J.M.B.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; et al. Update of the risk assessment of di-butylphthalate (DBP), butyl-benzyl-phthalate (BBP), bis(2-ethylhexyl)phthalate (DEHP), di-isononylphthalate (DINP) and di-isodecylphthalate (DIDP) for use in food contact materials. EFSA J. 2019, 17, e05838. [Google Scholar] [CrossRef]
- Krichen, F.; Hamed, M.; Karoud, W.; Bougatef, H.; Sila, A.; Bougatef, A. Essential oil from pistachio by-product: Potential biological properties and natural preservative effect in ground beef meat storage. J. Food Meas. Charact. 2020, 14, 3020–3030. [Google Scholar] [CrossRef]
- Jung, H.-J.; Song, Y.-S.; Lim, C.-J.; Park, E.-H. Evaluation on Pharmacological Activities of 2,4-Dihydroxybenzaldehyde. Biomol. Ther. 2009, 17, 263–269. [Google Scholar] [CrossRef][Green Version]
- Bensaad, M.S.; Dassamiour, S.; Hambaba, L.; Kahoul, M.A.; Sami, R.; Al Masoudi, L.M.; Al-Mushhin, A.A.M.; Benajiba, N. Chemical Profile by Gas Chromatography/Mass Spectrometry of Ethyl Acetate and N-butanol Extracts of Centaurea tougourensis Boiss. & Reut. J. Biobased Mater. Bioenergy 2022, 16, 140–149. [Google Scholar] [CrossRef]
- Salim, S.A. Identification of Active Pharmaceutical Ingredients in Thevetia neriifolia Juss Leaf Callus using Analysis of GC-MS. Indian J. Public Heal. Res. Dev. 2018, 9, 1019. [Google Scholar] [CrossRef]
- Khatiwora, E.; Adsul, V.B.; Kulkarni, M.; Deshpande, N.R.; Kashalkar, R.V. Antibacterial activity of Dibutyl Phthalate: A secondary metabolite isolated from Ipomoea carnea stem. J. Pharm Res. 2012, 5, 150–152. [Google Scholar]
- Rhetso, T.; Shubharani, R.; Roopa, M.S.; Sivaram, V. Chemical constituents, antioxidant, and antimicrobial activity of Allium chinense G. Don. Futur. J. Pharm. Sci. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Rajisha, K.; Fernandes, J. Identification of compounds from different fractions of Exacum Bicolor Roxb. by GC-MS analysis. Plant Arch. 2020, 20, 4531–4536. [Google Scholar]
- Ramya, B.; Malarvili, T.; Velavan, S. GC-MS analysis of bioactive compounds in Bryonopsis laciniosa fruit extract. Int. J. Pharm. Sci. Res. 2015, 6, 3375. [Google Scholar] [CrossRef]
- Banakar, P.; Jayaraj, M. GC-MS analysis of bioactive compounds from ethanolic leaf extract of Waltheria indica Linn. and their pharmacological activities. Int. J. Pharm Sci. Res. 2018, 9, 2005–2010. [Google Scholar] [CrossRef]
- Albratty, M.; Alhazmi, H.A.; Meraya, A.M.; Najmi, A.; Alam, M.S.; Rehman, Z.; Moni, S.S. Spectral analysis and Antibacterial activity of the bioactive principles of Sargassum tenerrimum J. Agardh collected from the Red sea, Jazan, Kingdom of Saudi Arabia. Braz. J. Biol. 2021, 83, e249536. [Google Scholar] [CrossRef] [PubMed]
- Sargunam, J.H.; Thilakavathy, S. GCMS Profile of Bioactive Compounds with Therapeutic Potential in Beta vulgaris (L.) Ethanolic Leaf Extracts. J. Pharm. Res. Int. 2021, 354–360. [Google Scholar] [CrossRef]



| Name of the Compounds | Molecular Formulae | Relative Peak Area (%) | |||
|---|---|---|---|---|---|
| FD | HAD | MWD | SD | ||
| Isobornyl acetate | C12H20O2 | 4.11 | 3.46 | 3.94 | 4.94 |
| Butylated hydroxytoluene | C15H24O | 0.58 | nd | nd | nd |
| Hexadecane | C16H34 | 0.50 | 0.71 | 0.57 | nd |
| Heptadecane | C17H36 | 0.88 | 1.16 | 0.97 | 1.21 |
| Sulfurous acid, cyclohexylmethyl heptyl ester | C14H28O3S | 6.59 | 11.94 | 7.6 | 5.37 |
| Hexadecanoic acid, methyl ester | C17H34O2 | 0.39 | 0.71 | 0.86 | 0.58 |
| 3,5-di-tert-Butyl-4-hydroxybenzaldehyde | C15H22O2 | 1.56 | nd | nd | nd |
| 1-Heptanal, 3,5,5-triethyl- | C13H26O | nd | 1.03 | 0.91 | 0.52 |
| Hexadecane, 2,6,10,14-tetramethyl- | C20H42 | 0.70 | 0.96 | 2.43 | 0.47 |
| Tetratetracontane | C44H90 | nd | 5.0 | 5.03 | nd |
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | C16H22O4 | 0.5 | nd | 0.34 | 0.47 |
| Benzoic acid, 2-hydroxy-, phenylmethyl ester | C14H12O3 | 0.71 | 0.57 | 0.58 | 0.94 |
| Heneicosane | C21H44 | 5.03 | 5.76 | 4.98 | 0.59 |
| 7,7-Diethylheptadecane | C21H44 | nd | nd | nd | 0.54 |
| Docosanoic acid, ethyl ester | C24H48O2 | nd | 0.92 | 0.89 | nd |
| 1-Eicosanol | C20H42O | 0.91 | nd | nd | nd |
| Dibutyl phthalate | C16H22O4 | 10.15 | 10.61 | 10.53 | 6.55 |
| Decane, 5,6-Bis(2,2-dimethylpropylidene)-, (E,Z)- | C20H38 | nd | 0.67 | 0.55 | nd |
| Nonadecane, 4-methyl- | C20H42 | 0.8 | nd | nd | nd |
| Hexadecanoic acid, ethyl ester | C18H36O2 | 0.77 | nd | nd | 0.53 |
| 1-Eicosanol, 2-hexadecyl- | C36H74O | 0.62 | nd | nd | nd |
| Methyl 9-cis,11-trans-octadecadienoate | C19H34O2 | 1.33 | 2.88 | 3.29 | 1.48 |
| Tridecane, 7-cyclohexyl- | C19H38 | 0.73 | nd | nd | nd |
| 9,12-Octadecadienoic acid (Z,Z)- | C18H32O2 | 28.85 | 9.39 | 12.52 | 41.42 |
| 17-Pentatriacontene | C35H70 | nd | 1.61 | nd | nd |
| Cyclohexadecane, 1,2-diethyl- | C20H40 | nd | 1.16 | nd | nd |
| Octadecanoic acid | C18H36O2 | 3.22 | 4.1 | 4.75 | 6.68 |
| Cyclopentane, heneicosyl- | C26H52 | nd | 0.78 | nd | nd |
| Heneicosane, 11-decyl- | C31H64 | nd | 1.38 | nd | nd |
| Oleic acid | C18H34O2 | nd | 5.86 | 9.01 | nd |
| Pentatriacontane | C35H72 | nd | 0.95 | 0.34 | nd |
| Eicosane | C20H42 | nd | 4.46 | 1.25 | 0.35 |
| Octadecane | C18H38 | nd | 0.54 | 1.58 | 0.57 |
| trans,trans-9,12-Octadecadienoic acid, propyl ester | C21H38O2 | 6.99 | nd | nd | nd |
| Tetracosane | C24H50 | nd | nd | nd | 0.56 |
| 9,12-Octadecadienoic acid (Z,Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester | C21H38O4 | nd | 3.44 | 3.26 | 5.48 |
| 2-Methyltetracosane | C25H52 | 1.88 | 0.92 | nd | nd |
| Hexadecanoic acid, butyl ester | C20H40O2 | 2.48 | 4.08 | 2.88 | 1.36 |
| Cyclopentane, decyl- | C15H30 | nd | nd | 0.71 | 0.89 |
| n-Heptadecylcyclohexane | C23H46 | nd | nd | nd | 1.53 |
| Erythro-9,10-dibromopentacosane | C25H50Br2 | nd | nd | nd | 1.06 |
| n-Pentadecylcyclohexane | C21H42 | 2.57 | 1.67 | nd | nd |
| 2-Methyloctacosane | C29H60 | 1.48 | nd | nd | nd |
| Tridecane, 3-cyclohexyl- | C19H38 | 2.81 | nd | nd | nd |
| Pentadecane, 2,6,10,14-tetramethyl- | C19H40 | 1.42 | 0.6 | 0.57 | 0.45 |
| Heptadecane, 3-methyl- | C18H38 | 1.62 | nd | nd | nd |
| 2-Methylhexacosane | C27H56 | 4.62 | 2.88 | 5.81 | nd |
| Methacrylic acid, nonadecyl ester | C23H44O2 | nd | nd | nd | 0.78 |
| Carbonic acid, eicosyl vinyl ester | C23H44O3 | nd | 1.38 | nd | nd |
| Bis(2-ethylhexyl) phthalate | C24H38O4 | nd | 0.6 | nd | nd |
| Tetrapentacontane, 1,54-dibromo- | C54H108Br2 | 1.74 | nd | nd | nd |
| Octadecanoic acid, butyl ester | C22H44O2 | 0.88 | 1.85 | 1.55 | 0.65 |
| 1-Decanol, 2-octyl- | C18H38O | nd | nd | nd | 0.88 |
| Diisooctyl phthalate | C24H38O4 | nd | nd | nd | 0.54 |
| N,N-dimethyldodecanamide | C14H29NO | nd | nd | nd | 0.71 |
| 9-Octadecenoic acid, methyl ester, (E)- | C19H36O2 | nd | nd | 1.83 | 0.8 |
| 4-t-Butyl-2-(1-methyl-2-nitroethyl)cyclohexanone | C13H23NO3 | nd | nd | nd | 0.52 |
| n-Hexadecanoic acid | C16H32O2 | nd | nd | nd | 7.58 |
| Anthracene | C14H10 | nd | nd | nd | 1.19 |
| Isopropyl myristate | C17H34O2 | 0.38 | nd | 0.35 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piskov, S.; Timchenko, L.; Avanesyan, S.; Siddiqui, S.A.; Sizonenko, M.; Kurchenko, V.; Rzhepakovsky, I.; Blinov, A.; Nagdalian, A.; Shariati, M.A.; et al. A Comparative Study on the Structural Properties and Lipid Profile of Mushroom (Pleurotus ostreatus) Powder Obtained by Different Drying Methods. Agriculture 2022, 12, 1590. https://doi.org/10.3390/agriculture12101590
Piskov S, Timchenko L, Avanesyan S, Siddiqui SA, Sizonenko M, Kurchenko V, Rzhepakovsky I, Blinov A, Nagdalian A, Shariati MA, et al. A Comparative Study on the Structural Properties and Lipid Profile of Mushroom (Pleurotus ostreatus) Powder Obtained by Different Drying Methods. Agriculture. 2022; 12(10):1590. https://doi.org/10.3390/agriculture12101590
Chicago/Turabian StylePiskov, Sergey, Lyudmila Timchenko, Svetlana Avanesyan, Shahida Anusha Siddiqui, Marina Sizonenko, Vladimir Kurchenko, Igor Rzhepakovsky, Andrey Blinov, Andrey Nagdalian, Mohammad Ali Shariati, and et al. 2022. "A Comparative Study on the Structural Properties and Lipid Profile of Mushroom (Pleurotus ostreatus) Powder Obtained by Different Drying Methods" Agriculture 12, no. 10: 1590. https://doi.org/10.3390/agriculture12101590
APA StylePiskov, S., Timchenko, L., Avanesyan, S., Siddiqui, S. A., Sizonenko, M., Kurchenko, V., Rzhepakovsky, I., Blinov, A., Nagdalian, A., Shariati, M. A., & Ibrahim, S. A. (2022). A Comparative Study on the Structural Properties and Lipid Profile of Mushroom (Pleurotus ostreatus) Powder Obtained by Different Drying Methods. Agriculture, 12(10), 1590. https://doi.org/10.3390/agriculture12101590









