Beyond Digestion: Can Animals Shape the Landscape According to Their Species–Specific Salivary Secretions?
Abstract
1. Introduction
2. Anatomical and Physiological Principles of Major Salivary Glands and Salivation
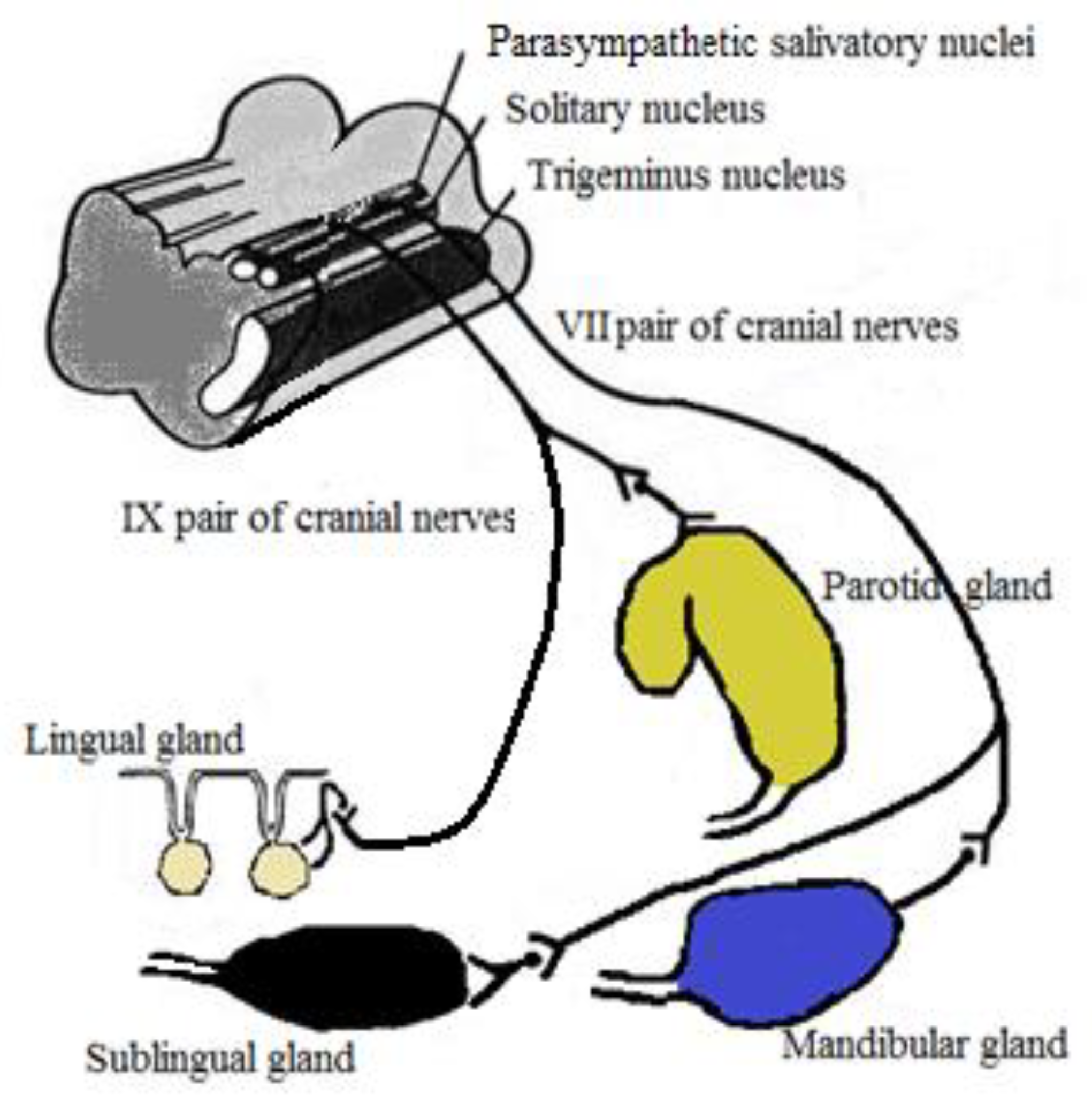
3. Autonomic Control of Salivary Secretion
4. Response to External Stimuli
5. Regulatory Neuroendocrine Systems of Salivary Composition Elicited by the Diet
6. Dynamics of Interaction between Natural Feeding Sources and Animals
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barone, R. Anatomie compareé des mammiféres domestique, 4th ed.; Edagricole: Bologna, Italy, 1996. [Google Scholar]
- Dukes, H.H. The Physiology of Domestic Animals; Comstock Publishing Associates: Ithaca, NY, USA, 1955. [Google Scholar]
- Pavlov, I.P. The Work of the Digestive Glands; Griffin C & Co. Ltd.: London, UK, 1910. [Google Scholar]
- Matsuo, R. Central connection for salivary innervation and efferent impulse formation. In Neural Mechanism of Salivary Gland Secretion; Frontiers oral biol; Garrett, J.R., Ekström, J., Anderson, L.C., Eds.; Karger: Basel, Switzerland, 1999. [Google Scholar]
- Cano, J.; Rodrìguez-Enchandia, E.L. Degenerating taste buds in sialectomized rats. Cells Tissues Organs 1980, 106, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, F.A.; Sweeney, E.A. The effects of surgical desalivation of the rat upon taste acuity. Arch. Oral Biol. 1972, 17, 1455–1465. [Google Scholar] [CrossRef]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007, 133, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B. Muscarinic receptors and salivary secretion. J. Appl. Physiol. 2006, 100, 1103–1104. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.H.; Moon, Y.W.; Hwang, S.; Kim, D.; Jo, S.H.; Oh, S.B.; Kim, J.S.; Jahng, J.W.; Lee, J.H.; et al. Histamine H1 receptor induces cytosolic calcium increase and aquaporine translocation in human salivary gland cells. J. Pharmacol. Experim. Ther. 2009, 330, 403–412. [Google Scholar] [CrossRef]
- Cappai, M.G.; Aboling, S. Toxic or harmful components of aromatic plants in animal nutrition. In Feed Additives: Aromatic Plants and Herbs in Animal Nutrition and Health; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 147–158. [Google Scholar]
- Cappai, M.G.; Pinna, W. Feed intake and selection behavior of the Asinara albino donkey: The fascination of adaptation. Tierarztl. Umsch. 2020, 75, 84–87. [Google Scholar]
- Ekström, J.; Khosravani, N.; Castagnola, M.; Messana, I. Saliva and the control of its secrection. In Dysphagia. Medical Radiology; Ekberg, O., Ed.; Springer: Cham, Switzerland, 2017; pp. 21–57. [Google Scholar]
- Ann, D.K.; Lin, H.H. Macaque salivary proline-rich protein: Structure, evolution, and expression. Crit. Rev. Oral Biol. Med. 1993, 4, 545–551. [Google Scholar] [CrossRef]
- Takahashi, T.; Shimada, T. Selective consumption of acorns by the Japanese wood mouse according to tannin content: A behavioral countermeasure against plant secondary metabolites. Ecol. Res. 2008, 23, 1033–1038. [Google Scholar] [CrossRef]
- Bennick, A. Salivary proline-rich proteins. Mol. Cell. Biochem. 1982, 45, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Mehansho, H.; Butler, L.; Carlson, D.M. Dietary tannins and salivary proline rich proteins: Interactions, Induction, and Defense Mechanism. Annu. Rev. Nutr. 1987, 7, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Bacon, J.R.; Rhoades, M.J. Development of a competition assay for the evaluation of the human parotid salivary proteins to dietary complex phenolics and tannins using a peroxidas-labelled tannin. J. Agric. Food Chem. 1998, 46, 5083–5088. [Google Scholar] [CrossRef]
- Clauss, M.; Gehrke, J.; Hatt, J.M.; Dierenfeld, E.S.; Flach, E.J.; Hermes, R.; Castell, J.; Streike, J.; Fickel, J. Tannin-binding salivary proteins in three captive rhinoceros species. Comp. Biochem. Physiol. Part A 2004, 140, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Cappai, M.G.; Wolf, P.; Dimauro, C.; Pinna, W.; Kamphues, J. The bilateral parotidomegaly (hypertrophy) induced by acorn consumption in pigs is dependent on individual’s age but not on intake duration. Livest. Sci. 2014, 167, 263–268. [Google Scholar] [CrossRef]
- Aboling, S.; Drotleff, A.M.; Cappai, M.G.; Kamphues, J. Contamination with ergot bodies (Claviceps purpurea sensu lato) of two horse pastures in Northern Germany. Mycotoxin Res. 2016, 32, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Cappai, M.G.; Wolf, P.; Pinna, W.; Kamphues, J. Pigs use endogenous proline to cope with acorn (Quercus pubescens Willd.) combined diets high in hydrolysable tannins. Livest. Sci. 2013, 155, 316–322. [Google Scholar] [CrossRef]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemlli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- Smart, D.; Jerman, J.C. The physiology and pharmacology of the orexins. Pharmacol. Ther. 2002, 94, 51–61. [Google Scholar] [CrossRef]
- Bernardis, L.L.; Bellinger, L.L. The lateral hypothalamic area revisited: Ingestive behavior. Neurosci. Biobehav. Rev. 1996, 20, 189–287. [Google Scholar] [CrossRef]
- Kukkonen, J.P.; Holmqvist, T.; Ammoun, S.; Åkerman, K.E.O. Functions of the orexinergic/hypocretinergic system. Am. J. Physiol.-Cell Physiol. 2002, 283, C1567–C1591. [Google Scholar] [CrossRef]
- Ehrström, M.; Gustafsson, T.; Finn, A.; Kirchgessner, A.; Grybäc, P.; Jacobsson, H.; Hellstrom, P.M.; Naslund, E. Inhibitory effect of exogenous orexin A on gastric emptying, plasma leptin and the distribution of orexin and orexin receptors in the gut and pancreas in man. J. Clin. Endocrinol. Metabolism. 2005, 90, 2370–2377. [Google Scholar] [CrossRef][Green Version]
- Nakabayashi, M.; Suzuki, T.; Takahashi, K.; Totsune, K.; Muramatsu, Y.; Kaneko, C.; Date, F.; Takeyama, J.; Darnel, A.D.; Moriya, T.; et al. Orexin-A expression in human peripheral tissues. Mol. Cell. Endocrinol. 2003, 205, 43–50. [Google Scholar] [CrossRef]
- Näslund, E.; Ehrström, M.; Ma, J.; Hellström, P.M.; Kirchgessner, A.L. Localization and effects of orexin on fasting motility in the rat duodenum. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 282, 470–479. [Google Scholar] [CrossRef][Green Version]
- Dall’Aglio, C.; Zannoni, A.; Mercati, F.; Forni, M.; Bacci, M.L.; Boiti, C. Differential gene expression and immune localization of the orexin system in the major salivary glands of pigs. Regul. Pept. 2011, 172, 51–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Akerman, F.; Lei, Z.M.; Rao, C.V. Human umbilical cord and fetal membranes co-express leptin and its receptor genes. Gynecol. Endocrinol. 2002, 16, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Señarís, R.; Garcia-Caballero, T.; Casabiell, X.; Gallego, R.; Castro, R.; Considine, R.V.; Dieguez, C.; Casanueva, F.F. Synthesis of leptin in human placenta. Endocrinology 1997, 138, 4501–4504. [Google Scholar] [CrossRef]
- Bado, A.; Levasseur, S.; Attoub, S.; Kermorgant, S.; Laigneau, J.P.; Bortoluzzi, M.N.; Moizo, L.; Lehy, T.; Guerre-Millo, M.; Le Marchand-Brustel, Y.; et al. The stomach is a source of leptin. Nature 1998, 394, 790–793. [Google Scholar] [CrossRef]
- Dall’Aglio, C.; Maranesi, M.; Pascucci, L.; Mercati, F.; Ceccarelli, P. Immunohistochemical distribution of leptin receptor in the major salivary glands of horses. Res. Vet. Sci. 2012, 93, 1116–1118. [Google Scholar] [CrossRef]
- Dall’Aglio, C.; Bazucchi, C.; Mercati, F.; Ceccarelli, P. Presence and distribution of leptin and its receptor in the minor salivary glands of the donkey. Acta Histochem. 2015, 117, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Cappai, M.G.; Dall’Aglio, C.; Sander, S.J.; Ratert, C.; Dimauro, C.; Pinna, W.; Kamphues, J. Different physical forms of one diet fed to growing pigs induce morphological changes in mandibular glands and local leptin (Ob) production and receptor (ObR) expression. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Buck, J. Cannabinoids protect cells from oxidative cell death: A receptor-independent mechanism. J. Pharmacol. Exp. Ther. 2000, 293, 807–812. [Google Scholar] [PubMed]
- Mercati, F.; Dall’Aglio, C.; Pascucci, L.; Boiti, C.; Ceccarelli, P. Identification of cannabinoid type 1 receptor in dog hair follicles. Acta Histochem. 2012, 114, 68–71. [Google Scholar] [CrossRef]
- Dall’Aglio, C.; Mercati, F.; De Felice, E.; Tardella, F.M.; Kamphue, J.; Cappai, M.G.; Scocco, P. Influence of different feed physical forms on mandibular gland in growing pigs. Animals 2020, 10, 910. [Google Scholar] [CrossRef]
- Jacobson, M.D.; Wusteman, M.; Downes, P. Muscarinic receptors and hydrolysis of inositol phospholipids in rat cerebral cortex and parotid gland. J. Neurochem. 1985, 44, 465–472. [Google Scholar] [CrossRef]
- Weiss, S.J.; Putney, J.W., Jr. The relationship of phosphatidylinositol turnover to receptors and calcium channels in rat parotid acinar cells. Biochem. J. 1981, 194, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Provenza, F.D.; Lynch, J.J.; Burritt, E.A.; Scott, C.B. How goats learn to distinguish between novel foods that differ in postingestive consequences. J. Chem. Ecol. 1994, 20, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, J.P. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: New York, NY, USA, 1994. [Google Scholar]
- Lamy, E.; da Costa, G.; e Silva, F.C.; Potes, J.; Coelho, A.V.; Baptista, E.S. Comparison of electroforetic protein profiles of sheep and goat parotid saliva. J. Chem. Ecol. 2008, 34, 388–397. [Google Scholar] [CrossRef]
- da Costa, G.; Lamy, E.; e Silva, F.C.; Andersen, J.; Baptista, E.S.; Coelho, A.V. Salivary amylase induction by tannin-enriched diets as a possible countermeasure against tannins. J. Chem. Ecol. 2008, 34, 376–387. [Google Scholar] [CrossRef]
- Patterson, J.; Brightling, P.; Titchen, D.A. β-Adrenergic effects on composition of parotid salivary secretion of sheep on feeding. Q. J. Exp. Physiol. 1982, 67, 57–67. [Google Scholar] [CrossRef]
- Narjisse, H.; Elhonsali, M.A.; Olsen, J.D. Effects of oak (Quercus ilex) tannins on digestion and nitrogen balance in sheep and goats. Small Rumin. Res. 1995, 18, 201–206. [Google Scholar] [CrossRef]
- Salem, A.Z.M.; Lòpez, S.; Ranilla, M.J.; Gonzàlez, J.S. Short- to medium-term effects of consumption of quebracho tannins on saliva production and composition in sheep and goats. J. Anim. Sci. 2013, 91, 1341–1349. [Google Scholar] [CrossRef]
- Fickel, J.; Göritz, F.; Joest, B.A.; Hildebrandt, T.; Hoffman, R.R.; Breves, G. Analysis of parotid and mixed saliva in roe deer. J. Comp. Physiol. B 1998, 168, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.J.; Suchar, L.A.; Robbins, C.T.; Hagerman, A.E. Tannin-binding proteins in saliva of deers and their absence in saliva of sheep and cattle. J. Chem. Ecol. 1989, 15, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Ridrigues, L.; Guerreiro, O.; Soldado, D.; Francisco, A.; Lima, M.; e Silva, F.C.; Jerònimo, E. Changes in salivary protein composition of lambs supplemented with aerial parts and condensed tannins: Extract from Cystus ladanifer L.—A preliminary study. Agrofor. Syst. 2020, 94, 1501–1509. [Google Scholar] [CrossRef]
- Wolf, P.; Cappai, M.G. Response of fattening rabbits with acorns (Quercus pubescens Will.) combined in the diet: First acquaintances on growth performance, carcass traits, and perirenal fatty acid profile. Animals 2020, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
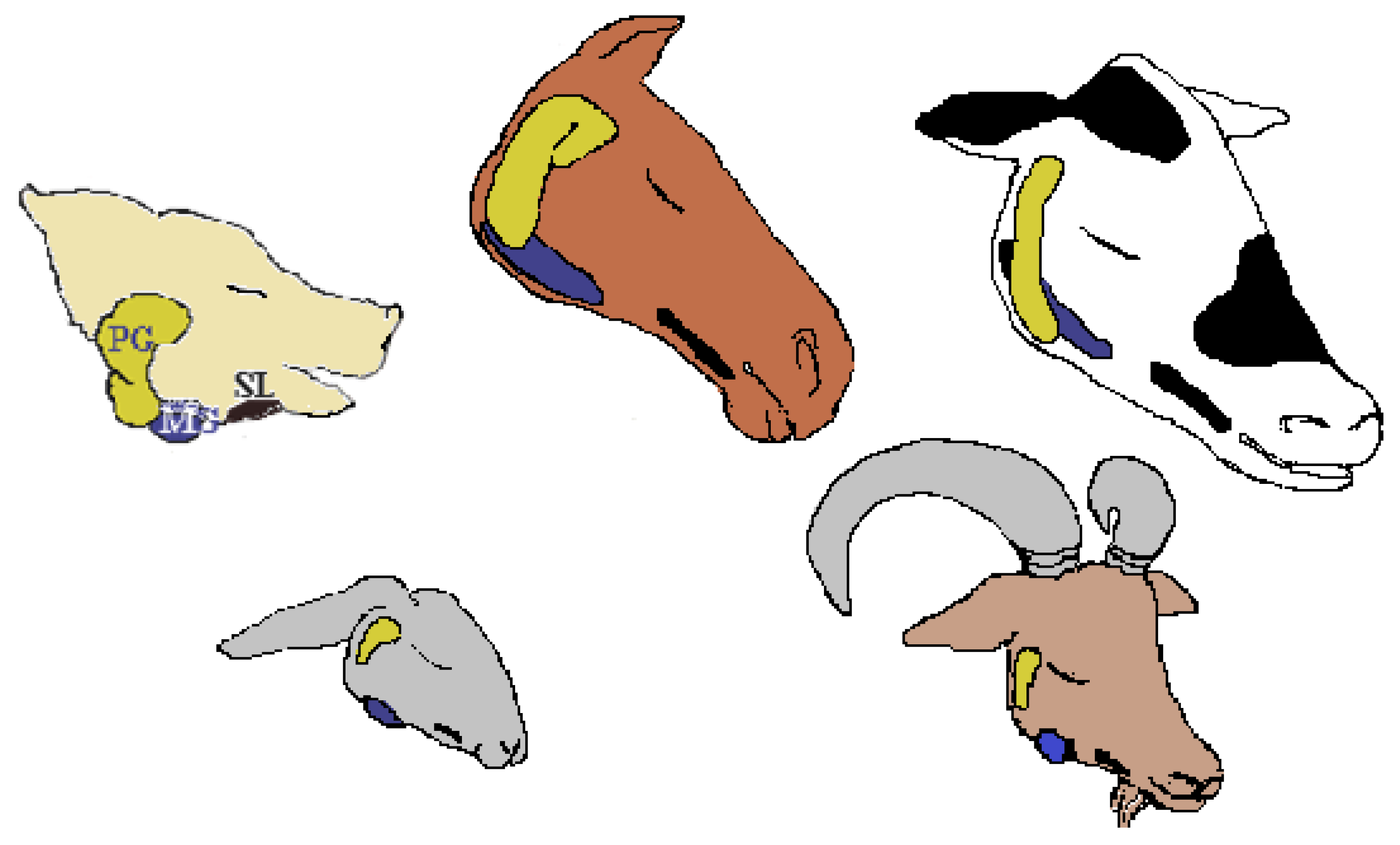
| Salivary Gland | Type of Gland | Type of Secretion |
|---|---|---|
| Parotid gland | Exocrine | Serous, pure |
| Mandibular gland | Exocrine | Mixed, mucous and serous, prevalently serous (demilune of Giannuzzi) |
| Sublingual gland | Exocrine | Mixed, mucous and serous, prevalently mucous |
| Animal Species | Salivary Response | Biologically Active Substance | Feed Sources |
|---|---|---|---|
| Pig | Parotid glands | Hydrolyzable tannins [19,20,21] | Oak acorns |
| Horse | Parotid glands | Ergot alkaloids [20] | Contaminated Poaceae by Claviceps purpurea |
| Goat | Parotid glands | Tannins [44,45,46,47] | Different seeds and foliage |
| Deer | Parotid glands and Mandibular glands | Tannins [48,49,50] | Different seeds and foliage; quebracho |
| Sheep | Parotid glands | Condensed tannins [51] | Cistus ladanifer L. |
| Rabbit | Parotid glands | Hydrolyzable tannins [52] | Oak acorns |
| Squirrel | Parotid glands | Tannins [14] | Oak acorns |
| Hamster, mouse | Parotid glands | Tannins [16] | Different seeds and nuts; isoproterenol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappai, M.G.; Aboling, S.; Dall’Aglio, C. Beyond Digestion: Can Animals Shape the Landscape According to Their Species–Specific Salivary Secretions? Agriculture 2021, 11, 817. https://doi.org/10.3390/agriculture11090817
Cappai MG, Aboling S, Dall’Aglio C. Beyond Digestion: Can Animals Shape the Landscape According to Their Species–Specific Salivary Secretions? Agriculture. 2021; 11(9):817. https://doi.org/10.3390/agriculture11090817
Chicago/Turabian StyleCappai, Maria Grazia, Sabine Aboling, and Cecilia Dall’Aglio. 2021. "Beyond Digestion: Can Animals Shape the Landscape According to Their Species–Specific Salivary Secretions?" Agriculture 11, no. 9: 817. https://doi.org/10.3390/agriculture11090817
APA StyleCappai, M. G., Aboling, S., & Dall’Aglio, C. (2021). Beyond Digestion: Can Animals Shape the Landscape According to Their Species–Specific Salivary Secretions? Agriculture, 11(9), 817. https://doi.org/10.3390/agriculture11090817







