Abstract
The study aimed to investigate the effect of the combination of year and season of breeding period, egg weight, the annual hatching order of chicks and the hatchability of eggs on the relative risk of total loss for chicks during their 48-week life period. The examination was conducted on one of the largest farms in Hungary working with sixty breeding birds, kept in trios (one male and two females). The research covered the growing information of 1606 chicks hatched in 2019, 2020 and 2021. The highest relative risk of total loss was revealed in autumn 2020 and 2021. In comparison with the reference group (2021 summer), the relative risk of total loss was significantly lower in summer 2019 (p < 0.05) and spring 2020 (p = 0.0049) 2020 (p = 0.0000) and 2021 (p = 0.0348) spring. Both between years and between seasons the tendency of relative risk was the same. The relative risk of total loss increased until the end of the third quart of the incubation period, then remained unchanged. Chicks from groups having weak hatchability (≤50%) had the highest relative risk (p < 0.05).
Keywords:
ostrich; chick; 48 weeks of age; survival analysis; year effect; month effect; hatchability 1. Introduction
Even though the ostrich (Struthio camelus) was domesticated in the 19th century [1] and has been living in the livelihood of humans, little is known about the exact needs of this species [2], and there is still a lack of information on the appropriate husbandry, feeding and incubation technology.
The embryo and chick mortality is extremely high (46.7 66.7%), as reported by many authors [3,4,5,6]. Cloete et al. [3] reported that chick mortality was 46.6% before four weeks of age and showed 30.7% between four and twelve weeks of age. This situation is similar in different continents. Smith et al. [7] found 50% mortality for three-month-old chicks in South Africa. In Australia, the mortality is 37% for three-month-old chicks and 15–50% in Israel [8]. In Europe, the average mortality rate is around 50% for ostrich chicks under four months of age [9].
Stress and diseases are the most sensitive factors for the chicks, especially during the first week of age, even up until two months of age. Accordingly, regular health monitoring is inevitable [5,10]. The heritability of ostrich chick livability is 10% [11], therefore the environmental factors, such as the mitigation of stress and, the appropriate husbandry conditions, stocking density, hygiene and feeding play key roles in the protection of chick health [12,13]. In South Africa, the breeding period starts at the onset of June and ends in late January. Brand et al. [14] found that the vitality of chicks hatched in winter was better compared to those hatched in summer and autumn. As the laying season proceeds, the depletion of the females’ nutritional reserves increases [15], which can decrease the egg production and the hatchability [16]. However, Cloete et al. [3] found that the beginning and the end of the laying season were the riskiest periods for mortality. In addition, the quality and survival of the hatched chicks depend on the feeding, hygiene, egg storage and incubation conditions [4]. Egg characteristics such as egg weight, shell thickness, structure and porosity indirectly affect the hatchability and the chick quality [17]. The higher than 18% water loss of eggs during incubation and the different than optimal (820–950 g) chick weight can be risk factors [3,18]. According to Tona et al. [19], there is no relationship between hatchability and chick vitality. However, Deeming [20] claimed that chicks hatched of higher quality have a better post-hatch performance. The sex of chicks, the incubation length or the nutrition of breeder animals had less effect on survival [3].
In Hungary ostriches have been kept for three decades, and the mortality rate is also high causing serious economic losses to the breeder. In our country, there are also many factors influencing the survival of chicks, of which we had some to evaluate. The year and season with different climatic effects can impact the vitality and survivability of chicks. Since incubators are disinfected only at the first incubation of each year in our situation, the effect of hatching order also needs to be investigated. The hatchability of a batch could be used as an indicator of fertility, embryo survival, hygiene and incubation conditions that influence chick vitality and survival. The study aimed to analyze the effects of the year and the season of the breeding period, the egg weight, the hatching (weekly batches) and the hatchability on the survival of ostrich chicks during their growing period from hatching to 48 weeks of age.
2. Materials and Methods
2.1. The Evaluated Farm
The study was carried out on a large farm in the central part of East Hungary, in Jász-Nagykun-Szolnok County, working with more than sixty breeding animals, kept in trios (one male with two females). The primary product of the farm is meat from slaughtered animals. The slaughtering age of ostriches on the farm varies but is generally at 48 weeks of age. Regarding the genotype, Zimbabwean blue-necked ostriches are bred at 90% and some South African black-necked can also be found on the farm.
2.2. Egg Production and Hatchability in the Three Evaluated Years
Table 1 presents the egg production and hatchability of eggs during the years 2019, 2020 and 2021. The number of trios differed in the three examined years, and it was the lowest in 2019; five more in 2020; and decreased by three in 2021. The length of the breeding period was the longest in 2020 and the shortest in 2021. In 2019, the breeding period started on 6 March and ended on 6 October. Compared to 2019, in 2020, the breeding period started earlier, on 1 February and lasted longer, until 8 October. In 2021, the breeding period started on 2 March and ended on 26 September. The earliest onset of the breeding period in 2020 can be explained by the mildest weather in winter in that year compared to 2019 and 2021. Conversely, the latest start of production in 2019 could be due to the coldest winter in that year. The earliest end of the breeding period in 2021 could be the result of the lowest monthly mean temperatures in September and October. The annual egg production was the highest in 2020 and the number of eggs produced was the lowest in 2019. Most eggs were incubated in 2020 and the least eggs were considered appropriate for setting in 2019. The selection criteria for hatching eggs are a clean, intact, and plain eggshell surface and a normal egg shape that is typical of the species. In 2020, the number of eggs that hatched was the highest and in 2019, it showed the lowest number. In 2019, we could observe the best hatchability and it was the weakest in 2021.

Table 1.
Egg production and hatchability in the examined years.
2.3. Climatic Information on the Evaluated Years
The mean annual temperature was the highest in 2019, being 0.84 °C higher than in 2021 and 0.19 °C higher than in 2020. Small differences in the mean annual humidity could be observed, being relatively lower in 2019 compared to 2020 and 2021 (Table 2). In January, the mean monthly temperature was the highest in 2021 compared to the earlier years. However, in 2020, the breeding period started earlier than in 2021. Even though the mean temperature was lower in October 2020 than in October 2019, the breeding period lasted two further days. The lower temperature in October 2021 resulted in the fact that the breeding period ended earlier compared to 2019 and 2020 (Table 1). The smallest month-by-month changes were similar in all the examined years (2019—4.22 °C, 2020—4.02 °C, 2021—4.37 °C). In April 2020, the relative humidity was especially low compared to all other data and that year October should be highlighted as presenting one of the highest percentages. The seasonal variation of temperature was as follows. The mean temperature in winter was 0.34 °C in 2019, 1.51 °C in 2020 and 0.78 °C in 2021. The spring was the coldest in 2021 (seasonal mean: 9.57 °C) and that autumn was also 1 °C lower than in 2019 and 2020. The summer in 2019 was similar to that in 2021, however, in summer 2020 we could demonstrate the lowest mean temperature of all. The highest mean relative humidity was observed in winter 2021(87.25%) and the lowest was shown in spring 2020 (58.47%).

Table 2.
The mean temperature (°C) and humidity (%) in the three examined years by seasons and months.
2.4. Feeding and Husbandry System of Breeders
In Hungary, the breeding period usually starts in mid-March and ends in mid-September, depending on the annual weather. The preparation of breeding animals for the laying season started in December when the quantity of feed was increased from 1.3 kg/bird/day to 1.8 kg/bird/day. From March and April—as the frost ceased—the ratio was 1.3 kg/bird/day. The quantity and composition of the feed were constant during the laying period. From September until February, it was the pre-laying feed, after that, the laying feed were provided for the breeders. Both kinds of feed were composed of 80% fodder and 20% grains (maize, wheat, oat and barley). The pre-laying fodder contained 16% raw protein, 2.6% raw fat, 10.10% fiber, 1.4% Ca, 0.77% P, 0.18% Na, 0.69% lysine, 0.35% methionine, 10 000 NE/kg vitamin-A, 3200 NE/kg vitamin-D3 and 45 mg/kg vitamin-E. The laying fodder contained 17% raw protein, 3% raw fat, 10.10% fiber, 2.5% Ca, 0.77% P, 0.18% Na, 1.00% lysine, 0.48% methionine, 10 000 NE/kg vitamin-A, 3200 NE/kg vitamin-D3, and 145 mg/kg vitamin-E. In colder weather, the ratio of maize was higher compared to other grains. Apart from the fodder and grains, alfalfa hay was also given to the birds at a 5 kg/bird/day ratio. Vitamin supplementation was started in January until August. Chicktonic, Aniselene, Phylamic and Tetravit products were used for vitamin and microelement supplementation, dissolved in drinking water. Apple vinegar and sunflower oil were also given in the case of digestive problems. The quantity and composition of feed were the same in the three examined years.
Pens were a size of 300 m2 with shelters of 15 m2. The feeding and husbandry technology was the same during the three years.
Breeding animals from two to eight years were involved in the study, however, we could not exactly identify which trio and breeder the eggs and chicks stemmed from. The relationship between the birds is not known, but was not excluded either.
2.5. Egg Collection and Storage
Eggs were collected daily in the evenings and were stored horizontally at 16 °C in a storage room for a maximum of seven days. After collection, dry eggs were cleaned with a sponge and then sprayed with Virocid 1% solution. Eggs were turned 180°, two or three times a day.
2.6. Incubation Conditions
Eggs were set in the incubator weekly and the incubators were continuously working during the whole breeding period each year. Three STR 120 incubators were used for the incubation with twelve trays, with ten eggs on each. During the three-year period, the same three incubators were applied with the same technology. Eggs were placed vertically in the incubator with the air chamber upwards. Eggs were incubated at 36.6 °C and 26–27% relative humidity without any change during the incubation period. The ventilation was continuous, and eggs were turned 90° every two hours. Disinfection was conducted only at the first incubation event. The incubator was filled continuously from the bottom to the top. Candling was performed weekly, eggs with non-developing embryos were culled. We did not have information on the egg weight loss.
The hatcher could manage sixty eggs in eight boxes. On the 38th day of incubation, the eggs were candled and those having live embryos were transferred to the hatcher. The hatchery was disinfected weekly with Biovet 2% wide-range solution and 1% Virocid solution before every placement of the eggs. Right after hatching, the navel of the chicks was disinfected and chicks were marked with a leg ring. For navel disinfection, Betadine solution was used in 2019, while Alamycin was applied in 2021. After hatching, chicks spent some days in the hatcher to dry and to enable navel closure.
For the first time, water and feed were provided for the chicks at one week of age. Until the age of eight weeks, the starter feed was added; from the age of nine weeks to the eleven weeks of age, both the starter and grower diet were given (Table 3). Between the ages of 12 and 24 weeks, only the grower diet was supplied. From the age of 25 weeks until the 52 weeks, a finisher was given for the slaughter birds and the pre-breeder diet was fed from 25th weeks of age until maturation for breeders. Dried leaves of nettle and lemongrass were also fed to the one- and two–week-old chicks. Until the age of 11 months, probiotics and joint protectors were also added to their drinking water. Cut alfalfa hay was given from the age of 12 weeks.

Table 3.
Feeding and husbandry system of chicks.
In 2019 and 2020, stables with zeolite deep litter were used for all ages. At the age of one week, 40 to 50 chicks were placed in each pen on the ground. From 2021, battery cages were used until twelve weeks of age. Each hatching group was kept on different batteries. It was possible to rear a maximum of twenty chicks on batteries independent of the chicks’ age. The size of the batteries was increased in proportion to the age and size of the chicks. One- and two-week-old chicks were in the same stable but kept in different pens and battery cages. For the one-week-old chicks, the temperature in the stable was 28 to 30 °C. From the age of three weeks, chicks were transported to another stable for two weeks. From three weeks to six weeks, chicks were kept in a stable with a yard at 22–23 °C. After six weeks of age, they resided outside the pens. Battery cages for one- and two-week-old chicks were placed higher (one meter from the ground), while for the three-week-old chicks they were lower (15–20 cm from the ground) to facilitate management. Battery cages were washed with high-pressure machines and disinfected with Virocid 1% solution.
From the third week, chicks spent one or two weeks on each battery cage depending on the weather and the stocking density. From the fifth week, chicks were allowed to go to a yard but closed in for the night. The farm applied insect and rodent control.
2.7. Data Analysis
Data for the analyses were provided by the owner. A total of 1606 chicks was examined during the three-year research period. Only those batches (weekly hatching groups) were analyzed in which there were at least ten chicks alive at 48 weeks of age. This is why the number of chicks hatched in the three examined years (in the whole population) was larger than the size of the studied group. As our aim was to evaluate the growing period of ostriches, the monitoring of chicks was concluded at 48 weeks of age, as the international literature points out that the earliest age that ostriches can reach the optimal slaughter yield is at 48 weeks (12 months) of age [21]. Animals finishing the growing period or still alive at the end of the data collection were censored, with a total of 38.36% right-censored records.
The following factors were taken into account during the analyses:
- -
- The combination of year and season (year-season) of egg production;
- -
- Hatching egg weight (small: <1430 g; medium: 1431–1456 g; large: >1457 g). The egg weight categories were set according to the number of elements in one group. The analysis was based on the dataset of the farm. Being non-significant (p = 0.1019, this factor was not included in the model;
- -
- The hatching order increased by week due to the weekly egg incubation. However, some are missing since only those with at least ten chicks that survived were used in the model. Altogether 20 groups were analyzed. Since the incubation period, depending on the onset of egg production, started on different dates in the three examined years, it will later be referred to as “hatching order in the year”.
- -
- Hatchability (weak: ≤50%, average: 51–70%, good: ≥70%), including all examined years and groups. The hatchability was calculated by the following formula: hatching %
The survival analysis was carried out with the Survival Kit using the following piecewise Weibull proportional hazards model [22]:
where, = hazard function (instantaneous probability of culling) for an ostrich chick at time t; = Weibull baseline hazard function with scale parameter λ; = the fixed time-independent effect of the combination of year and season of egg production; = the fixed time-independent effect of the hatching egg weight; = the fixed time-independent effect of the hatching order-batches; = the fixed time-independent effect of hatchability.
The effect of the different factors (year and season of production, egg weight, hatching order in the year and hatchability) on the relative risk of total loss was estimated using the Weibull model [22]. The risk ratios showed the relative risk of total loss, including mortality and culling due to serious injuries or illness, compared with the reference class (where the risk ratio = 1.00). The relative risk of total loss indicates the probability of a population being dead or culled in comparison with the reference group. Egg production and hatchability results were calculated using the different mathematical functions of the Microsoft Office Excel program.
3. Results
The life of censored individuals ranged between 19 and 48 weeks with a mean of 24.28 weeks (Table 4). The minimum life of uncensored individuals was remarkably shorter than that of the censored ones. The maximum life showed a slightly lower value for uncensored birds compared to censored animals, while the mean value was very similar in both cases.

Table 4.
The minimum, average and maximum life of censored and uncensored individuals (week).
Table 5 shows the p-value of factors as effects on the relative risk of total loss. The year-season, hatching order and hatchability showed p-values lower than 0.05, so the effect of these factors on the relative risk of total loss was considered significant (Table 5). The egg weight took on a p-value of 0.1019 and as a non-significant factor, it was excluded from the model and further analysis.

Table 5.
The p-values of factors affecting the relative risk of chick survival.
Figure 1 shows the influence of the combination of the year and season of production on the survival of chicks until the age of 48 weeks. The relative risk of loss which is the number of birds that died or were culled under 48 weeks of age was the highest in autumn 2020 and 2021 and the lowest in summer 2019 and spring 2020. In comparison with the reference group (2021 summer), the relative risk of total loss was significantly lower in summer 2019 and 2020 and spring 2020 and 2021. Between years in spring, the tendency of relative risk of total loss decreased from 2019 to 2020, and then increased by 2021. Regarding summer, the relative risk of total loss revealed the lowest value in 2019 and showed an increasing tendency in the following years. The relative risk of total loss for autumn was the lowest in 2019 but it was extremely high in 2020 and 2021. In summary, it means that in those years and months that are described with significantly high relative risk, chicks have a higher probability of dying or being culled. Within years between seasons, we can suggest the same tendency as it was presented between years.
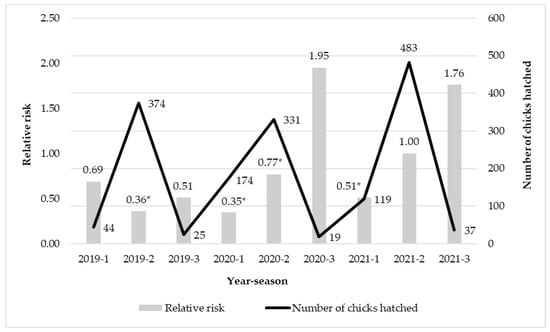
Figure 1.
The effect of the year of the breeding period on the relative risk of total loss. The seasons are numbered: -1: spring, -2: summer, -3: autumn. * Statistically significant difference from the reference group (p ≤ 0.05).
Figure 2 presents the effect of the annual hatching batch order on the relative risk of total loss (being culled or die) until the 48th weeks of age. It can be stated that the relative risk showed an increasing tendency until the 16th week of incubation/batch (Figure 3). Week 17 was considered the reference group, having 1.00 as relative risk. After the 17th week, the relative risk did not change. The relative risk had a similar tendency as the hatching rate. The more chick hatched, the higher the risk of loss before the 48th week was. This means that the higher the number of chicks that had to be nursed in a batch, the more likely were the chicks to be culled or died.
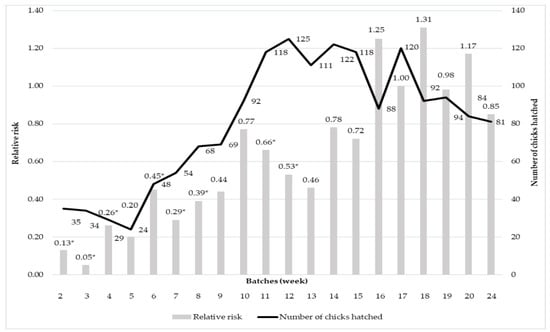
Figure 2.
The effect of hatching order in the year on the relative risk of total loss. * Statistically significant difference from the reference group (p ≤ 0.05).
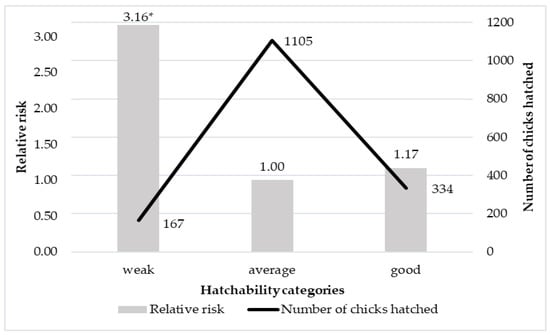
Figure 3.
The effect of the mean hatchability of the batch on the relative risk of total loss. * Statistically significant difference from the reference group (p ≤ 0.05).
Figure 3 shows the effect of the different hatchability results of eggs on the relative risk of the total chick loss (being culled or die). Chicks from groups with weak hatchability (≤50%) had the highest relative risk of losses (Figure 3). However, the average hatchability was the most advantageous, resulting in not significantly but lower risk than good and a significantly lower risk than weak hatchability. This means that chicks hatched in a weak batch were more likely to be culled or die before the 48th week than the average and good hatching ones.
4. Discussion
In the study, we used a 95% confidence interval for the estimation of relative risk. Only factors showing significant effects (p ≤ 0.05) on the risk ratios are presented and discussed in depth (Table 4).
The relative risk of total loss was defined as the probability of an individual being culled due to health problems or dying up until the 48th week of age. The relative risk of total loss was the highest in autumn 2020 and 2021and the lowest in spring 2019 and summer 2020 (Figure 1). In our study, the better survival at the onset of the laying season can be explained by an early preparation of the female for the laying season and, more advantageous egg composition [15]. During summer, breeding birds consume less due to the hot weather [23,24], so the chemical composition of eggs can also be unfavorably affected. In autumn, the lower hatchability and higher relative risk of chicks could be due to the nutritional depletion of hens [15] and the rainy weather and wet environment which is the hotbed of bacterial infection. In South Africa, Bonato et al. [25] found that the fertility of males was the best in spring and early summer, resulting in better egg hatchability. In Hungary, the hatchability of eggs is the best at the end of spring and during summer [16] resulting in a better quality of chicks [20]. The seasonal climate can have an indirect effect on chick vitality (i.e., results in better hatchability and survival in summer and weaker in autumn) [14,16]. However, we could not find a reference for the effect of annual and season climate on chick vitality in the international literature, nor could we observe a relation between the seasonal climate and relative risk of loss. Even though there were differences in the husbandry technology in the examined years, we could not find a relationship between the applied technology and the relative risk of total loss. In 2019 and 2020 zeolite deep litter was used for chicks, while in 2021 they were kept in battery cages. Furthermore, in 2019 and 2020, the navel of the chicks was treated with Betadine, while in 2021 Alamycin was used for this purpose. Since the relative risk was high in both 2020 and 2021 compared to 2019, we could not obviously attribute the differences to the technological changes.
We conducted a bacterial examination on dead chicks in 2021 and found that half of the samples were contaminated with different bacteria (personal observation). Enterococcus faecalis, Escherichia coli, Coliform spp., Pseudomonas spp. and Salmonella spp. were present in the blood of heart and liver of dead ostrich chicks. The literature reports that poor nutrition, hygiene, ventilation and overcrowding can increase the mortality rates [26]. Furthermore, leg problems arising from dietary deficiencies can result in higher mortality, primarily between two and 26 weeks of age [27]. In 2021, the breeder showed that the main causes of chick loss were the technological defects in feeding, toxicosis, and leg deformity which could also stem from feeding failures (nutritional deficiencies). Leg deformities were also frequent in other years. It is also supported by Musa et al. [28] that ostriches have an extremely high growth rate during the first two to four months of age which can lead to leg deformity and a mortality as high as 41.20%. The annual climate (temperature and humidity) can influence the climate on survival.
Even though the effect of egg weight on the relative risk of total loss was not significant (p = 0.1019), El-Safty [18] found that the best hatchability results could be observed in the group with the smallest eggs, weighing ≤ 1350 g and the weakest hatchability in the largest weigh category (≥1450 g).
Regarding the effect of hatching order in the year, the relative risk showed an increasing tendency until the 16th setting, when we could see fluctuating changes (Figure 2). This means that by the increase in the number of batches, as the mean of the three examined years, the relative risk of total loss was even higher. The increasing risk ratio can be interpreted by the weaker egg composition due to the nutritional depletion of females [15] and also by the higher assumed bacterium count due to the lack of systematic disinfection of the incubator and hatcher resulting in weaker chicks [29].
The hatchability of ostrich eggs in Hungary is the best from late spring to mid-summer [16]. In our case, the seasonal hatchability results showed a similar tendency to the seasonal risk ratio (Figure 3). Additionally, Deeming [20] supports the fact that the post-performance of chicks with good quality at hatching is better, compared to the lower vitality chicks. However, Tona [19] stated that there was no correlation between chick quality and vitality. Poor hatchability and chick vitality can be the consequence of the inappropriate incubation conditions which cause stress for the developing embryo [30].
5. Conclusions
It can be concluded that the combination of the year and season of production, the hatching order and the hatchability of eggs can have a significant effect on chick survival.
Regarding the effect of the combination of the year and season of production on the relative risk of total loss, the autumn of 2020 and 2021 was the riskiest period for chicks to reach 48 weeks of age. Conversely, summer 2019 and spring 2020 were considered as the most advantageous periods resulting in the lowest relative risk.
Until the 16th week of incubation, the relative risk of total loss showed an increasing tendency, then remained unchanged.
Chicks in a group with weak hatchability had the highest relative risk of total loss compared to the average hatchability group. The relative risk of the good hatching group did not differ from the average hatching group.
Feed supplementation of breeders at the end of summer, incubator disinfection after and before each setting with wide-range disinfectants and optimal chick nursing technology (feeding and hygiene, etc.) can help to maintain or enhance chick vitality.
In summary, the results help to understand which parameters have the largest effect and pose the greatest risk to chick survival, thus enhancing the application of the right husbandry and incubation technology.
Author Contributions
Conceptualization, L.D.B., E.T., I.K. and J.P.; Methodology, I.K. and J.P.; Software, E.T. and J.P.; Validation, L.D.B., E.T., I.K. and J.P.; Formal analysis, E.T. and J.P.; Writing-original draft preparation, L.D.B.; Writing-review and editing, L.D.B., E.T., I.K. and J.P.; Visualization, L.D.B., E.T., I.K. and J.P.; Supervision, I.K. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was supported by the EFOP-3.6.3-VEKOP-16-2017-00008 project. The project was co-financed by the European Union and the European Social Fund.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Engelbrecht, A. Establishing Genetic and Environmental Parameters for Ostrich (Struthio camelus domesticus) Growth and Slaughter Characteristics. Ph.D. Thesis, Faculty of Agri Sciences at Stellenbosch University, Stellenbosch, South Africa, 2013; p. 1. [Google Scholar]
- Cloete, S.W.P.; Brand, T.S.; Hoffman, L.; Brand, Z.; Engelbrecht, A.; Bonato, M.; Malecki, I.A. The development of ratite production through continued research. World’s Poult. Sci. 2012, 68, 323–334. [Google Scholar] [CrossRef]
- Cloete, S.W.P.; Lambrechts, H.; Punt, K.; Brand, Z. Factors related to high levels of ostrich chick mortality from hatching to 90 days of age in an intensive rearing system. S. Afr. Vet. Assoc. 2001, 72, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Glatz, P.; Miao, Z. Reducing Mortality Rates in Ostrich Chicks; Rural Industries Research and Development Corporation: Kingston, Australia, 2008; pp. 1–27. [Google Scholar]
- Adewumi, A.; Samuel, A.; Samman, A. Performance Traits and Survival Rate of Ostrich (Struthio camelus Linnaeus, 1758) Chicks in Captivity. Nig. Agric. Food Environ. 2017, 13, 45–49. [Google Scholar]
- Muvhali, P.T.; Bonato, M.; Engelbrecht, A.; Malecki, I.A.; Cloete, S.W.P. Extensive human presence and regular gentle handling improve growth, survival and immune competence in ostrich chicks. Appl. Anim. Welf. Sci. 2019, 23, 95–107. [Google Scholar] [CrossRef]
- Smith, W.A.; Cilliers, S.C.; Mellett, F.D.; van Schalkwyk, S.J. Ostrich production—A South African perspective. In Proceedings of the Biotechnology in the Feed Industry, Proceedings of the 11th Alltech Annual Symposium, UK, 1995; Lyons, T.P., Jacques, K.A., Eds.; Nottingham University Press: Nottingham, UK; pp. 175–197.
- More, S.J. The performance of farmed ostrich chicks in eastern Australia. Prev. Vet. Med. 1996, 29, 91–106. [Google Scholar] [CrossRef]
- Adams, J.; Revell, B.J. Ostrich Farming: A Review and Feasibility Study of Opportunities in the EU; School of Management, Harper Adams University College: Newport, UK, 1998. [Google Scholar]
- Barri, F.R.; Navarro, J.L.; Maceira, N.O.; Martella, M.B. Rearing Greater Rhea (Rhea americana) chicks: Is adoption more effective than the artificial intensive system? Br. Poult. Sci. 2005, 46, 22–25. [Google Scholar] [CrossRef]
- Cogburn, D. What Is Commercial Ostrich? Rooster Cogburn Ranch: Picacho Peak, AZ, USA, 2006. [Google Scholar]
- Elobeid, E.A.E.; Salih, M.T.; Faki, A.E.; Amin, A.E. Captive Red-Necked Ostrich (Struthio camelus camelus) Intensive Starter/Grower Chick Rearing; University of Khartoum: Khartoum, Sudan, 2014. [Google Scholar]
- Tully, T.N., Jr.; Shane, S.M. (Eds.) Infectious diseases. In Ratite Management, Medicine and Surgery; Krieger Publishing Company: Malabar, FL, USA, 1996; pp. 127–146. [Google Scholar]
- Brand, Z.; Cloete, S.W.P.; Malecki, I.A.; Brown, C.R. Influence of incubation management on pipping position, hatching ability and survival of ostrich chicks. S. Afr. J. Anim. Sci. 2011, 41, 265–274. [Google Scholar] [CrossRef]
- Ankney, C.D.; MacInnes, C.D. Nutrient Reserves and Reproductive Performance of Female Lesser Snow Geese. Auk 1978, 95, 459–471. [Google Scholar]
- Brassó, D.L.; Komlósi, I.; Varga, É.; Várszegi, Z.; Béri, B. Egy magyar struccállomány tojástermelésének értékelése (előzetes közlemény). Hung. J. Anim. Prod. 2021, 70, 284–297. [Google Scholar]
- Brand, Z.; Cloete, S.W.P.; Malecki, I.A.; Brown, C.R. Genetic parameters for ostrich incubation traits in South Africa. S. Afr. Anim. Sci. 2008, 39, 253–259. [Google Scholar] [CrossRef]
- El-Safty, S.A.E. Effect of egg weight grades, porosity and their interaction on some hatching traits of ostrich eggs. Egypt. Poult. Sci. 2012, 32, 725–733. [Google Scholar]
- Tona, K.; Onagbesan, O.; De Ketelaere, B.; Decuypere, E.; Bruggeman, V. Effects of age of broiler breeders and egg storage on egg quality, hatchability, chick quality, chick weight and chick post-hatch growth to 42 days. Appl. Poult. Res. 2004, 13, 10–18. [Google Scholar] [CrossRef]
- Deeming, D.C. Production, fertility and hatchability of ostrich (Struthio camelus) eggs on a farm in the United Kingdom. Anim. Sci. 1996, 63, 329–336. [Google Scholar] [CrossRef]
- Pollok, K.D.; Hale, D.S.; Miller, R.K.; Angel, R.; Blue-Mclendon, A.; Baltmanis, B.; Keeton, J.T. Ostrich slaughter and by-product yields. Am. Ost. 1997, 4, 31–35. [Google Scholar]
- Mészáros, G.; Kadlečík, O.; Kasarda, R.; Sölkner, J. Analysis of longevity in the Slovak Pinzgau population—Extension to the animal model. Czech J. Anim. Sci. 2013, 58, 289–295. [Google Scholar] [CrossRef]
- Ruuskanen, S.; Hsu, B.-Y.; Nord, A. Endocrinology of thermoregulation in birds in a changing climate. Mol. Cell Mol. Cell. Endocrinol. 2021, 519, 111088. [Google Scholar] [CrossRef]
- Di Meo, C.; Stanco, G.; Cutrignelli, M.I.; Castaldo, S.; Nizza, A. Physical and chemical quality of ostrich eggs during the laying season. Br. Poult. Sci. 2003, 44, 386–390. [Google Scholar] [CrossRef]
- Bonato, M.; Malecki, I.A.; Rybnik-Trzaskowska, P.K.; Cornwallis, C.K.; Cloete, S.W. Predicting ejaculate quality and libido in male ostriches: Effect of season and age. Anim. Reprod. Sci. 2014, 151, 49–55. [Google Scholar] [CrossRef]
- Shivaprasad, H.L. Neonatal Mortality in Ostriches: An Overview of Possible Causes; Association of Avian Veterinarians: Teaneck, NJ, USA, 1993; pp. 282–285. [Google Scholar]
- Bezuidenhout, A.J.; Penrith, M.L.; Burger, W.P. Prolapse of the phallus and cloaca in the ostrich (Struthio camelus). J. South Afr. Vet. Assoc. 1993, 64, 156–158. [Google Scholar]
- Musa, H.H.; Suleiman, A.H.; Lanyasunya, T.P.; Olowofeso, O.; Mekki, D.M. Feeding Practices, Growth Rate, and Management of Ostrich Chicks in Sudan. Pak. J. Nutr. 2005, 4, 154–157. [Google Scholar]
- Mushi, E.Z.; Isa, J.W.; Binta, M.G.; Kgotlhane, M.C.G. Physical characteristics of ostrich (Struthio camelus) eggs from Botswana. Anim. Vet. Adv. 2007, 6, 676–677. [Google Scholar]
- Deeming, D.C.; Ar, A. Factors affecting the success of commercial incubation. In The Ostrich: Biology, Production and Health; Deeming, D.C., Ed.; CABI Publishing, CAB International: Wallingford, UK, 1999; pp. 159–190. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).