Exotic and Emergent Citrus Viruses Relevant to the Mediterranean Region
Abstract
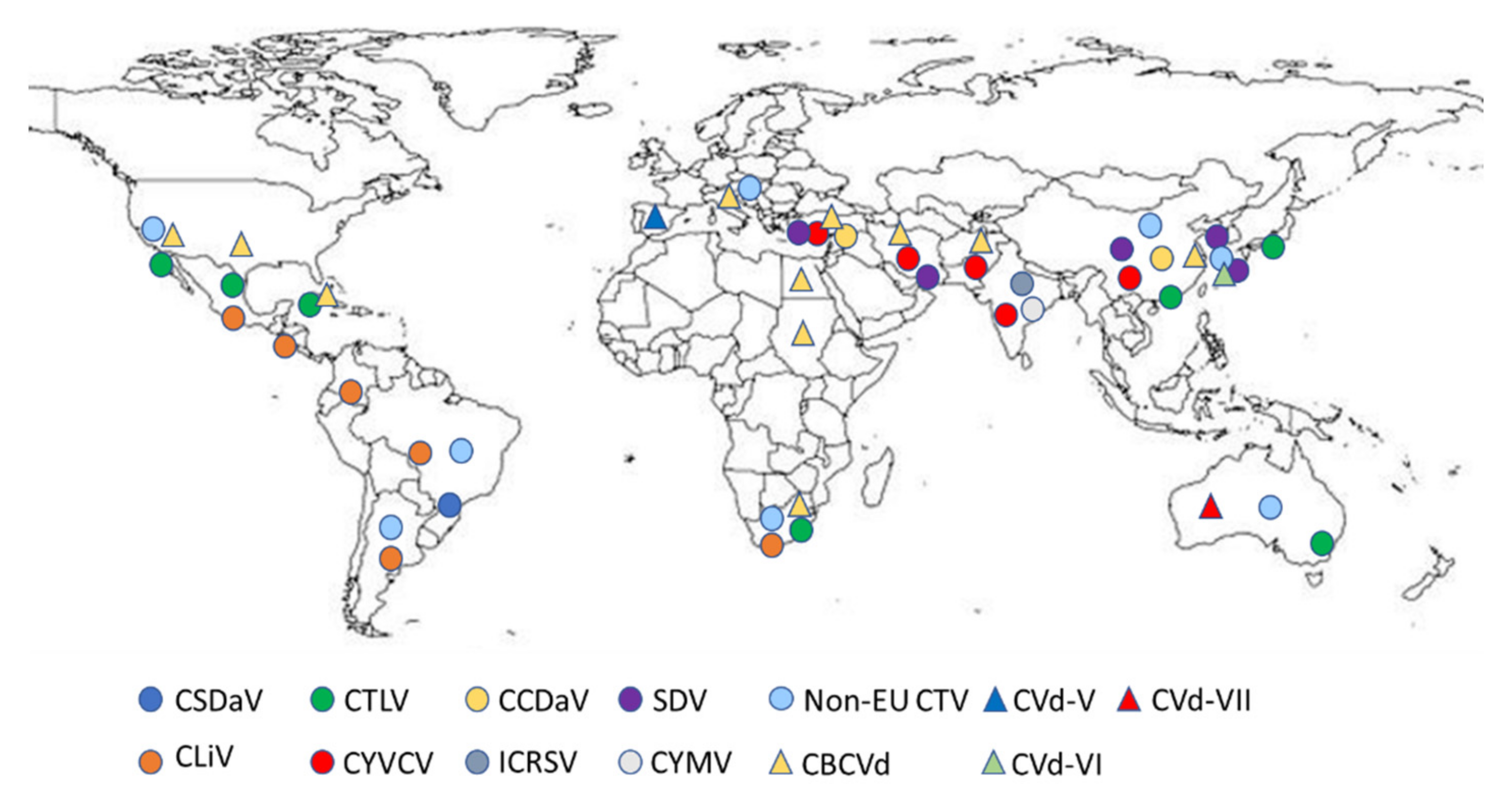
1. Introduction
2. Citrus Viruses Recommended for Regulation as Quarantine Pests
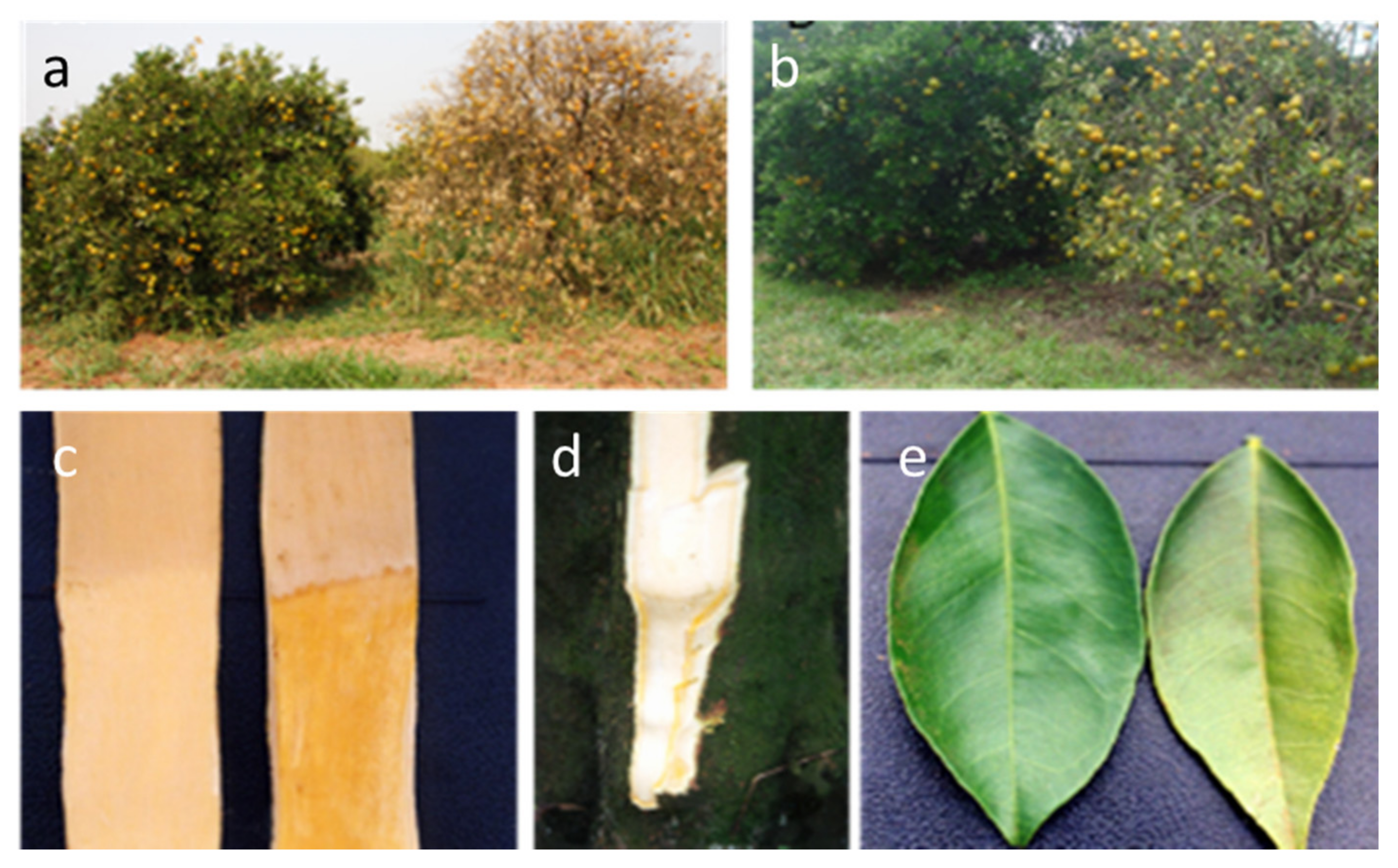
2.1. Citrus Tristeza Virus (Closterovirus, Closteroviridae)
2.2. Citrus Tatter Leaf Virus, a Strain of Apple Stem Grooving Virus (Capillovirus, Betaflexiviridae)
2.3. Satsuma Dwarf Virus (Sadwavirus, Secoviridae) and Related Diseases
2.4. Citrus Leprosis Viruses (Cilevirus, Kitaviridae, Dichorhavirus and Rhabdoviridae)
2.5. Citrus Yellow Mosaic Virus (Badnavirus, Caulimoviridae)
3. Citrus Viruses Not Yet Recommended for Regulation as Quarantine Pests
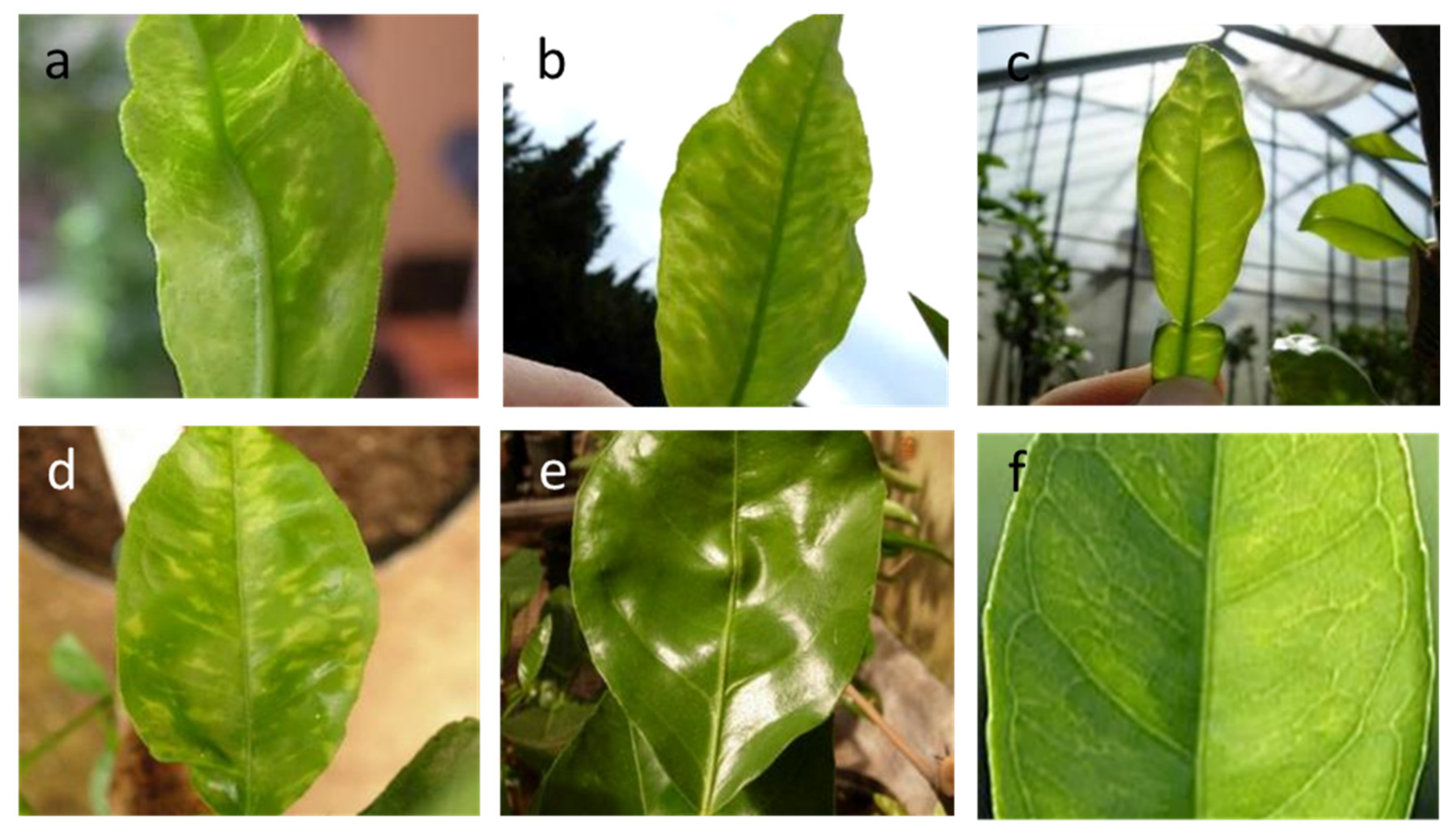
3.1. Citrus Yellow Vein Clearing Virus (Mandarivirus, Alphaflexiviridae)
3.2. Indian Citrus Ringspot Virus (Mandarivirus, Alphaflexiviridae)
3.3. Citrus Chlorotic Dwarf-Associated Virus (Geminiviridae)
3.4. Citrus Sudden Death-Associated Virus (Marafivirus, Tymoviridae)
4. Control Measures
4.1. Surveillance Is the Priority
4.2. Towards Better Detection Tools, for Fast and Large Surveys
4.3. Production of Healthy Plants for Planting
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bar-Joseph, M.; Batuman, O.; Roistacher, N. The history of citrus tristeza virus–Revisited. In Citrus Tristeza Virus Complex and Tristeza Diseases; Karasev, A.V., Hilf, M., Eds.; APS Press: Saint Paul, MN, USA, 2009; pp. 3–26. [Google Scholar]
- Bar-Joseph, M.; Catara, A. Endemic and emerging vector-borne Mediterranean citrus diseases and their epidemiological consequences. In Integrated Control of Citrus Pests in the Mediterranean Region; Vacante, V., Gerson, V., Eds.; Bentham Science Pubblishers Ltd.: Sharjah, UAE, 2010; pp. 137–155. [Google Scholar]
- Davino, S.; Willemsen, A.; Panno, S.; Davino, M.; Catara, A.; Elena, S.F.; Rubio, L. Emergence and phylodynamics of Citrus tristeza virus in Sicily, Italy. PLoS ONE 2013, 8, e66700. [Google Scholar] [CrossRef]
- Reichert, I. EPPO study mission to investigate virus diseases of citrus in the Mediterranean countries. In Report of the International Conference on Virus Diseases of Citrus (Acireale, Sicily, 15–17 September 1959); European and Mediterranean Plant Protection Organisation: Paris, France, 1960; pp. 13–44. [Google Scholar]
- Moreno, P.; Ambros, S.; Albiach-Martì, M.R.; Guerri, J.; Peña, L. Citrus tristeza virus: A pathogen that changed the course of the citrus industry. Mol. Plant Path. 2008, 9, 251–268. [Google Scholar] [CrossRef]
- Zhou, C.; da Graça, J.V.; Freitas-Astúa, J.; Vidalakis, G.; Duran-Vila, N.; Lavagi, I. Citrus viruses and viroids. In The Genus Citrus, 2nd ed.; Talon, M., Caruso, M., Gmitter, F., Jr., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 391–410. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). Plant Pest Surveillance: A Guide to Understand the Principal Requirements of Surveillance Programmes for National Plant Protection Organizations; FAO: Rome, Italy, 2016. [Google Scholar]
- ISPM 5. Glossary of Phytosanitary Terms; IPPC/FAO: Rome, Italy, 2017. [Google Scholar]
- ISPM 6. Surveillance; IPPC/FAO: Rome, Italy, 2018. [Google Scholar]
- EFSA (European Food Safety Authority); Schrader, G.; Camilleri, M.; Diakaki, M.; Vos., S. Pest survey card on non-European isolates of citrus tristeza virus. EFSA Support. Publ. 2019, 16, 1600E. [Google Scholar]
- EPPO. EPPO Global Database. 2021. Available online: https://gd.eppo.int/ (accessed on 28 August 2021).
- Kitajima, E.W.; Silva, D.M.; Oliveira, A.R.; Müller, G.W.; Costa, A.S. Thread-like particles associated with tristeza disease of citrus. Nature 1964, 201, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Martelli, G.P.; Agranosky, A.A.; Bar Joseph, M.; Boscia, D.; Candresse, T.; Coutts, R.H.A.; Dolja, V.V.; Hu, J.S.; Jelkmann, W.; Karasev, A.V.; et al. Family Closteroviridae. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier-Academic Press: Amsterdam, The Netherlands, 2011; pp. 987–1001. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health). Scientific opinion on the pest categorisation of Citrus tristeza virus (non-European isolates). EFSA J. 2017, 15, 5031. [Google Scholar]
- Harper, S.J. Citrus tristeza virus: Evolution of complex and varied genotypic groups. Front. Microbiol. 2013, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Yokomi, R.K.; Selvaraj, V.; Maheshwari, Y.; Saponari, M.; Giampetruzzi, A.; Chiumenti, M.; Hajeri, S. Identification and characterization of CTV RB strains Citrus tristeza virus isolates breaking resistance in trifoliate orange in California. Phytopathology 2017, 107, 901–908. [Google Scholar] [CrossRef]
- Targon, M.L.P.N.; Machado, M.A.; Muller, G.W.; Coletta Filho, H.D.; Manjunath, K.L.; Lee, R.F. Sequence of coat protein gene of the severe Citrus tristeza virus complex Capão Bonito. In Proceedings of the 14th Conference of International Organization of Citrus Virologists (IOCV), Campinas, Brazil, 13–18 September 1998; da Graca, J.V., Lee, R.F., Yokomi, R.K., Eds.; IOCV: Riverside, CA, USA, 2000; pp. 121–126. [Google Scholar]
- Roy, A.; Ananthakrishnan, G.; Hartung, J.S.; Brlansky, R.H. Development and application of a multiplex reverse transcription polymerase chain reaction assay for screening a global collection of Citrus tristeza virus isolates. Phytopathology 2010, 100, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Bester, R.; Cook, G.; Maree, H.J. Citrus tristeza virus genotype detection using high-throughput sequencing. Viruses 2021, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Yokomi, R.; Selvaraj, V.; Maheshwari, Y.; Chiumenti, M.; Saponari, M.; Giampetruzzi, A.; Weng, Z.; Xiong, Z.; Hajeri, S. Molecular and biological characterization of a novel mild strain of citrus tristeza virus in California. Arch. Virol. 2018, 163, 1795–1804. [Google Scholar] [CrossRef]
- Karasev, A.V.; Boyko, V.P.; Gowda, S.; Nikolaeva, O.V.; Hilf, M.E.; Koonin, E.V.; Niblett, C.L.; Cline, K.; Gumpf, D.J.; Lee, R.F.; et al. Complete sequence of Citrus tristeza virus RNA genome. Virology 1995, 208, 511–520. [Google Scholar] [CrossRef]
- Chen, A.Y.S.; Watanabe, S.; Yokomi, R.; Ng, J.C.K. Nucleotide heterogeneity at the terminal ends of the genomes of two California Citrus tristeza virus strains and their complete genome sequence analysis. Virol. J. 2018, 15, 141. [Google Scholar] [CrossRef]
- Pappu, H.R.; Karasev, A.V.; Anderson, E.J.; Pappu, S.S.; Hilf, M.E.; Febres, V.J.; Eckloff, R.M.; McCaffery, M.; Boyko, V.; Gowda, S.; et al. Nucleotide sequence and organization of eight 3’ open reading frames of the citrus tristeza closterovirus genome. Virology 1994, 199, 35–46. [Google Scholar] [CrossRef]
- Scuderi, G.; Russo, M.; Davino, S.; Ferraro, R.; Catara, A.; Licciardello, G. Occurrence of the T36 Genotype of Citrus tristeza virus in Citrus Orchards in Sicily, Italy. Plant Dis. 2016, 100, 1253. [Google Scholar] [CrossRef]
- Weng, Z.; Barthelson, R.; Gowda, S.; Hilf, M.E.; Dawson, W.O.; Galbraith, D.W.; Xiong, Z. Persistent infection and promiscuous recombination of multiple genotypes of an RNA virus within a single host generate extensive diversity. PLoS ONE 2007, 2, e917. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Brlansky, R.H. Genome analysis of an orange stem pitting Citrus tristeza virus isolate reveals a novel recombinant genotype. Virus Res. 2010, 151, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.; Breytenbach, J.H.J.; Steyn, C.; de Bruyn, R.; van Vuuren, S.P.; Burger, J.T.; Maree, H.J. Grapefruit field trial evaluation of Citrus tristeza virus T68-strain sources. Plant Dis. 2021, 105, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.J.; Dawson, T.E.; Pearson, M.N. Complete genome sequences of two distinct and diverse Citrus tristeza virus isolates from New Zealand. Arch. Virol. 2009, 154, 1505–1510. [Google Scholar] [CrossRef]
- Zablocki, O.; Pietersen, G. Characterization of a novel citrus tristeza virus genotype within three cross-protecting source GFMS12 sub-isolates in South Africa by means of Illumina sequencing. Arch. Virol. 2014, 159, 2133–2139. [Google Scholar] [CrossRef]
- Licciardello, G.; Scuderi, G.; Ferraro, R.; Giampetruzzi, A.; Russo, M.; Lombardo, A.; Raspagliesi, D.; Bar-Joseph, M.; Catara, A. Deep sequencing and analysis of small RNAs in sweet orange grafted on sour orange infected with two Citrus tristeza virus isolates prevalent in Sicily. Arch. Virol. 2015, 160, 2583–2589. [Google Scholar] [CrossRef]
- Mawassi, M.; Mietkiewska, E.; Gofman, R.; Yang, G.; Bar-Joseph, M. Unusual sequence relationships between two isolates of Citrus tristeza virus. J. Gen. Virol. 1996, 77, 2359–2364. [Google Scholar] [CrossRef]
- Licciardello, G.; Ferraro, R.; Scuderi, G.; Russo, M.; Catara, A.F. A simulation of the use of high throughput sequencing as pre-screening assay to enhance the surveillance of citrus viruses and viroids in the EPPO region. Agriculture 2021, 11, 400. [Google Scholar] [CrossRef]
- Varveri, C.; Olmos, A.; Pina, J.A.; Marroquín, C.; Cambra, M. Biological and molecular characterization of a distinct Citrus tristeza virus isolate originating from a lemon tree in Greece. Plant Pathol. 2015, 64, 792–798. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, S.; Moreno, P.; Guerri, J.; Ambrós, S. The complete nucleotide sequence of a severe stem pitting isolate of Citrus tristeza virus from Spain: Comparison with isolates from different origins. Arch. Virol. 2006, 151, 387–398. [Google Scholar] [CrossRef]
- Biswas, K.K.; Tarafdar, A.; Sharma, S.K. Complete genome sequence of mandarin decline Citrus tristeza virus of the north-Eastern Himalayan hill region of India: Comparative analyses determine recombinant. Arch. Virol. 2012, 157, 579–583. [Google Scholar] [CrossRef]
- Matsumura, E.E.; Coletta-Filho, H.D.; Nouri, S.; Falk, B.W.; Nerva, L.; Oliveira, T.S.; Dorta, S.O.; Machado, M.A. Deep sequencing analysis of RNAs from citrus plants grown in a citrus sudden death-affected area reveals diverse known and putative novel viruses. Viruses 2017, 9, 92. [Google Scholar] [CrossRef]
- Yang, Z.N.; Mathews, D.M.; Dodds, J.A.; Mirkov, T.E. Molecular characterization of an isolate of citrus tristeza virus that causes severe symptoms in sweet orange. Virus Genes 1999, 19, 131–142. [Google Scholar] [CrossRef]
- Albiach-Marti, M.R.; Mawassi, M.; Gowda, S.; Satanarayana, T.; Hilf, M.E.; Shanker, S.; Almira, E.C.; Vives, M.C.; López, C.; Guerri, J.; et al. Sequences of Citrus tristeza virus separated in time and space are essentially identical. J. Virol. 2000, 74, 6856–6865. [Google Scholar] [CrossRef]
- Vives, M.C.; Rubio, L.L.; Pez, C.; Navas-Castillo, J.; Albiach-Mart, M.R.; Dawson, W.O.; Guerri, J.; Flores, R.; Moreno, P. The complete genome sequence of the major component of a mild citrus tristeza virus isolate. J. Gen. Virol. 1999, 80, 811–816. [Google Scholar] [CrossRef][Green Version]
- Roy, A.; Choudhary, N.; Hartung, J.S.; Brlansky, R.H. The prevalence of the Citrus tristeza virus trifoliate resistance breaking genotype among Puerto Rican isolates. Plant Dis. 2013, 97, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.J.; Dawson, T.E.; Pearson, M.N. Isolates of Citrus tristeza virus that overcome Poncirus trifoliata resistance comprise a novel strain. Arch. Virol. 2010, 155, 471–480. [Google Scholar] [CrossRef]
- Melzer, M.J.; Borth, W.B.; Sether, D.M.; Ferreira, S.; Gonsalves, D.; Hu, J.S. Genetic diversity and evidence for recent modular recombination in Hawaiian Citrus tristeza virus. Virus Genes 2010, 40, 111–118. [Google Scholar] [CrossRef]
- Maccheroni, W.; Alegria, M.C.; Greggio, C.C.; Piazza, J.P.; Kamla, R.F.; Zacharias, P.R.A.; Bar-Joseph, M.; Kitajima, E.W.; Assumpção, L.C.; Camarotte, G.; et al. Identification and genomic characterization of a new virus (Tymoviridae family) associated with citrus sudden death disease. J. Virol. 2005, 79, 3028–3037. [Google Scholar] [CrossRef] [PubMed]
- Afloukou, F.M.; Çalişkan, F.; Önelge, N. Aphis gossypii Glover is a vector of citrus yellow vein clearing virus. J. Gen. Plant Pathol. 2021, 87, 83–86. [Google Scholar] [CrossRef]
- Onelge, N.; Satar, S.; Elibüyük, O.; Bozan, O.; Kamberoglu, M. Transmission studies on Citrus yellow vein clearing virus. In Proceedings of the 18th Conference International Organization of Citrus Virology, Campinas, Brazil, 7–12 November 2010; da Graca, J.V., Lee, R.F., Yokomi, R.K., Eds.; IOCV: Riverside, CA, USA, 2011. [Google Scholar]
- Zhang, Y.H.; Wang, Y.L.; Qin, W.; Cao, M.J.; Zhou, C.Y.; Yan, Z. Identification of Aphis spiraecola as a vector of Citrus yellow vein clearing virus. Eur. J. Plant Pathol. 2018, 152, 841–844. [Google Scholar] [CrossRef]
- Bastianel, M.; Novelli, V.M.; Kitajima, E.W.; Kubo, K.S.; Bassanezi, R.B.; Machado, M.A.; Freitas-Astua, J. Citrus Leprosis: Centennial of an unusual mite-virus pathosystem. Plant Dis. 2010, 94, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.S.; Novelli, V.M.; Bastianel, M.; Locali-Fabris, E.C.; Antonioli-Luizon, R.; Machado, M.A.; Freitas-Astua, J. Detection of Brevipalpus-transmitted viruses in their mite vectors by RT–PCR. Exp. Appl. Acarol. 2011, 54, 33–39. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Liu, C.H.; Wang, Q.; Wang, Y.L.; Zhou, C.Y.; Zhou, Y. Identification of Dialeurodes citri as a vector of citrus yellow vein clearing virus in China. Plant Dis. 2019, 103, 65–68. [Google Scholar] [CrossRef]
- Korkmaz, S.; Çinar, A.; Kersting, U.; Garnsey, S.M. Citrus chlorotic dwarf: A new whitefly-transmitted virus-like disease of citrus in Turkey. Plant Dis. 1995, 79, 1074. [Google Scholar] [CrossRef]
- Garnsey, S.M.; Behe, C.G.; Lockhart, B.E.L. Transmission of citrus yellow mosaic badnavirus by the citrus mealybug. Phytopathology 1998, 9, S43. [Google Scholar]
- Raccah, B.; Loebenstein, G.; Bar-Joseph, M. Transmission of citrus tristeza virus by melon aphid. Phytopathology 1976, 66, 1102–1104. [Google Scholar] [CrossRef]
- Bar-Joseph, M.; Loebenstein, G. Effects of strain, source plant, and temperature on the transmissibility of citrus tristeza virus by the melon aphid. Phytopathology 1973, 63, 716–720. [Google Scholar] [CrossRef]
- Costa, A.S.; Muller, G.W. Tristeza control by cross protection: A U.S.-Brasil cooperative success. Plant Dis. 1980, 64, 538–541. [Google Scholar] [CrossRef]
- Cook, G.; van Vuuren, S.P.; Breytenbach, J.H.J.; Steyn, C.; Burger, J.T.; Maree, H.J. Characterization of Citrus tristeza virus single-variant sources in grapefruit in greenhouse and field trials. Plant Dis. 2016, 100, 2251–2256. [Google Scholar] [CrossRef] [PubMed]
- Albiach-Marti, M.R.; Grosser, J.W.; Gowda, S.; Mawassi, M.; Satyanarayana, T.; Garnsey, S.M.; Dawson, W.O. Citrus tristeza virus replicates and forms infections virions in protoplasts of resistant citrus relatives. Mol. Breed. 2004, 14, 117–128. [Google Scholar] [CrossRef]
- Harper, S.J.; Pearson, M.N. Citrus tristeza virus strains present in New Zealand and the South Pacific. J. Cit. Pathol. 2015, 2, 1–5. [Google Scholar]
- Zhou, Y.; Liu, Y.; Liu, K.; Yang, F.; Zhou, C. Distribution and population structure of Citrus tristeza virus in Poncirus trifoliata. Austral. Plant Pathol. 2017, 46, 351–355. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, T.; Cao, M.; Zhou, Y.; Li, Z. First report of citrus tristeza virus trifoliate resistance-breaking (RB) genotype in Citrus grandis in China. J. Plant Pathol. 2019, 101, 451. [Google Scholar] [CrossRef]
- Afechtal, M.; D’Onghia, A.M.; Cocuzza, G.E.M.; Djelouah, K. First report of the Citrus tristeza virus resistance-breaking strain in Morocco. J. Plant Path. 2018, 100, 351. [Google Scholar] [CrossRef]
- Cook, G.; van Vuuren, S.P.; Breytenbach, J.H.J.; Burger, J.T.; Maree, H.J. Expanded strain-specific RT-PCR assay for differential detection of currently known Citrus tristeza virus strains: A useful screening tool. J. Phytopathol. 2016, 164, 847–851. [Google Scholar] [CrossRef]
- Scuderi, G.; Catara, A.F.; Licciardello, G. Genotyping citrus tristeza virus isolates by sequential multiplex RT-PCR and microarray hybridization in a lab-on-chip device. In Citrus Tristeza Virus: Methods in Molecular Biology; Catara, A., Bar-Joseph, M., Licciardello, G., Eds.; Humana: New York, NY, USA, 2019; Volume 2015, pp. 127–142. [Google Scholar]
- Saponari, M.; Giampetruzzi, A.; Selvaraj, V.; Maheshwari, Y.; Yokomi, R. Identification and characterization of resistance-breaking (RB) isolates of citrus tristeza virus. In Citrus Tristeza Virus: Methods in Molecular Biology; Catara, A., Bar-Joseph, M., Licciardello, G., Eds.; Humana: New York, NY, USA, 2019; Volume 2015, pp. 105–126. [Google Scholar]
- Tatineni, S.; Afunian, M.R.; Hilf, M.E.; Gowda, S.; Dawson, W.O.; Garnsey, S.M. Molecular characterization of Citrus tatter leaf virus historically associated with Meyer lemon trees: Complete genome sequence and development of biologically active in vitro transcripts. Phytopathology 2009, 99, 423–431. [Google Scholar] [CrossRef]
- EFSA PLH Panel (European Food Safety Authority). Scientific Opinion on the pest categorisation of Tatter leaf virus. EFSA J. 2017, 15, 5033. [Google Scholar]
- Yan, J.; Zhang, S.; Wu, J.; Yang, F.; Zhou, Y.; Zhou, C.; Cao, M. Complete genome sequences and recombination analysis of three divergent Satsuma dwarf virus isolates. Trop. Plant Pathol. 2021, 46, 26–30. [Google Scholar] [CrossRef]
- Roy, A.; Stone, A.; Otero-Colina, G.; Wei, G.; Choudhary, N.; Achor, D.; Shao, J.; Levy, L.; Nakhla, M.K.; Hollingsworth, C.R.; et al. Genome assembly of Citrus leprosis virus nuclear type reveals a close association with Orchid Fleck Virus. Genome Announc. 2013, 1, e00519-13. [Google Scholar] [CrossRef] [PubMed]
- Locali-Fabris, E.C.; Freitas-Astúa, J.; Souza, A.A.; Takita, M.A.; Astúa-Monge, G.; Antonioli-Luizon, R.; Rodrigues, V.; Targon, M.L.P.N.; Machado, M.A. Complete nucleotide sequence, genomic organization and phylogenetic analysis of Citrus leprosis virus cytoplasmic type. J. Gen. Virol. 2006, 87, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Pascon, R.C.; Kitajima, J.P.; Breton, M.C.; Assumpção, L.; Greggio, C.; Zanca, A.S.; Okura, V.K.; Alegria, M.C.; Camargo, M.E.; Silva, G.G.; et al. The complete nucleotide sequence and genomic organization of Citrus Leprosis associated Virus, Cytoplasmatic type (CiLV-C). Virus Genes 2006, 32, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Hartung, J.S. Cloning and sequence analysis of an infectious clone of Citrus yellow mosaic virus that can infect sweet orange via Agrobacterium-mediated inoculation. J. Gen. Virol. 2001, 82, 2549–2558. [Google Scholar] [CrossRef]
- Borah, B.K.; Johnson, A.M.; Sai Gopal, D.V.; Dasgupta, I. Sequencing and computational analysis of complete genome sequences of Citrus yellow mosaic badna virus from acid lime and pummelo. Virus Genes 2009, 39, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, G.; Önelge, N.; Potere, O.; Giampetruzzi, A.; Bozan, O.; Satar, S.; De Stradis, A.; Savino, V.; Yokomi, R.K.; Saponari, M. Identification and characterization of Citrus yellow vein clearing virus, a putative new member of the genus Mandarivirus. Phytopathology 2012, 102, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, Q.; Su, H.; Wang, X.; Cao, M.; Zhou, C. Small RNA deep sequencing reveals full-length genome of Citrus yellow vein clearing virus in Chongqing, China. J. Int. Agric. 2017, 16, 503–508. [Google Scholar] [CrossRef]
- Song, Z.; Kurth, E.G.; Peremyslov, V.V.; Zhou, C.Y.; Dolja, V.V. Molecular characterization of a Citrus yellow vein clearing virus strain from China. Arch. Virol. 2015, 160, 1811–1813. [Google Scholar]
- Cao, M.J.; Wu, Q.; Atta, S.; Su, H.N.; Yu, Y.Q.; Chen, H.M.; Zhou, C.Y. First molecular evidence of Citrus yellow vein clearing virus from citrus in Punjab, Pakistan. Plant Dis. 2016, 100, 504. [Google Scholar] [CrossRef]
- Cui, T.; Bin, Y.; Yan, J.; Mei, P.; Li, Z.; Zhou, C.; Song, Z. Development of infectious cDNA clones of citrus yellow vein clearing virus using a novel and rapid strategy. Phytopathology 2018, 108, 1212–1218. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.M.; Cao, M.J.; Wang, X.F.; Jin, X.; Liu, K.H.; Zhou, C.Y. Occurrence, distribution, and molecular characterization of Citrus yellow vein clearing virus in China. Plant Dis. 2017, 101, 137–143. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Yang, Z.; He, S.; Wu, Q.; Li, J.; Li, Z.; Zhou, Y. Distribution and molecular characterization of Citrus yellow vein clearing virus in Yunnan Province of China. Chil. J. Agric. Res. 2020, 80, 133–139. [Google Scholar] [CrossRef]
- Afloukou, F.; Önelge, N. Occurrence and molecular characterisation of citrus yellow vein clearing virus isolates in Adana province, Turkey. Physiol. Mol. Plant Pathol. 2021, 115, 101655. [Google Scholar] [CrossRef]
- Rustici, G.; Milne, R.G.; Accotto, G.P. Nucleotide sequence, genome organization and phylogenetic analysis of Indian citrus ringspot virus. Arch. Virol. 2002, 147, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Prabha, K.; Baranwal, V.K. The Genome Sequence of an Isolate of Indian Citrus Ringspot Virus Infecting the Sweet Orange in India. J. Virol. 2012, 86, 12446–12447. [Google Scholar]
- Loconsole, G.; Saldarelli, P.; Doddapaneni, H.; Savino, V.; Martelli, G.P.; Saponari, M. Identification of a single-stranded DNA virus associated with citrus chlorotic dwarf disease, a new member in the family Geminiviridae. Virology 2012, 432, 162–172. [Google Scholar] [CrossRef]
- Karanfil, A.; Korkmaz, S. Geographic distribution and molecular characterization of Turkish isolates of the citrus chlorotic dwarf-associated virus. J. Plant Pathol. 2019, 3, 621–628. [Google Scholar] [CrossRef]
- Guo, J.; Lai, X.P.; Li, J.X.; Yue, J.Q.; Zhang, S.Y.; Li, Y.Y.; Gao, J.Y.; Wang, Z.R.; Duan, H.F.; Yang, J.D. First Report on citrus chlorotic dwarf associated virus on lemon in Dehong Prefecture, Yunnan, China. Plant Dis. 2015, 99, 1287. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Liu, Y.; Chen, H.; Li, T.; Zhou, C. Distribution and molecular characterization of citrus chlorotic dwarf-associated virus in China. Australas. Plant Pathol. 2017, 46, 227–229. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, L.; Zhao, J.; Li, T.; Liu, Q.; Cao, M.; Zhou, Y. First Report of Citrus Chlorotic Dwarf-Associated Virus on Pomelo in Nakhon, Thailand. Plant Dis. 2020, 104, 1262. [Google Scholar] [CrossRef]
- Wallace, J.M. Virus and virus like diseases. In The Citrus Industry; Reuther, W., Calavan, C., Carman, G.E., Eds.; Division of Agricultural Science, University of California: Berkeley, CA, USA, 1978; Volume IV, pp. 67–184. [Google Scholar]
- Zhang, T.M.; Liang, X.Y.; Roistacher, C.N. Occurrence and detection of Citrus tatter leaf virus (CTLV) in Huangyan, Zhejiang-Province, China. Plant Dis. 1988, 72, 543–545. [Google Scholar] [CrossRef]
- Song, Z.; Liu, K.; Li, Z.; Zhou, C. RT-PCR-RFLP for genetic diversity analysis of the citrus tatter leaf virus strain of Apple stem grooving virus. Eur. J. Plant Pathol. 2016, 144, 687–692. [Google Scholar] [CrossRef]
- Cowell, S.J.; Harper, S.J.; Dawson, W.O. A real-time RT-qPCR assay for the detection of citrus tatter leaf virus. J. Virol. Methods 2017, 244, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Hailstones, D.; Bryant, K.L.; Broadbent, P.; Zhou, C. Detection of Citrus tatter leaf virus with reverse transcription-polymerase chain reaction (RT-PCR). Austral. Plant Pathol. 2002, 29, 240–248. [Google Scholar] [CrossRef]
- Marais, L.J.; Lee, R.F. Citrange stunt virus associated with decline of Shamouti on Swingle citrumelo rootstock in South Africa. Plant Dis. 1987, 70, 892. [Google Scholar] [CrossRef]
- Alas, T.; Baloglu, S.; Kemal Caglar, B.; Gunes, A. Detection and characterization of citrus tatter leaf virus (CTLV) and citrus yellow vein clearing virus (CYVCV) in citrus trees from Cyprus. Saudi J. Biol. Sci. 2019, 26, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.D.; Kunta, M.; da Graça, J.V.; Skaria, M.; Nelson, S.D. Evidence of a low rate of seed transmission of Citrus tatter leaf virus in Citrus. In Proceedings of the 18th Conference International Organization of Citrus Virology, Campinas, Brazil, 7–12 November 2010; da Graca, J.V., Lee, R.F., Yokomi, R.K., Eds.; IOCV: Riverside, CA, USA, 2011. [Google Scholar]
- Kawai, A.; Nishio, T. Detection of citrus tatter leaf virus by enzyme-linked immunosorbent assay (ELISA). Ann. Phytopathol. Soc. Jpn. 1990, 56, 342–345. [Google Scholar] [CrossRef]
- Hilf, M. An immunocapture RT-PCR procedure using Apple stem grooving virus antibodies facilitates analysis of Citrus tatter leaf virus from the original Meyer lemon host. Plant Dis. 2008, 92, 746–750. [Google Scholar] [CrossRef]
- Park, J.W.; Kunta, M.; McCollum, G.; Gonzalez, M.; Vedasharan, P.; da Graça, J. Development of a sensitive real-time PCR detection method for citrus tatter leaf virus. J. Plant Pathol. 2018, 100, 62–73. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, C.; Song, Z.; Li, Z.; Tang, K. Detection of citrus tatter leaf virus (CTLV) by real-time PCR. J. Fruit Sci. 2009, 26, 748–751. (In Chinese) [Google Scholar]
- Roy, A.; Fayad, A.; Barthe, G.; Brlansky, R. A multiplex PCR assay for the simultaneous detection of seven citrus viruses. J. Virol. Methods 2005, 129, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ieki, H.; Ozaki, K. Simultaneous detection of six citrus viroids and Apple stem grooving virus from citrus plants by multiplex reverse transcription polymerase chain reaction. J. Virol. Methods 2002, 106, 235–239. [Google Scholar] [CrossRef]
- Bester, R.; Cook, G.; Breytenbach, J.H.J.; Steyn, C.; De Bruyn, R.; Maree, H.J. Towards the validation of high-throughput sequencing (HTS) for routine plant virus diagnostics: Measurement of variation linked to HTS detection of citrus viruses and viroids. Virol. J. 2021, 18, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Iwanami, T. Properties and control of Satsuma dwarf virus. Jpn. Agric. Res. Q. 2010, 44, 1–6. [Google Scholar] [CrossRef]
- Roistacher, C.N. Diagnosis and management of virus and virus like diseases of Citrus. In Diseases of Fruits and Vegetables; Naqvi, S.A.M.H., Ed.; Springer: Dordrecht, The Netherlands, 2004; Volume I, pp. 109–189. [Google Scholar]
- Iwanami, T.; Kondo, Y.; Karasev, A.V. Nucleotide sequences and taxonomy of Satsuma dwarf virus. J. Gen. Virol. 1999, 80, 793–797. [Google Scholar] [CrossRef][Green Version]
- EFSA Panel on Plant Health (PLH). Scientific Opinion on the pest categorisation of Satsuma dwarf virus. EFSA J. 2017, 15, 5032. [Google Scholar] [CrossRef]
- Hyun, J.W.; Hwang, R.Y.; Jung, K.E. Development of multiplex PCR for simultaneous detection of citrus viruses and the incidence of citrus viral diseases in late-maturity citrus trees in Jeju Island. Plant Pathol. J. 2017, 33, 307. [Google Scholar] [CrossRef]
- Dang, T.; Ramirez, B.; Osman, F.; Bodaghi, S.; Vidalakis, G. QuantiGene Plex: A non-PCR, high throughput, multiplex detection assay for citrus pathogens. In Proceedings of the 20th Conference of the International Organization of Citrus Virologists, Chongqing, China, 10–15 April 2016; IOCV: Riverside, CA, USA, 2016; p. 5. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health). Scientific Opinion on the pest categorisation of Citrus leprosis viruses. EFSA J. 2017, 15, 5110. [Google Scholar] [CrossRef]
- Tassi, A.D.; Garita-Salazar, L.C.; Amorim, L.; Novelli, V.M.; Freitas-Astúa, J.; Childers, C.C.; Kitajima, E.W. Virus-vector relationship in the citrus leprosis pathosystem. Exper. Appl. Acarol. 2017, 71, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Roy, A.; Guillermo, L.M.; Picton, D.D.; Wei, G.; Nakhla, M.K.; Levy, L.; Brlansky, R.H. Immunodiagnosis of Citrus leprosis virus C using a polyclonal antibody to an expressed putative coat protein. J. Virol. Methods 2013, 193, 548–553. [Google Scholar] [CrossRef]
- Choudhary, N.; Roy, A.; Govindarajulu, A.; Nakhla, M.K.; Levy, L.; Brlansky, R.H. Production of monoclonal antibodies for detection of Citrus leprosis virus C in enzyme-linked immuno-assays and immunocapture reverse transcription-polymerase chain reaction. J. Virol. Methods 2014, 206, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Locali, E.C.; Freitas-Astua, J.; de Souza, A.A.; Takita, M.A.; Astua-Monge, G.; Antonioli, R.; Kitajima, E.W.; Machado, M.A. Development of a molecular tool for the diagnosis of leprosis, a major threat to citrus production in the Americas. Plant Dis. 2003, 87, 1317–1321. [Google Scholar] [CrossRef][Green Version]
- Choudhary, N.; Roy, A.; Leon, M.G.; Wei, G.; Nakhla, M.K.; Levy, L.; Brlansky, R.H. Production of mono-and polyclonal antibodies to Citrus leprosis virus C2 and their application in triple antibody sandwich ELISA and immunocapture RT-PCR diagnostic assays. J. Virol. Methods 2017, 243, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Wei, G.; Govindarajulu, A.; Roy, A.; Li, W.; Picton, D.D.; Nakhla, M.K.; Levy, L.; Brlansky, R.H. Detection of Citrus leprosis virus C using specific primers and TaqMan probe in one-step real-time reverse transcription polymerase chain reaction assays. J. Virol. Methods 2015, 224, 105–109. [Google Scholar] [CrossRef]
- Novelli, V.M.; Freitas-Astua, J.; Bastianel, M.; Locali-Fabris, E.C.; Antonioli-Luizon, R.; Ribeiro, B.M.; Machado, M.A. Detecção vìrus da leprose dos citros no àcaro vetor. Laranja 2007, 28, 39–46. [Google Scholar]
- Roy, A.; Stone, A.; Shao, J.; Otero-Colina, G.; Wei, G.; Choudhary, N.; Achor, D.; Levy, L.; Nakhla, M.K.; Hartung, J.S.; et al. Identification and characterization of nuclear Citrus leprosis virus, an unassigned Dichorhavirus genus associated with citrus leprosis disease in Mexico. Phytopathology 2015, 105, 564–575. [Google Scholar] [CrossRef]
- Ramos-González, P.L.; Chabi-Jesus, C.; Guerra-Peraza, O.; Tassi, A.D.; Kitajima, E.W.; Harakava, R.; Salaroli, R.B.; Freitas-Astúa, J. Citrus leprosis virus N: A new dichorhavirus causing citrus leprosis disease. Phytopathology 2017, 107, 963–976. [Google Scholar] [CrossRef]
- Ahlawat, Y.S.; Pant, R.P.; Lockhart, B.E.L.; Srivastava, M.; Chakraborty, N.K.; Varma, A. Association of badnavirus with citrus mosaic disease in India. Plant Dis. 1996, 80, 590–592. [Google Scholar] [CrossRef]
- Johnson, A.A.; Borah, B.; Gopal, D.S.; Dasgupta, I. Analysis of full-length sequences of two Citrus yellow mosaic badnavirus isolates infecting Citrus jambhiri (rough lemon) and Citrus sinensis L. Osbeck (sweet orange) from a nursery in India. Virus Genes 2012, 45, 600–605. [Google Scholar] [CrossRef]
- Baranwal, V.K.; Majumdar, S.; Ahlawat, Y.S.; Singh, R.P. Sodium sulphite yields improved DNA of higher stability for PCR detection of Citrus yellow mosaic virus from citrus leaves. J. Virol. Methods 2003, 112, 153–156. [Google Scholar] [CrossRef]
- Aparna, G.; Gopal, K.; Subbaiah, K.V.; Reddy, M.; Sreenivasulu, M. First report of herbaceous hosts for Citrus yellow mosaic badnavirus from India. Plant Dis. 2002, 86, 920. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, V.K.; Majumder, S.; Ahlawat, V.S.; Singh, R.P. A novel approach for simultaneous detection of citrus yellow mosaic virus and citrus greening bacterium by multiplex polymerase chain reaction. Indian J. Biotechnol. 2005, 4, 528–533. [Google Scholar]
- Chung, K.R.; Brlansky, R.H. Citrus Diseases Exotic to Florida: Citrus Yellow Mosaic; University of Florida, Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2012; p. 293. Available online: http://edis.ifas.ufl.edu (accessed on 28 August 2021).
- Kumar, P.V.; Sharma, S.K.; Rishi, N.; Baranwal, V.K. Efficient immunodiagnosis of Citrus yellow mosaic virus using polyclonal antibodies with an expressed recombinant virion-associated protein. 3 Biotech. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.K.; Aglave, B.; Bhanare, K.; Baranwal, V.K. PCR based detection of Citrus yellow mosaic disease from Vidarbha region of Maharastra. Indian Phytopathol. 2007, 60, 520–526. [Google Scholar]
- Borah, B.K.; Johnson, A.M.A.; Gopal, D.V.R.S.; Dasgupta, I. A comparison of four DNA extraction methods for the detection of Citrus yellow mosaic Badnavirus from two species of citrus using PCR and dot-blot hybridization. J. Virol. Methods 2008, 151, 321–324. [Google Scholar] [CrossRef]
- Johnson, A.M.A.; Dasgupta, I.; Sai Gopal, D.V.R. Development of loop-mediated isothermal amplification and SYBR green real-time PCR methods for the detection of Citrus yellow mosaic badnavirus in citrus species. J. Virol. Methods 2014, 203, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.V.; Sharma, S.K.; Rishi, N.; Ghosh, D.K.; Baranwal, V.K. An isothermal based recombinase polymerase amplification assay for rapid, sensitive and robust indexing of citrus yellow mosaic virus. Acta Virol. 2018, 62, 104–108. [Google Scholar] [CrossRef]
- Grimaldi, V.; Catara, A. Association of a filamentous virus with yellow vein clearing of lemon. In Proceedings of the 13th Conference of International Organization of Citrus Virologists (IOCV), Fouzhou, China, 16–23 November 1995; Da Graca, J.V., Moreno, P., Yokomi, R.K., Eds.; IOCV: Riverside, CA, USA, 1996; pp. 343–345. [Google Scholar]
- Catara, A.; Azzaro, A.; Davino, M.; Polizzi, G. Yellow vein clearing of lemon in Pakistan. In Proceedings of the 12th Conference of the International Organization of Citrus Virologist (IOCV), India; Moreno, P., Da Graca, J.V., Timmer, L.W., Eds.; IOCV: Riverside, CA, USA, 1993; pp. 363–367. Available online: https://escholarship.org/content/qt2v63f19n/qt2v63f19n.pdf (accessed on 31 July 2021).
- Alshami, A.A.A.; Ahlawat, Y.S.; Pant, R.P. A hitherto unreported yellow vein clearing disease of citrus in India and its viral etiology. Indian Phytopathol. 2003, 56, 422–427. [Google Scholar]
- Ahlawat, Y.S.; Pant, R.P. Major virus and virus-like diseases of citrus in India, their diagnosis and management. Annu. Rev. Plant Pathol. 2003, 41, 447–474. [Google Scholar]
- Chen, H.M.; Li, Z.A.; Wang, X.F.; Zhou, Y.; Tang, K.Z.; Zhou, C.Y.; Yue, J.Q. First Report of Citrus yellow vein clearing virus on lemon in Yunnan, China. Plant Dis. 2014, 98, 1747. [Google Scholar] [CrossRef] [PubMed]
- Bani Hashmian, S.M.; Aghajanzade, S. Occurrence of citrus yellow vein clearing virus in citrus species in Iran. J. Plant Pathol. 2017, 99, 287–304. [Google Scholar]
- Önelge, N. First report of yellow vein clearing of lemons in Turkey. J. Turk. Phytopath. 2003, 32, 53–55. [Google Scholar]
- Iftikhar, Y.; Iqbal, Z.; Ahmed, S.; Awan, A.R.; Saleem, U.; Sarwar, G. Effect of environmental factors on yellow vein clearing virus incidence in lemon. J. Agr. Res. 2010, 48, 87–92. [Google Scholar]
- Li, X.T.; Su, H.N.; Tan, K.G.; Tong, X.N.; Zhong, B.L. Fruit malformation of satsuma mandarin (Citrus unshiu Marc.) infected by citrus yellow vein clearing virus. J. Phytopathol. 2017, 165, 283–288. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.M.; Wang, X.F.; Li, Z.A.; Tang, M.; Zhou, C.Y. Lack of evidence for seed transmission of Citrus yellow vein clearing virus despite its frequent detection in seed tissues. J. Plant Pathol. 2015, 97, 1–3. [Google Scholar]
- Liu, Y.; Wang, Y.; Wang, Q.; Zhang, Y.; Shen, W.; Li, R.; Cao, M.; Chen, L.; Li, X.; Zhou, C.; et al. Development of a sensitive and reliable reverse transcription droplet digital PCR assay for the detection of citrus yellow vein clearing virus. Arch. Virol. 2019, 164, 691–697. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, D.D.; Chen, H.M.; Wang, X.F.; He, S.; Zhou, C.Y. A rapid and efficient purification of Citrus yellow vein clearing virus by sucrose cushion ultracentrifugation. J. Plant Pathol. 2016, 98, 159–161. [Google Scholar]
- Bin, Y.; Li, Z.A.; Wu, J.X.; Wang, X.F.; Zhou, Y.; Li, T.; Yang, F.; Zhou, C.; Song, Z. Development of an immunochromatographic strip test for rapid detection of Citrus yellow vein clearing virus. Arch. Virol. 2018, 163, 349–357. [Google Scholar] [CrossRef]
- Bin, Y.; Song, Z.; Li, Z.A.; Zhou, C.Y. Direct tissue blot immunoassay for detection of Citrus yellow vein clearing virus. Acta Hortic. Sin. 2015, 42, 1843–1850. (In Chinese) [Google Scholar]
- Liu, Z.; Sun, Y.J.; Zhou, X.P.; Hong, J.; Wu, J.X. Monoclonal antibody-based serological detection of Citrus yellow vein clearing virus in citrus groves. J. Integr. Agr. 2017, 16, 884–891. [Google Scholar] [CrossRef][Green Version]
- Liu, K.H.; Chen, H.M.; Zhou, Y.; Li, Z.A. Establishment of RT-LAMP assay for detection of Citrus yellow vein clearing virus. Acta Hortic. Sin. 2015, 42, 997–1002. [Google Scholar]
- Zhou, Y.; Chen, H.M.; Wang, X.F.; Li, Z.A.; Zhou, C.Y. Development and application of nested RT-PCR assay for detection of Citrus yellow vein clearing virus. J. Plant Prot. 2016, 43, 255–259. [Google Scholar]
- Chen, H.; Zhou, Y.; Wang, X.; Zhou, C.; Yang, X.; Li, Z. Detection of Citrus yellow vein clearing virus by Quantitative Real-time RT-PCR. Hortic. Plant J. 2016, 2, 188–192. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, S.; Atta, S.; Yang, C.; Zhou, Y.; Di Serio, F.; Zhou, C.; Cao, M. Discovery and survey of a new Mandarivirus associated with leaf yellow mottle disease of citrus in Pakistan. Plant Dis. 2020, 104, 1593–1600. [Google Scholar] [CrossRef]
- Adams, M.J.; Antoniw, J.F.; Bar-Joseph, M.; Brunt, A.A.; Candresse, T.; Foster, G.D.; Martelli, G.P.; Milne, R.G.; Zavriev, S.K.; Fauquet, C.M. The new plant virus family Flexiviridae and assessment of molecular criteria for species demarcation. Arch. Virol. 2004, 149, 1045–1060. [Google Scholar] [CrossRef]
- Byadgi, A.S.; Ahlawat, Y.S. A new viral ringspot disease of citrus (Citrus species) in India. Indian J. Agric. Sci. 1995, 65, 763–770. [Google Scholar]
- Sharma, S.; Singh, B.; Rani, G.; Zaidi, A.A.; Hallan, V.; Virk, G.S. Current status of Indian citrus ringspot virus (ICRSV) in kinnow orchards of Punjab and neighbouring states. J. Curr. Biosci. 2004, 2, 132–135. [Google Scholar]
- Kokane, A.D.; Kokane, S.B.; Warghane, A.J.; Gubyad, M.G.; Sharma, A.K.; Reddy, M.K.; Ghosh, D.K. A rapid and sensitive reverse transcription–loop-mediated isothermal amplification (RT-LAMP) assay for the detection of Indian Citrus Ringspot Virus. Plant Dis. 2020, 105, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Çinar, A.; Korkmaz, S.; Kersting, U. Presence of new whitefly-borne citrus disease of possible viral etiology in Turkey. FAO Plant Prot. Bull. 1994, 42, 73–75. [Google Scholar]
- Matsumura, E.E.; Coletta-Filho, H.D.; Dorta, S.O.; Nouri, S.; Machado, M.A. Genetic structure and molecular variability analysis of Citrus sudden death-associated virus isolates from infected plants grown in Brazil. Viruses 2016, 8, 330. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, P.T.; Bassanezi, R.B.; Wulff, N.A.; Santos, M.A.; Sanches, A.L.; Toloy, R.S.; Gimenes-Fernandes, N.; Ayres, A.J.; Jesus Junior, W.C.; Nagata, T.; et al. Citrus sudden death is transmitted by graft-inoculation and natural transmission is prevented by individual insect-proof cages. Plant Dis. 2010, 95, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, S.; Mei, S.; Zhou, Y.; Wang, J.; Han, G.-Z.; Chen, L.; Zhou, C.; Cao, M. Viromics unveils extraordinary genetic diversity of the family Closteroviridae in wild citrus. PLoS Pathog. 2021, 17, e1009751. [Google Scholar] [CrossRef]
- Boonham, N.; Kreuze, J.; Winter, S.; Van der Vlugt, R.; Bergervoet, J.; Tomlinson, J.; Mumford, R. Methods in virus diagnostics: From ELISA to next generation sequencing. Virus Res. 2014, 186, 20–31. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, S.; Moreno, P.; Guerri, J.; Ambrós, S. Discrimination between mild and severe Citrus tristeza virus isolates with a rapid and highly specific real–time reverse transcription-polymerase chain reaction method using TaqMan LNA probes. Phytopathology 2009, 99, 307–315. [Google Scholar] [CrossRef]
- Panno, S.; Matić, S.; Tiberini, A.; Caruso, A.G.; Bella, P.; Torta, L.; Stassi, R.; Davino, A.S. Loop Mediated Isothermal Amplification: Principles and Applications in Plant Virology. Plants 2020, 6, 461. [Google Scholar] [CrossRef] [PubMed]
- Maree, H.J.; Fox, A.; Al Rwahnih, M.; Boonham, N.; Candresse, T. Application of HTS for routine plant virus diagnostics: State of the art and challenges. Front. Plant Sci. 2018, 9, 1082. [Google Scholar] [CrossRef]
- Olmos, A.; Boonham, N.; Candresse, T.; Gentit, P.; Giovani, B.; Kutnjak, D.; Liefting, L.; Maree, H.J.; Minafra, A.; Moreira, A.; et al. High-throughput sequencing technologies for plant pest diagnosis: Challenges and opportunities. Bull. OEPP/EPPO Bull. 2018, 48, 219–224. [Google Scholar] [CrossRef]
- Al Rwahnih, M.; Daubert, S.; Golino, D.; Islas, C.; Rowhani, A. Comparison of next-generation sequencing versus biological indexing for the optimal detection of viral pathogens in grape-vine. Phytopathology 2015, 105, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Massart, S.; Chiumenti, M.; De Jonghe, K.; Glover, R.; Haegeman, A.; Koloniuk, I.; Komínek, P.; Kreuze, J.; Kutnjak, D.; Lotos, L.; et al. High throughput sequencing of small RNAs and virus detection: Do sequence analysis strategies really matter? Phytopathology 2018, 109, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Vidalakis, G.; da Graça, J.V.; Dixon, W.N.; Ferrin, D.; Kesinger, M.; Krueger, R.; Lee, R.F.; Olive, J.; Polek, M.; Sieburth, P.; et al. Citrus quarantine sanitary and certification programs in the USA, prevention of introduction and distribution of citrus diseases, Part 1 Citrus quarantine and introduction programs. Citrograph 2010, 3, 26–35. [Google Scholar]
- Navarro, N.; Pina, J.A.; Juárez, J.; Ballester-Olmos, J.F.; Duran-Vila, N.; Guerri, J.; Moreno, P.; Ortega, C.; Navarro, A.; Arregui, J.M.; et al. The Spanish Varietal Certification System. In Proceedings of the CIRAD/CLAM 2002 Meeting. The Quality of Fresh and Processed Citrus Fruits. New Responses to the Expectation of Professionals, Montpellier, France, 10–11 October 2002; Available online: http://citrus2002.cirad.fr/en/resumes_en.html (accessed on 31 July 2021).
- EPPO. EPPO Standards, Certification Schemes: Pathogen-Tested Citrus Trees and Rootstocks; EPPO: Paris, France, 1998; PM 4/12 (1). [Google Scholar]
- EPPO. EPPO Standards, Schemes for the Production of Healthy Plants for Planting: Nursery Requirements; EPPO: Paris, France, 2000; PM 4/7(2). [Google Scholar]






| Virus | Host Plants | Mediter Ranean Distribution | Categorization | ||
|---|---|---|---|---|---|
| Rutaceous | Non-Rutaceous | Experimental | |||
| Citrus tristeza virus Decline | Citrus, Fortunella, Poncirus and hybrids, Aeglopsis, Afraegle, Pambulus, Clausena, Citropsis | Arracacia xanthorrhiza | Passiflora gracilis, P. coerulea | Widely Present | Egypt, A2 list; Morocco, Quarantine pest; Tunisia, Quarantine pest; Turkey, A2 list; EPPO, A2 list; EU, NRQP; IAPSC, A2 list |
| Citrus tristeza virus Stem pitting | Grapefruit, sweet oranges | Absent | EU, A1 quarantine pest (Annex IIA) | ||
| Citrus tristeza virus Resistance breaking | Poncirus and some hybrids | Absent (a field report in Morocco) | EU, A1 quarantine pest (Annex IIA) | ||
| Citrus tatter leaf virus (Apple stem grooving virus) | Citrus, Fortunella, Poncirus and hybrids | Apple, pear and others | Many vegetables and herbaceous hosts | Absent | Egypt, A1 list; Morocco, Quarantine pest; Tunisia, Quarantine pest; Turkey, A1 list; EPPO, A1 list |
| Citrus leprosis viruses | Citrus, Fortunella, Poncirus and hybrids, Glycosmis, Swinglea glutinosa | Dieffenbachia, Hibiscus, others | Many herbaceous hosts species | Absent | Egypt, NRQP; Morocco, Quarantine pest; Tunisia, Quarantine pest; Turkey, A1 list; EPPO, A1 list; EU, A1 Quarantine pest; NAPPO, Alert list |
| Satsuma dwarf virus | Citrus, Fortunella, Poncirus and hybrids | Fabaceae, Fragaria | Many herbaceous hosts | Turkey | Egypt, RNQP; Morocco, Quarantine pest;Tunisia, Quarantine pest; Turkey, A2 list; EPPO, A2 list; EU, A1 quarantine pest |
| Citrus yellow mosaic virus | Citrus, Fortunella, Poncirus and hybrids | Several non citrus | Absent | Egypt, RNQP; Morocco, Quarantine pest; Turkey, A1 list; EPPO, A1 list | |
| Citrus yellow vein clearing virus | Lemon, sour orange, Satsuma mandarins and others | Vitis, wild herbaceous plants | Some weeds | Diffused in Turkey (few introduced trees in Cyprus) | Not regulated |
| Indian citrus ringspot virus | Sweet orange, Kinnow mandarins, acid limes and grapefruit | French bean, soybean, Chenopodium quinoa and C. amaranticolor | No information | Not regulated | |
| Citrus chlorotic dwarf associated virus | (Lemon, grapefruit, mandarin, and others) | (Turkey) | Not regulated | ||
| Citrus sudden death associated virus | Volkameriana lemon, alemow, other citrus | Absent | EPPO, Alert list | ||
| Virus | Genome Organization * | Host | Country | Target Molecule | Sequencing Strategy | Reference |
|---|---|---|---|---|---|---|
| Citrus tatter leaf virus | ssRNA(+) Mono- | Citrus sp., C. maxima | China, Japan, Florida, California, Texas | RNA | HTS | [unpublished] |
| Mandarin, Citrus sp., Kumquat, C. sinensis, Meyer lemon | Taiwan, China, Florida | RNA | Sanger | [65] | ||
| Satsuma dwarf virus | ssRNA(+) Bi- | Sour orange and tangor | China | RNA | HTS | [66] |
| Citrus leprosis virus-N | ssRNA(-) Bi- | Citrus reticulata | Mexico | sRNA | HTS | [67] |
| Citrus leprosis virus-C/C2 | ssRNA(+) Bi- | Citrus sinensis, Citrus sp. | Brazil, Colombia, Panama | RNA | Sanger | [67,68,69] |
| Citrus yellow mosaic virus | dsDNA-RT Mono- | Sweet orange, rangpur lime, acid lime, rough lemon | India | DNA | Sanger | [70,71] |
| Citrus yellow vein clearing virus | ssRNA(+) Mono- | Lemon | Turkey, China, Pakistan | sRNA | HTS | [72,73] |
| Lemon, Citrus sp. | China, Pakistan, India | RNA | Sanger | [74,75,76,77,78] | ||
| Sour orange | Turkey, China | sRNA | HTS | [32,79] | ||
| Navel orange, Satsuma mandarin | China | RNA | Sanger | [77] | ||
| Indian citrus ringspot virus | ssRNA(+) Mono- | Mandarin, Sweet orange | India | RNA | Sanger | [80,81] |
| Citrus chlorotic dwarf-associated virus | ssDNA Mono- | Lemon | Turkey | sRNA | HTS | [82] |
| Lemon, mandarin, sweet orange, grapefruit, sour orange, Minneola tangelo hybrid | Turkey | DNA | Sanger | [83] | ||
| Lemon | China | DNA | Sanger | [84,85] | ||
| Pomelo | Thailand | DNA | Sanger | [86] | ||
| Citrus sudden death-associated virus | ssRNA(+) Mono- | Sweet orange | Brazil | sRNA, RNA | HTS | [36] |
| Sweet orange | Brazil | RNA | Sanger | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catara, A.F.; Bar-Joseph, M.; Licciardello, G. Exotic and Emergent Citrus Viruses Relevant to the Mediterranean Region. Agriculture 2021, 11, 839. https://doi.org/10.3390/agriculture11090839
Catara AF, Bar-Joseph M, Licciardello G. Exotic and Emergent Citrus Viruses Relevant to the Mediterranean Region. Agriculture. 2021; 11(9):839. https://doi.org/10.3390/agriculture11090839
Chicago/Turabian StyleCatara, Antonino F., Moshe Bar-Joseph, and Grazia Licciardello. 2021. "Exotic and Emergent Citrus Viruses Relevant to the Mediterranean Region" Agriculture 11, no. 9: 839. https://doi.org/10.3390/agriculture11090839
APA StyleCatara, A. F., Bar-Joseph, M., & Licciardello, G. (2021). Exotic and Emergent Citrus Viruses Relevant to the Mediterranean Region. Agriculture, 11(9), 839. https://doi.org/10.3390/agriculture11090839






