Growth and Energy Use Efficiency of Grafted Tomato Transplants as Affected by LED Light Quality and Photon Flux Density
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
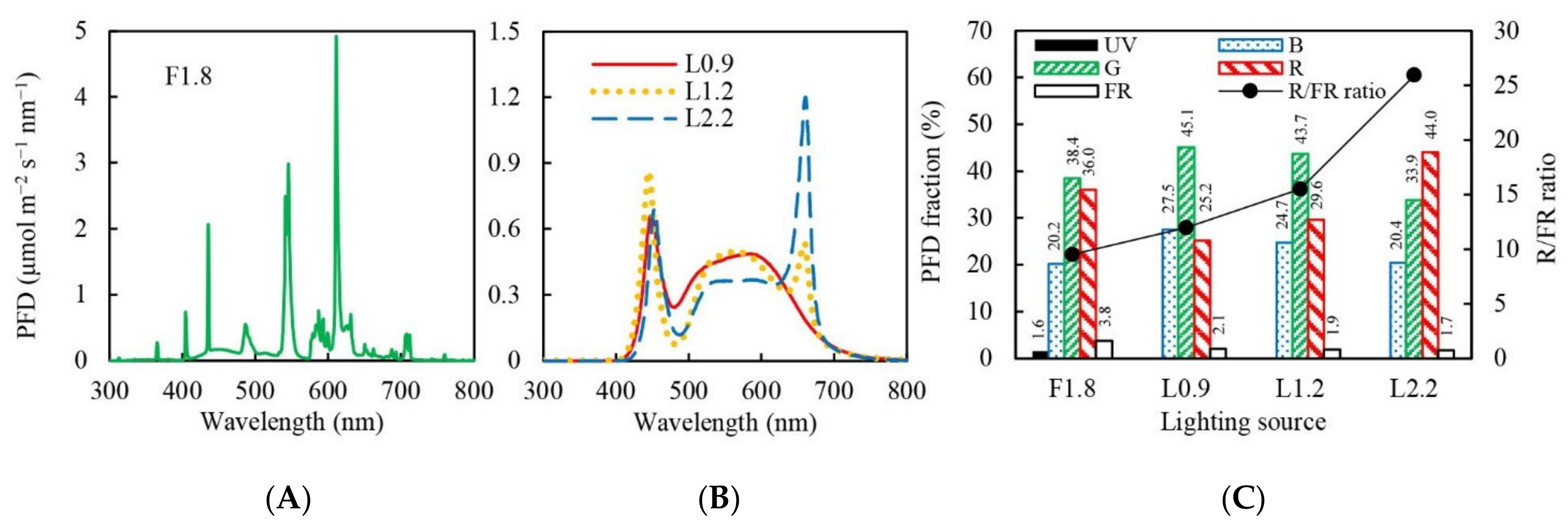
2.2. Lighting Treatments
2.3. Measurements and Calculations
2.4. Data Statistics and Analysis
3. Results
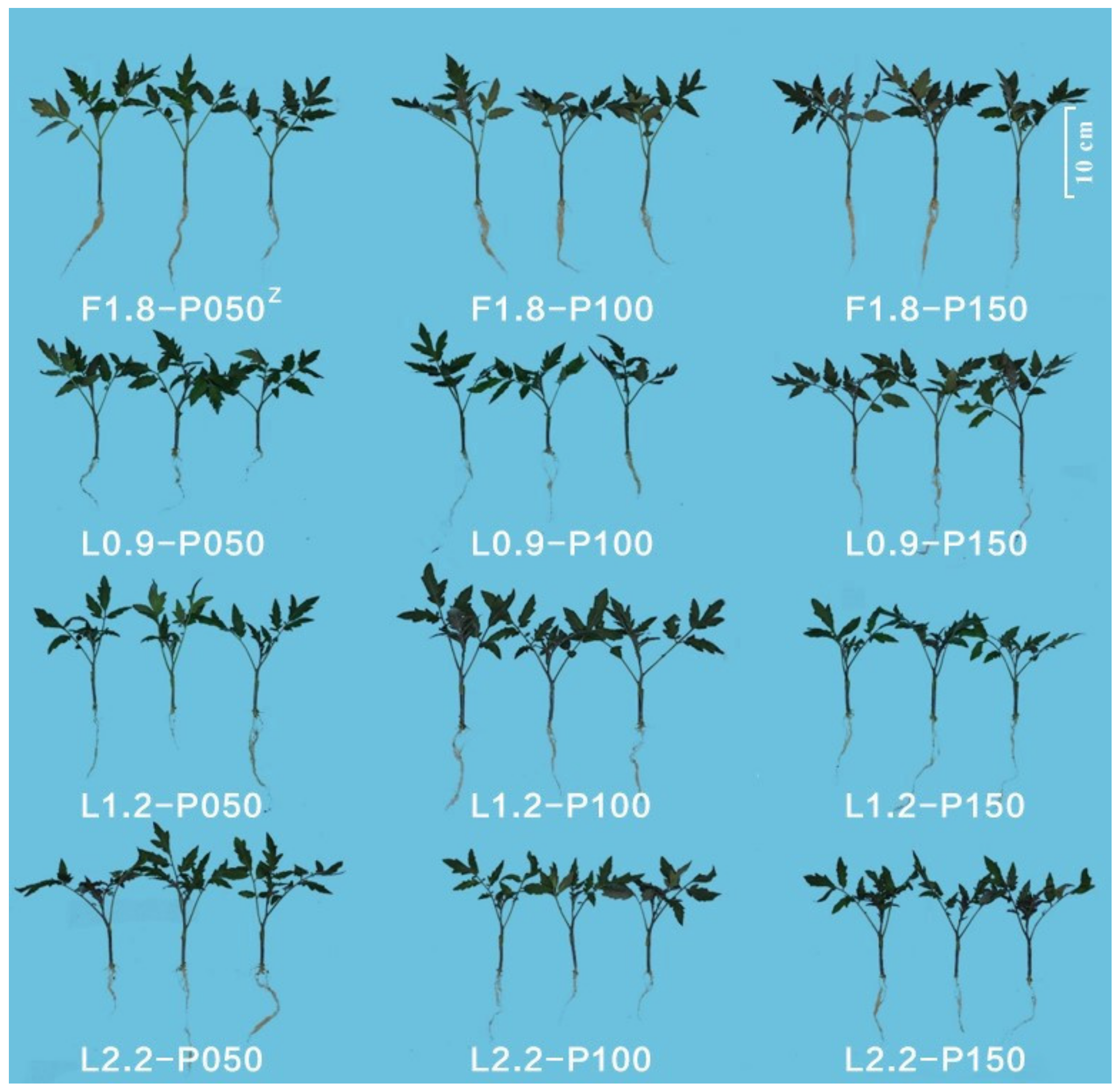
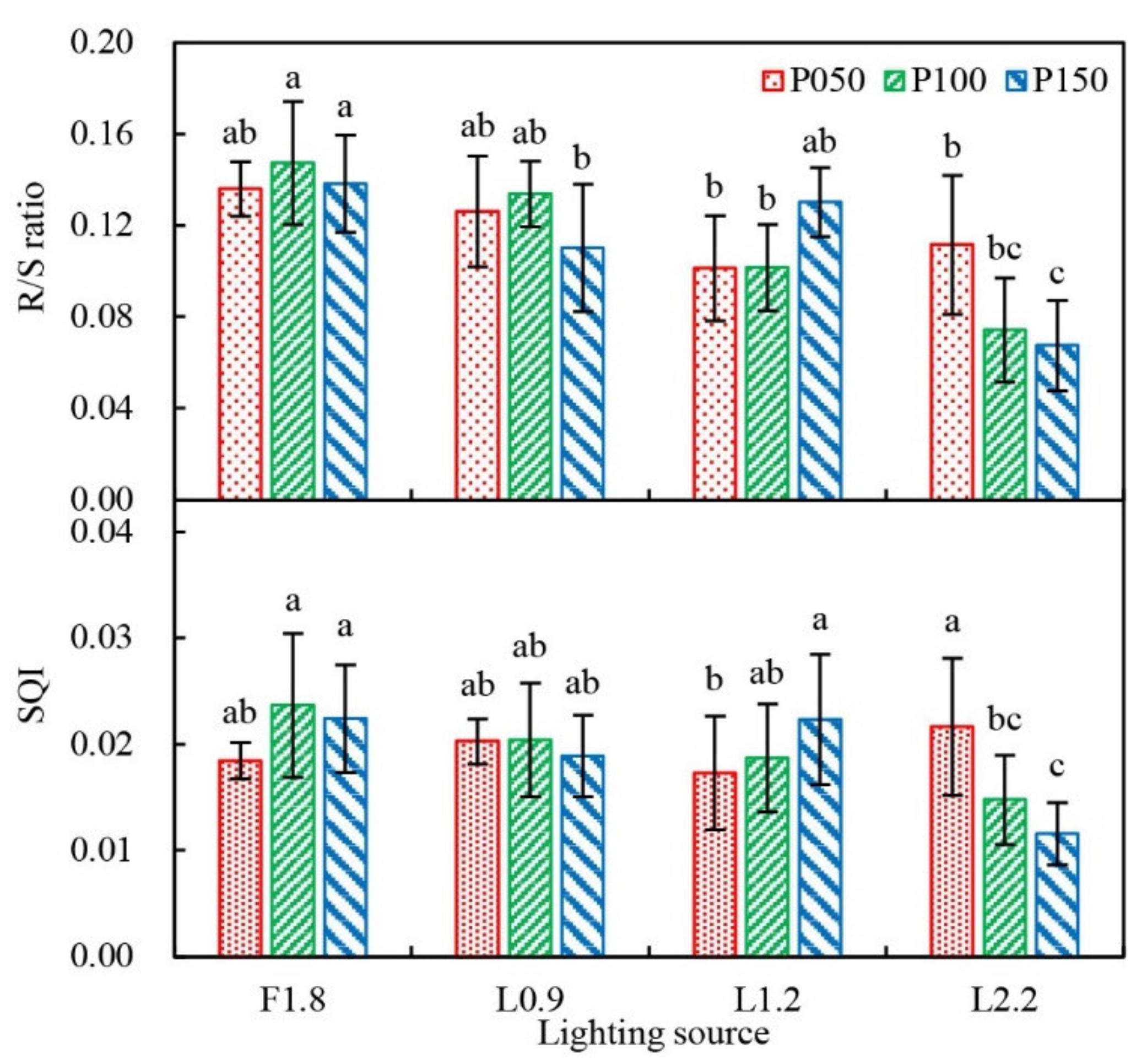
3.1. Growth Characteristics of Grafted Tomato Transplants
3.2. Energy Use Efficiency
4. Discussion
4.1. Growth of Grafted Tomato Transplants as Affected by Light Quality and PFD
4.2. Energy Use Efficiency as Affected by LED Quality
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Colla, G.; Pérez-Alfocea, F.; Schwarz, D. Vegetable Grafting: Principles and Practices; CAB International: Oxfordshire, UK, 2017. [Google Scholar]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Hoyos Echevarria, P.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Huang, Y.; Kong, Q.S.; Chen, F.; Bie, Z.L. The history, current status and future prospects of vegetable grafting in China. Acta Hortic. 2015, 1086, 31–40. [Google Scholar] [CrossRef]
- Dong, W.; Zhou, Z.C.; Bu, Y.L.; Zhuo, J.Q.; Chen, L.Z.; Li, Y.Z. Research and application of grafted seedlings healing room. Acta Hortic. 2015, 1086, 51–58. [Google Scholar] [CrossRef]
- Lee, K.M.; Lim, C.S.; Muneer, S.; Jeong, B.R. Functional vascular connections and light quality effects on tomato grafted unions. Sci. Hortic. 2016, 201, 306–317. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A.; Siomos, A.S.; Fotelli, M.N.; Kintzonidis, D. Bichromatic red and blue LEDs during healing enhance the vegetative growth and quality of grafted watermelon seedlings. Sci. Hortic. 2020, 261, 109000. [Google Scholar] [CrossRef]
- Lang, K.M.; Nair, A.; Litvin, A.G. An alternative healing method for grafted tomato transplants: The effect of light exclusion and substrate temperature on plant survival and growth. HortTechnology 2020, 30, 677–684. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yang, C.S. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G.H., Takagaki, M., Eds.; BERG EARTH Co. Ltd.: Uwajima, Japan; Academic Press: London, UK, 2016; pp. 382–386. [Google Scholar]
- Kozai, T.; Niu, G.H. Introduction. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G.H., Takagaki, M., Eds.; Academic Press: London, UK, 2020; pp. 3–6. [Google Scholar]
- Kozai, T. PFAL business and R&D in Asia and North America: Status and perspectives. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G.H., Takagaki, M., Eds.; Academic Press: London, UK, 2020; pp. 35–39. [Google Scholar]
- Sharakshane, A. White LED lighting for plants. BioRxiv 2016, e215095, (Preprint). [Google Scholar]
- Croce, R.; Müller, M.G.; Bassi, R.; Holzwarth, A.R. Carotenoid-to-chlorophyll energy transfer in recombinant major light-harvesting complex (LHCII) of higher plants. I. Femtosecond transient absorption measurements. Biophys. J. 2001, 80, 901–915. [Google Scholar] [CrossRef] [Green Version]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Aagric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red-and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef] [Green Version]
- Hogewoning, S.W.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; Van Ieperen, W.; Croce, R.; Harbinson, J. Photosynthetic quantum yield dynamics: From photosystems to leaves. Plant Cell 2012, 24, 1921–1935. [Google Scholar] [CrossRef] [Green Version]
- Nozue, H.; Shirai, K.; Kajikawa, K.; Gomi, M.; Nozue, M. White LED light with wide wavelength spectrum promotes high-yielding and energy-saving indoor vegetable production. Acta Hortic. 2018, 1227, 585–592. [Google Scholar] [CrossRef]
- Cho, J.; Park, J.H.; Kim, J.K.; Schubert, E.F. White light-emitting diodes: History, progress, and future. Laser Photonics Rev. 2017, 11, 1600147. [Google Scholar] [CrossRef]
- Avercheva, O.V.; Berkovich, Y.A.; Konovalova, I.O.; Radchenko, S.G.; Lapach, S.N.; Bassarskaya, E.M.; Kochetova, G.V.; Zhigalova, T.V.; Yakovleva, O.S.; Tarakanov, I.G. Optimizing LED lighting for space plant growth unit: Joint effects of photon flux density, red to white ratios and intermittent light pulses. Life Sci. Space Res. 2016, 11, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Urrestarazu, M.; Nájera, C.; Gea, M.D.M. Effect of the spectral quality and intensity of light-emitting diodes on several horticultural crops. HortScience 2016, 53, 268–271. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Hu, J.; Liu, C.; Wang, M.; Zhao, J.; Kang, D.I.; Jeong, B.R. Effect of supplementary light source on quality of grafted tomato seedlings and expression of two photosynthetic genes. Agronomy 2018, 8, 207. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.; Goto, E.; Ishigami, Y.; Mun, B.; Chun, C. Effects of light intensity and relative humidity on photosynthesis, growth and graft-take of grafted cucumber seedlings during healing and acclimatization. Hort. Environ. Biotechnol. 2011, 52, 331–338. [Google Scholar] [CrossRef]
- Hu, B.; Ling, P.; Kleinhenz, M. Grafted tomato propagation: Effects of light intensity and temperature on graft healing and plant regrowth. Acta Hortic. 2016, 1140, 327–330. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A.; Siomos, A.; Radoglou, K.; Dangitsis, C. Optimal LED wavelength composition for the production of high-quality watermelon and interspecific squash seedlings used for grafting. Agronomy 2019, 9, 870. [Google Scholar] [CrossRef] [Green Version]
- Hunt, R. Basic Growth Analysis: Plant Growth Analysis for Beginners; Unwin Hyman Ltd.: London, UK, 1990. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef] [Green Version]
- Goto, E. Plant production in a closed plant factory with artificial lighting. Acta Hortic. 2012, 956, 37–49. [Google Scholar] [CrossRef]
- Jang, Y.; Mun, B.; Seo, T.; Lee, J.; Chun, C. Effects of light quality and intensity on the carbon dioxide exchange rate, growth, and morphogenesis of grafted pepper transplants during healing and acclimatization. Kor. J. Hort. Sci. Technol. 2013, 31, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Hernández, R.; Eguchi, T.; Deveci, M.; Kubota, C. Tomato seedling physiological responses under different percentages of blue and red photon flux ratios using LEDs and cool white fluorescent lights. Sci. Hortic. 2016, 213, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F. Light adaptation and senescence of the photosynthetic apparatus. changes in pigment composition, chlorophyll fluorescence parameters and photosynthetic activity. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 713–736. [Google Scholar]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origin. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- McCree, K.J. Significance of enhancement for calculations based on the action spectrum for photosynthesis. Plant Physiol. 1972, 49, 704–706. [Google Scholar] [CrossRef] [Green Version]
- Wollaeger, H.M.; Runkle, E.S. Growth of impatiens, petunia, salvia, and tomato seedlings under blue, green, and red light-emitting diodes. HortScience 2014, 49, 734–740. [Google Scholar] [CrossRef]
- Cope, K.R.; Snowden, M.C.; Bugbee, B. Photobiological interactions of blue light and photosynthetic photon flux: Effects of monochromatic and broad-spectrum light sources. Photochem. Photobiol. 2014, 90, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Clifford, S.C.; Runkle, E.S.; Langton, F.A.; Mead, A.; Foster, S.A.; Pearson, S.; Heins, R.D. Height control of poinsettia using photoselective filters. HortScience 2004, 39, 383–387. [Google Scholar] [CrossRef] [Green Version]
- Ciolfi, A.; Sessa, G.; Sessi, M.; Possenti, M.; Salvucci, S.; Carabelli, M.; Morelli, G.; Ruberti, I. Dynamics of the shade-avoidance response in arabidopsis. Plant Physiol. 2013, 163, 331–353. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Schenkels, L.; Saeys, W.; Lauwers, A.; Proft, M.P.D. Green light induces shade avoidance to alter plant morphology and increases biomass production in Ocimum basilicum L. Sci. Hortic. 2020, 261, e109002. [Google Scholar] [CrossRef]
- Kwesiga, F.; Grace, J. The role of the red/far-red ratio in the response of tropical tree seedlings to shade. Ann. Bot. 1986, 57, 283–290. [Google Scholar] [CrossRef]
- Vu, N.T.; Kim, Y.S.; Kang, H.M.; Kim, I.S. Influence of short-term light irradiation under pre-and post-grafting period on the graft-take ratio and quality of tomato seedlings. Hort. Environ. Biotechnol. 2014, 55, 27–35. [Google Scholar] [CrossRef]
- Wei, H.; Wang, M.; Jeong, B.R. Effect of supplementary lighting duration on growth and activity of antioxidant enzymes in grafted watermelon seedlings. Agronomy 2020, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Currey, C.J.; Hutchinson, V.A.; Lopez, R.G. Growth, morphology, and quality of rooted cuttings of several herbaceous annual bedding plants are influenced by photosynthetic daily light integral during root development. HortScience 2012, 47, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Ma, S.; Gao, L.; Qu, M.; Tian, Y. Enhancing root regeneration and nutrient absorption in double-rootcutting grafted seedlings by regulating light intensity and photoperiod. Sci. Hortic. 2020, 264, 109192. [Google Scholar] [CrossRef]
- Yokoi, S.; Kozai, T.; Ohyama, K.; Hasegawa, T.; Chun, C.; Kubota, C. Effects of leaf area index of tomato seedling populations on energy utilization efficiencies in a closed transplant production system. J. Soc. High Technol. Agr. 2003, 15, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Ji, F.; Gan, P.D.; Liu, N.; He, D.X.; Yang, P. Effects of LED spectrum and daily light integral on growth and energy use efficiency of tomato seedlings. Trans. Chin. Soc. Agric. Eng. 2020, 36, 231–238. [Google Scholar]
- Kusuma, P.; Pattison, P.M.; Bugbee, B. From physics to fixtures to food: Current and potential LED efficacy. Hortic. Res. 2020, 7, e56. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Treatment | Light Source for the Whole Seedling Period | Photon Flux Density (μmol m−2 s−1) | Photoperiod (h d−1) | Daily Light Integral (mol m−2 d−1) | ||
|---|---|---|---|---|---|---|
| Healing Stage (7 d) Z | Pre-Grafting/Post-Healing Stage (15 d/3 d) | Healing Stage | Pre-Grafting/Post-Healing Stage | |||
| F1.8-P050Y | F1.8 | 50 | 250 | 14 | 2.5 | 12.6 |
| F1.8-P100 | 100 | 5.0 | ||||
| F1.8-P150 | 150 | 7.6 | ||||
| L0.9-P050 | L0.9 | 50 | 250 | 14 | 2.5 | 12.6 |
| L0.9-P100 | 100 | 5.0 | ||||
| L0.9-P150 | 150 | 7.6 | ||||
| L1.2-P050 | L1.2 | 50 | 250 | 14 | 2.5 | 12.6 |
| L1.2-P100 | 100 | 5.0 | ||||
| L1.2-P150 | 150 | 7.6 | ||||
| L2.2-P050 | L2.2 | 50 | 250 | 14 | 2.5 | 12.6 |
| L2.2-P100 | 100 | 5.0 | ||||
| L2.2-P150 | 150 | 7.6 | ||||
| Treatment | Plant Height (cm) | Stem Diameter (mm) | Leaf Number | Leaf Area (cm2) | Total Dry Matter (g) | Net Photosynthetic Rate (μmol m−2 s−1) |
|---|---|---|---|---|---|---|
| F1.8-P050 Z | 7.6 ± 0.6 ab | 2.7 ± 0.1 b | 3.8 ± 0.4 a | 46.5 ± 4.3 a | 0.19 ± 0.02 b | 11.9 ± 1.4 ab |
| F1.8-P100 | 7.9 ± 0.4 a | 3.0 ± 0.2 a | 3.8 ± 0.4 a | 46.0 ± 5.4 a | 0.22 ± 0.03 ab | 12.2 ± 1.1 ab |
| F1.8-P150 | 7.2 ± 0.2 b | 2.9 ± 0.2 ab | 3.7 ± 0.5 ab | 46.9 ± 7.4 a | 0.22 ± 0.04 ab | 14.1 ± 1.2 a |
| L0.9-P050 | 6.2 ± 0.8 cd | 2.9 ± 0.2 ab | 3.5 ± 0.5 ab | 43.4 ± 4.7 ab | 0.20 ± 0.03 b | 12.0 ± 1.7 ab |
| L0.9-P100 | 6.0 ± 0.4 cd | 2.9 ± 0.2 ab | 3.2 ± 0.4 b | 41.9 ± 8.2 ab | 0.20 ± 0.03 b | 12.8 ± 1.7 ab |
| L0.9-P150 | 6.6 ± 0.7 c | 2.9 ± 0.2 ab | 3.8 ± 0.4 a | 46.6 ± 6.8 a | 0.24 ± 0.03 a | 12.3 ± 2.3 ab |
| L1.2-P050 | 5.4 ± 0.3 e | 2.7 ± 0.1 b | 3.0 ± 0.0 b | 36.6 ± 4.1 b | 0.19 ± 0.02 b | 13.8 ± 1.2 a |
| L1.2-P100 | 5.5 ± 0.3 e | 3.0 ± 0.2 a | 3.3 ± 0.5 ab | 44.2 ± 5.1 ab | 0.23 ± 0.03 a | 14.1 ± 1.3 a |
| L1.2-P150 | 5.6 ± 0.2 de | 3.0 ± 0.1 a | 3.3 ± 0.5 ab | 36.7 ± 1.7 b | 0.23 ± 0.02 a | 11.0 ± 1.0 b |
| L2.2-P050 | 6.3 ± 0.4 cd | 2.9 ± 0.1 ab | 3.2 ± 0.4 b | 46.4 ± 3.0 a | 0.24 ± 0.03 a | 10.9 ± 2.6 b |
| L2.2-P100 | 6.7 ± 0.5 c | 2.8 ± 0.2 ab | 3.2 ± 0.4 b | 42.4 ± 8.6 ab | 0.24 ± 0.05 a | 12.7 ± 1.6 ab |
| L2.2-P150 | 6.0 ± 0.6 d | 2.8 ± 0.2 ab | 3.6 ± 0.5 ab | 42.4 ± 6.4 ab | 0.24 ± 0.04 a | 10.9 ± 0.8 b |
| LQ | * | NS | * | * | * | * |
| PFD | NS | * | NS | NS | * | NS |
| LQ × PFD | * | NS | NS | NS | NS | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Gan, P.; Ji, F.; He, D.; Yang, P. Growth and Energy Use Efficiency of Grafted Tomato Transplants as Affected by LED Light Quality and Photon Flux Density. Agriculture 2021, 11, 816. https://doi.org/10.3390/agriculture11090816
Zheng J, Gan P, Ji F, He D, Yang P. Growth and Energy Use Efficiency of Grafted Tomato Transplants as Affected by LED Light Quality and Photon Flux Density. Agriculture. 2021; 11(9):816. https://doi.org/10.3390/agriculture11090816
Chicago/Turabian StyleZheng, Jianfeng, Peidian Gan, Fang Ji, Dongxian He, and Po Yang. 2021. "Growth and Energy Use Efficiency of Grafted Tomato Transplants as Affected by LED Light Quality and Photon Flux Density" Agriculture 11, no. 9: 816. https://doi.org/10.3390/agriculture11090816
APA StyleZheng, J., Gan, P., Ji, F., He, D., & Yang, P. (2021). Growth and Energy Use Efficiency of Grafted Tomato Transplants as Affected by LED Light Quality and Photon Flux Density. Agriculture, 11(9), 816. https://doi.org/10.3390/agriculture11090816






