The Impact of LED Light Spectrum on the Growth, Morphological Traits, and Nutritional Status of ‘Elizium’ Romaine Lettuce Grown in an Indoor Controlled Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Experimental Combinations—Light
2.3. Experimental Combinations—Composition of Nutrient Solution
2.4. Growth and Morphological Characteristics of Plants
2.5. Chlorophyll, Flavonol, and Nitrogen Balance Indices
2.6. Experimental Design and Statistical Analysis
3. Results
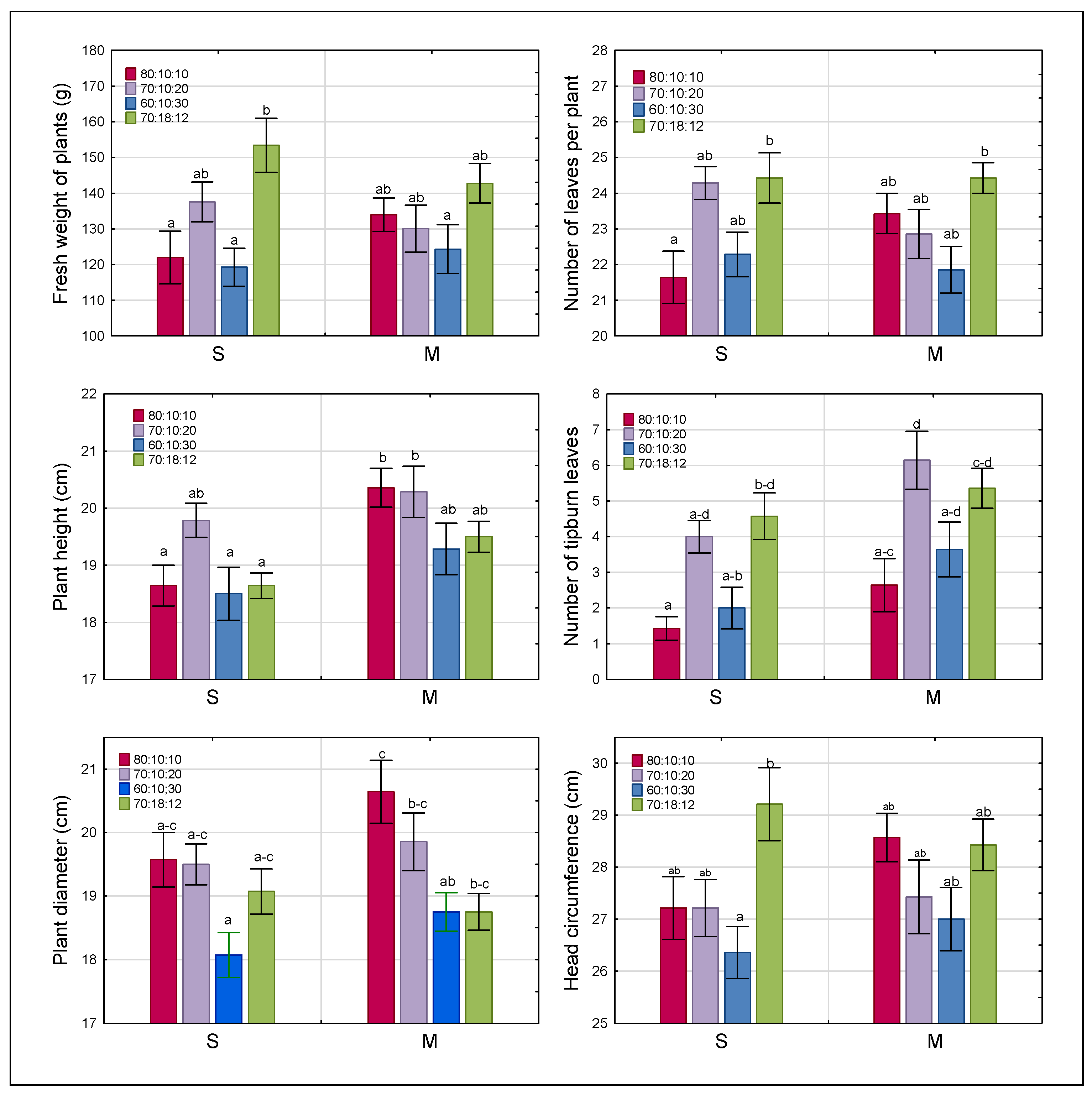
3.1. Growth and Morphological Characteristics of Plants
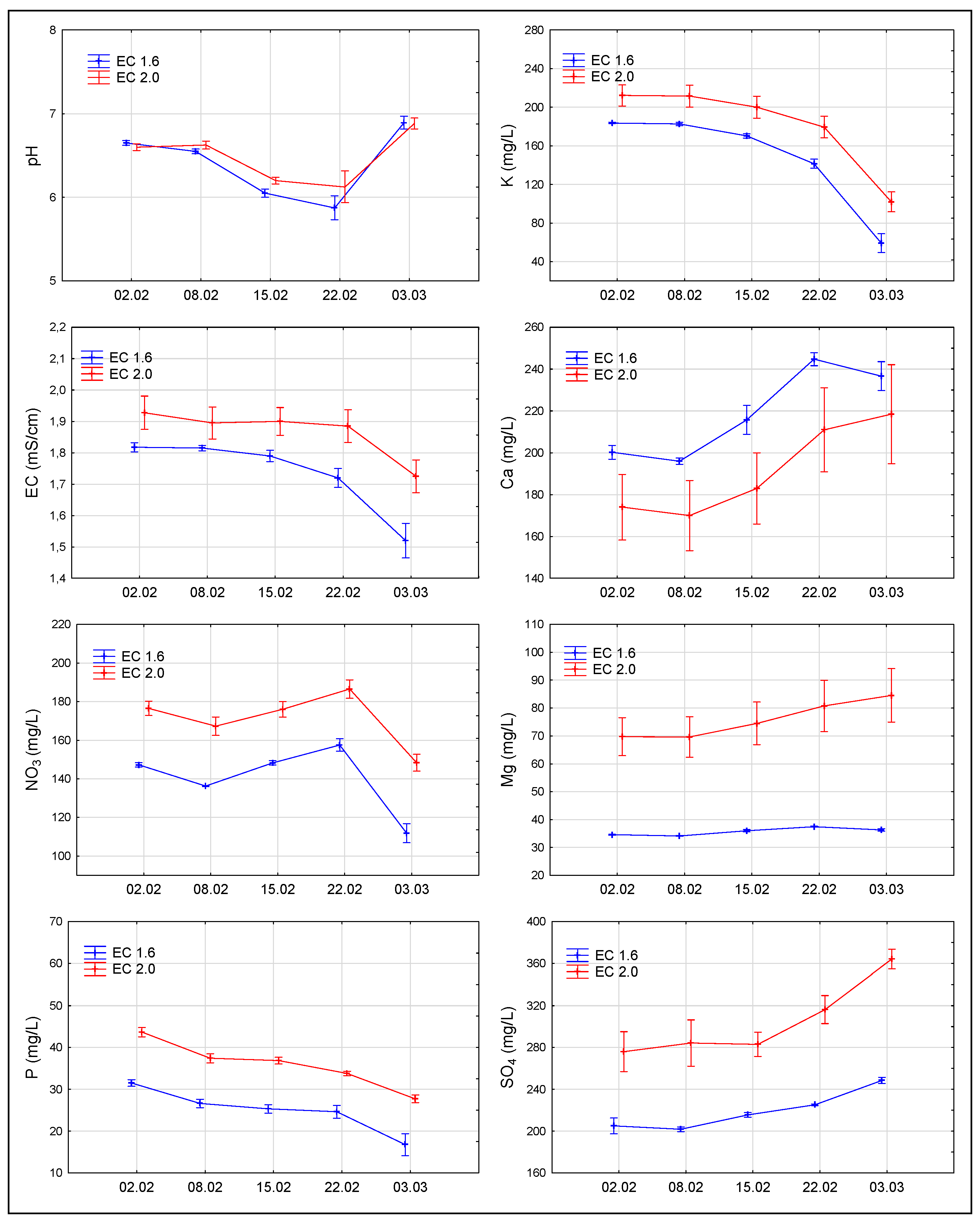
3.2. Changes in the Composition of Nutrient Solution
3.3. Mineral Composition of Plants
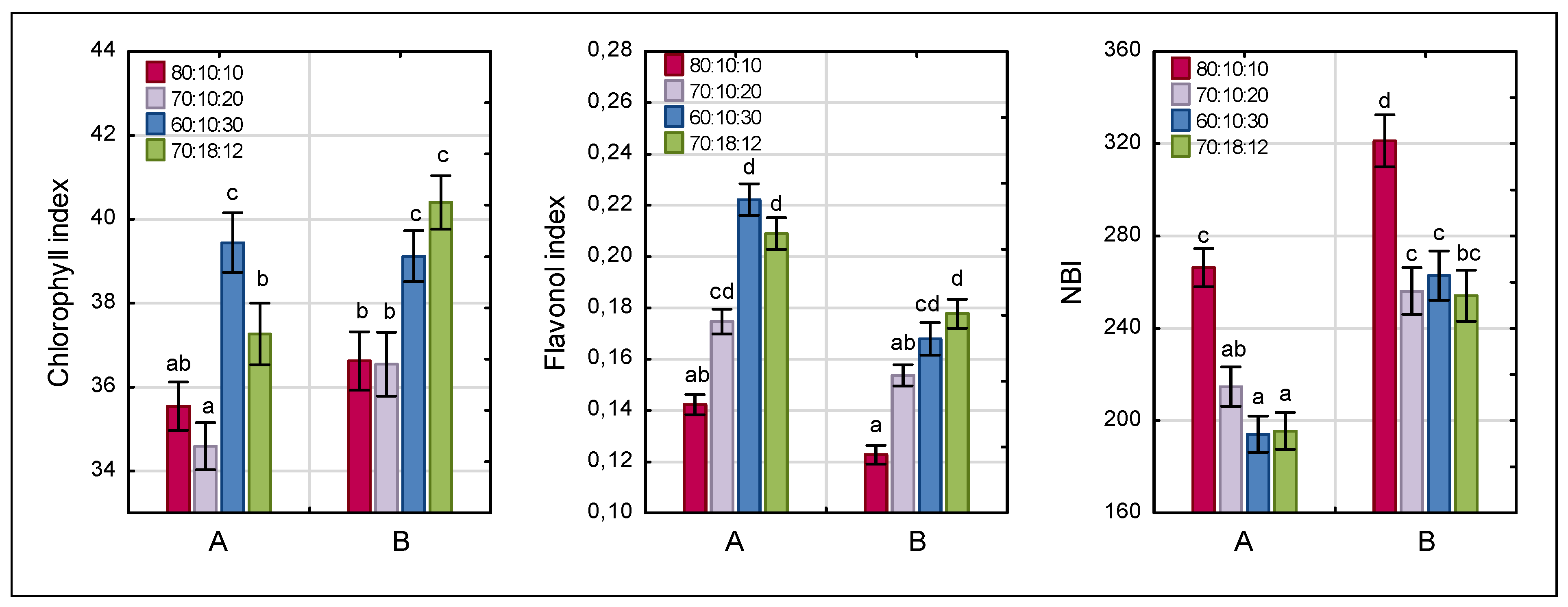
3.4. Chlorophyll, Flavonol, and Nitrogen Balance Indices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kozai, T.; Niu, G. Plant factory as a resource-efficient closed plant production system. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: Cambridge, MA, USA, 2016; pp. 69–90. [Google Scholar]
- Kalantari, F.; Mohd Tahir, O.; Mahmoudi Lahijani, A.; Kalantari, S. A review of vertical farming technology: A guide for implementation of building integrated agriculture in cities. Adv. Eng. Forum 2017, 24, 76–91. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K. Functional components in leafy vegetables. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: Cambridge, MA, USA, 2016; pp. 177–185. [Google Scholar]
- Gómez, C.; Jiménez, J. Effect of end-of-production high-energy radiation on nutritional quality of indoor-grown red-leaf lettuce. HortScience 2020, 55, 1055–1060. [Google Scholar] [CrossRef]
- Graamans, L.; Baeza, E.; van den Dobbelsteen, A.; Tsafaras, I.; Stanghellini, C. Plant factories versus greenhouses: Comparison of resource use efficiency. Agric. Syst. 2018, 160, 31–43. [Google Scholar] [CrossRef]
- van Iersel, M.W. Optimizing LED lighting in controlled environment agriculture. In Light Emitting Diodes for Agriculture; Springer: Singapore, 2017; pp. 59–80. [Google Scholar]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hort. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Kang, W.H.; Park, J.S.; Park, K.S.; Son, J.E. Leaf photosynthetic rate, growth, and morphology of lettuce under different fractions of red, blue, and green light from light-emitting diodes (LEDs). Hortic. Environ. Biotechnol. 2016, 57, 573–579. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fujiwara, K.; Kurata, K. Effect of light quality on growth and vegetable quality in leaf lettuce, spinach and komatsuna. Environ. Control Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Lee, J.S.; Lim, T.G.; Kim, Y.H. Growth and phytochemicals in lettuce as affected by different ratios of blue to red LED radiation. Acta Hortic. 2014, 1037, 843–848. [Google Scholar] [CrossRef]
- Shen, Y.Z.; Guo, S.S.; Ai, W.D.; Tang, Y.K. Effects of illuminants and illumination time on lettuce growth, yield and nutritional quality in a controlled environment. Life Sci. Space Res. 2014, 2, 38–42. [Google Scholar] [CrossRef]
- Han, T.; Vaganov, V.; Cao, S.; Li, Q.; Ling, L.; Cheng, X.; Pen, L.; Zhang, C.; Yakovlev, A.N.; Zhong, Y.; et al. Improving “color rendering” of LED lighting for the growth of lettuce. Sci. Rep. 2017, 7, 45944. [Google Scholar] [CrossRef]
- Liu, H.; Fu, Y.; Hu, D.; Yu, J.; Liu, H. Effect of green, yellow and purple radiation on biomass, photosynthesis, morphology and soluble sugar content of leafy lettuce via spectral wavebands “knock out”. Sci. Hortic. 2018, 236, 10–17. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Chow, W.S. Impacts of LED spectral quality on leafy vegetables: Productivity closely linked to photosynthetic performance or associated with leaf traits? Int. J. Agric. Biol. Eng. 2019, 12, 16–25. [Google Scholar] [CrossRef]
- Chang, C.L.; Chang, K.P. The growth response of leaf lettuce at different stages to multiple wavelength-band light-emitting diode lighting. Sci. Hortic. 2014, 179, 78–84. [Google Scholar] [CrossRef]
- Chen, X.L.; Guo, W.Z.; Xue, X.Z.; Wang, L.C.; Qiao, X.J. Growth and quality responses of ‘Green Oak Leaf’ lettuce as affected by monochromic or mixed radiation provided by fluorescent lamp (FL) and light-emitting diode (LED). Sci. Hortic. 2014, 172, 168–175. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [Green Version]
- Azad, O.K.; Kjaer, K.H.; Adnan, M.; Naznin, M.T.; Lim, J.D.; Sung, I.J.; Park, C.H.; Lim, Y.S. The evaluation of growth performance, photosynthetic capacity, and primary and secondary metabolite content of leaf lettuce grown under limited irradiation of blue and red LED light in an urban plant factory. Agriculture 2020, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Son, K.H.; Lee, J.H.; Oh, Y.; Kim, D.; Oh, M.M.; In, B.C. Growth and bioactive compound synthesis in cultivated lettuce subject to light-quality changes. HortScience 2017, 52, 584–591. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef] [Green Version]
- Muneer, S.; Kim, E.-J.; Park, J.-S.; Lee, J.-H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 515, 4657–4670. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red-and blue light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef] [Green Version]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.E.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Jishi, T.; Kimura, K.; Matsuda, R.; Fujiwara, K. Effects of temporally shifted irradiation of blue and red LED light on cos lettuce growth and morphology. Sci. Hortic. 2016, 198, 227–232. [Google Scholar] [CrossRef]
- Mickens, M.A.; Skoog, E.J.; Reese, L.E.; Barnwell, P.L.; Spencer, L.E.; Massa, G.D.; Wheeler, R.M. A strategic approach for investigating light recipes for ‘Outredgeous’ red romaine lettuce using white and monochromatic LED. Life Sci. Space Res. 2018, 19, 53–62. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Kopsell, D.A.; Park, S.; Tou, J.C.; Waterland, N.L. Nutritional value of crisphead ‘Iceberg’ and romaine lettuces (Lactuca sativa L.). J. Agric. Sci. 2016, 8, 734. [Google Scholar] [CrossRef] [Green Version]
- Sago, Y. Effects of light intensity and growth rate on tipburn development and leaf calcium concentration in butterhead lettuce. HortScience 2016, 51, 1087–1091. [Google Scholar] [CrossRef]
- Jenni, S.; Hayes, R.J. Genetic variation, genotype × environment interaction, and selection for tipburn resistance in lettuce in multi-environments. Euphytica 2010, 171, 427–439. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y.; Tang, J. Effects of different light intensities on anti-oxidative enzyme activity, quality and biomass in lettuce. Sci. Hort. 2012, 39, 129–134. [Google Scholar]
- Brazaitytè, A.; Miliauskienè, J.; Vaštakaitè-Kairienè, V.; Sutulienè, R.; Laužikè, K.; Duchovskis, P.; Małek, S. Effect of different ratios of blue and red LED light on Brassicaceae microgreens under a controlled environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Genotype-specific modulatory effects of select spectral bandwidths on the nutritive and phytochemical composition of microgreens. Front. Plant Sci. 2019, 10, 1501. [Google Scholar] [CrossRef]
- Sabat, T.; Kaniszewski, S.; Dyśko, J. Flood fertigation of leaf lettuce grown in various substrates. J. Elem. 2019, 24, 19–29. [Google Scholar] [CrossRef]
- Sabat, T.; Kaniszewski, S.; Dyśko, J. Effect of flood fertigation on yield of greenhouse lettuce grown in different substrates. J. Elem. 2015, 20, 407–416. [Google Scholar] [CrossRef] [Green Version]
- European Commission. European Commission Regulation EC No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Boss, C.H.; Fredeen, K.J. Concepts, Instrumentation, and Techniques in Inductively Coupled Plasma Optical Emission Spectrometry, 3rd ed.; Perkin Elmer: Shelton, CT, USA, 2004; Available online: https://www.perkinelmer.com/lab-solutions/resources/docs/GDE_Concepts-of-ICP-OES-Booklet.pdf (accessed on 5 January 2021).
- Latimer, G., Jr. Official Methods of Analysis, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012; ISBN 978-0-935584-83-7. [Google Scholar]
- Amoozgar, A.; Mohammadi, A.; Sabzalian, M.R. Impact of light-emitting diode irradiation on photosynthesis, phytochemical composition and mineral element content of lettuce cv. Grizzly. Photosynthetica 2017, 55, 85–95. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Growth responses of red-leaf lettuce to temporal spectral changes. Front. Plant Sci. 2020, 11, 571788. [Google Scholar] [CrossRef]
- Wissemeier, A.H.; Zühlke, G. Relation between climatic variables, growth and the incidence of tipburn in field-grown lettuce as evaluated by simple, partial and multiple regression analysis. Sci. Hortic. 2002, 93, 193–204. [Google Scholar] [CrossRef]
- Assimakopoulou, A.; Kotsiras, A.; Nifakos, K. Incidence of lettuce tipburn as related to hydroponic system and cultivar. J. Plant Nutr. 2013, 36, 1383–1400. [Google Scholar] [CrossRef]
- Carotti, L.; Graamans, L.; Puksic, F.; Butturini, M.; Meinen, E.; Heuvelink, E.; Stanghellini, C. Plant factories are heating up: Hunting for the best combination of light intensity, air temperature and root-zone temperature in lettuce production. Front. Plant Sci. 2021, 11, 592171. [Google Scholar] [CrossRef] [PubMed]
- Jenni, S.; Truco, M.J.; Michelmore, R.W. Quantitative trait loci associated with tipburn, heat stress-induced physiological disorders, and maturity traits in crisphead lettuce. Theor. Appl. Genet. 2013, 126, 3065–3079. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.C.; Wells, D.E.; Pickens, J.M.; Kemble, J.M. Selection of heat tolerant lettuce (Lactuca sativa L.) cultivars grown in deep water culture and their marketability. Horticulturae 2019, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Macias-González, M.; Truco, M.J.; Bertier, L.D.; Jenni, S.; Simko, I.; Hayes, R.J.; Michelmore, R.W. Genetic architecture of tipburn resistance in lettuce. Theor. Appl. Genet. 2019, 132, 2209–2222. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red:blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef] [PubMed]
- Maynard, D.N.; Hochmuth, G.J. Knott’s Handbook for Vegetable Growers, 5th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2013; p. 642. ISBN 978-1-118-68610-2. [Google Scholar]
- Iammarino, M.; Taranto, A.; Cristino, M. Monitoring of nitrites and nitrates levels in leafy vegetables (spinach and lettuce): A contribution to risk assessment. J. Sci. Food Agric. 2014, 94, 773–778. [Google Scholar] [CrossRef]
- Pinho, P.; Jokinen, K.; Halonen, L. The influence of the LED light spectrum on the growth and nutrient uptake of hydroponically grown lettuce. Lighting Res. Technol. 2017, 49, 866–881. [Google Scholar] [CrossRef]
- Loconsole, D.; Cocetta, C.; Santoro, P.; Ferrante, A. Optimization of LED lighting and quality evaluation of Romaine Lettuce grown in an innovative indoor cultivation system. Sustainability 2019, 11, 841. [Google Scholar] [CrossRef] [Green Version]
- Samuolienė, G.; Samuolienė, A.; Duchovskis, P.; Bliznikas, Z.; Vitta, P.; Žukauskas, A. Decrease in nitrate concentration in leafy vegetables under a solid-state illuminator. HortScience 2009, 44, 1857–1860. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhou, Q.; Qu, Y. Growth, nutritional quality, and energy use efficiency of hydroponic lettuce as influenced by daily light integrals exposed to white versus white plus red light-emitting diodes. Hortscience 2019, 54, 1737–1744. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Fu, Y.; Yu, J.; Liu, H. Accumulation and primary metabolism of nitrate in lettuce (Lactuca sativa L. var. Youmaica) grown under three different light sources. Commun. Soil Sci. Plant Anal. 2016, 47, 1994–2002. [Google Scholar] [CrossRef]
- Clavijo-Herrera, J.; van Santen, E.; Gómez, C. Growth, water-use efficiency, stomatal conductance, and nitrogen uptake of two lettuce cultivars grown under different percentages of blue and red light. Horticulturae 2018, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Boldt, J.; Runkle, E.S. Blue radiation interacts with green radiation to influence growth and predominantly controls quality attributes of lettuce. J. Am. Soc. Hortic. Sci. 2020, 145, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Ashkar, S.A.; Ries, S.K. Lettuce tipburn as related to nutrient imbalance and nitrogen composition. J. Am. Soc. Hortic. Sci. 1971, 96, 448–452. [Google Scholar]
- Saure, M.C. Causes of the tipburn disorder in leaves of vegetables. Sci. Hortic. 1998, 76, 131–147. [Google Scholar] [CrossRef]
- Barta, D.J.; Tibbitts, T.W. Calcium localization and tipburn development in lettuce leaves during early enlargement. J. Am. Soc. Hortic. Sci. 2000, 125, 294–298. [Google Scholar] [CrossRef] [Green Version]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef] [PubMed]
- Birlanga, V.; Acosta-Motos, J.R.; Pérez-Pérez, J.M. Genotype-dependent tipburn severity during lettuce hydroponic culture is associated with altered nutrient leaf content. Agronomy 2021, 11, 616. [Google Scholar] [CrossRef]
- Lee, J.G.; Choi, C.S.; Jang, Y.A.; Jang, S.W.; Lee, S.G.; Um, Y.C. Effects of air temperature and air flow rate control on the tipburn occurrence of leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 303–310. [Google Scholar] [CrossRef]
- Ouzounis, T.; Fretté, X.; Rosenqvist, E.; Ottosen, C.-O. Spectral effects of supplementary lighting on the secondary metabolites in roses, chrysanthemums, and campanulas. J. Plant Physiol. 2014, 171, 1491–1499. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B. Proximal optical sensing of cucumber N status using chlorophyll fluorescence indices. Eur. J. Agron. 2015, 73, 83–97. [Google Scholar] [CrossRef]
- Agati, G.; Tuccio, L.; Kusznierewicz, B.; Chmiel, T.; Bartoszek, A.; Kowalski, A.; Grzegorzewska, M.; Kosson, R.; Kaniszewski, S. Nondestructive optical sensing of flavonols and chlorophyll in white head cabbage (Brassica oleracea L. var. capitata subvar. alba) grown under different nitrogen regimens. J. Agric. Food Chem. 2016, 64, 85–94. [Google Scholar] [CrossRef]
- Kaniszewski, S.; Kowalski, A.; Dyśko, J.; Agati, G. Application of a combined transmittance /fluorescence leaf clip sensor for the non-destructive determination of nitrogen status in white cabbage plants. Sensors 2021, 21, 482. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, B.; Kowalski, A. The growth, photosynthetic parameters and nitrogen status of basil, coriander and oregano grown under different led light spectra. Acta Sci. Pol. Hortorum Cultus 2021, 20, 13–22. [Google Scholar] [CrossRef]
- Hooks, T.; Masabni, J.; Sun, L.; Niu, G. Effect of pre-harvest supplemental UV-A/Blue and Red/Blue LED lighting on lettuce growth and nutritional quality. Horticulturae 2021, 7, 80. [Google Scholar] [CrossRef]
- Deng, B.; Li, Y.; Xu, D.; Qingqing, Y.; Guihua, L. Nitrogen availability alters flavonoid accumulation in Cyclocarya paliurus via the effects on the internal carbon/nitrogen balance. Sci. Rep. 2019, 9, 2370. [Google Scholar] [CrossRef]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Liu, H. Nutritional quality, mineral and antioxidant content in lettuce affected by interaction of light intensity and nutrient solution concentration. Sci. Rep. 2020, 10, 2796. [Google Scholar] [CrossRef] [Green Version]
- Wan, H.; Zhang, J.; Song, T.; Tian, J.; Yao, Y. Promotion of flavonoid biosynthesis in leaves and calli of ornamental crab apple (Malus sp.) by high carbon to nitrogen ratios. Front. Plant Sci. 2015, 6, 673. [Google Scholar] [CrossRef] [Green Version]
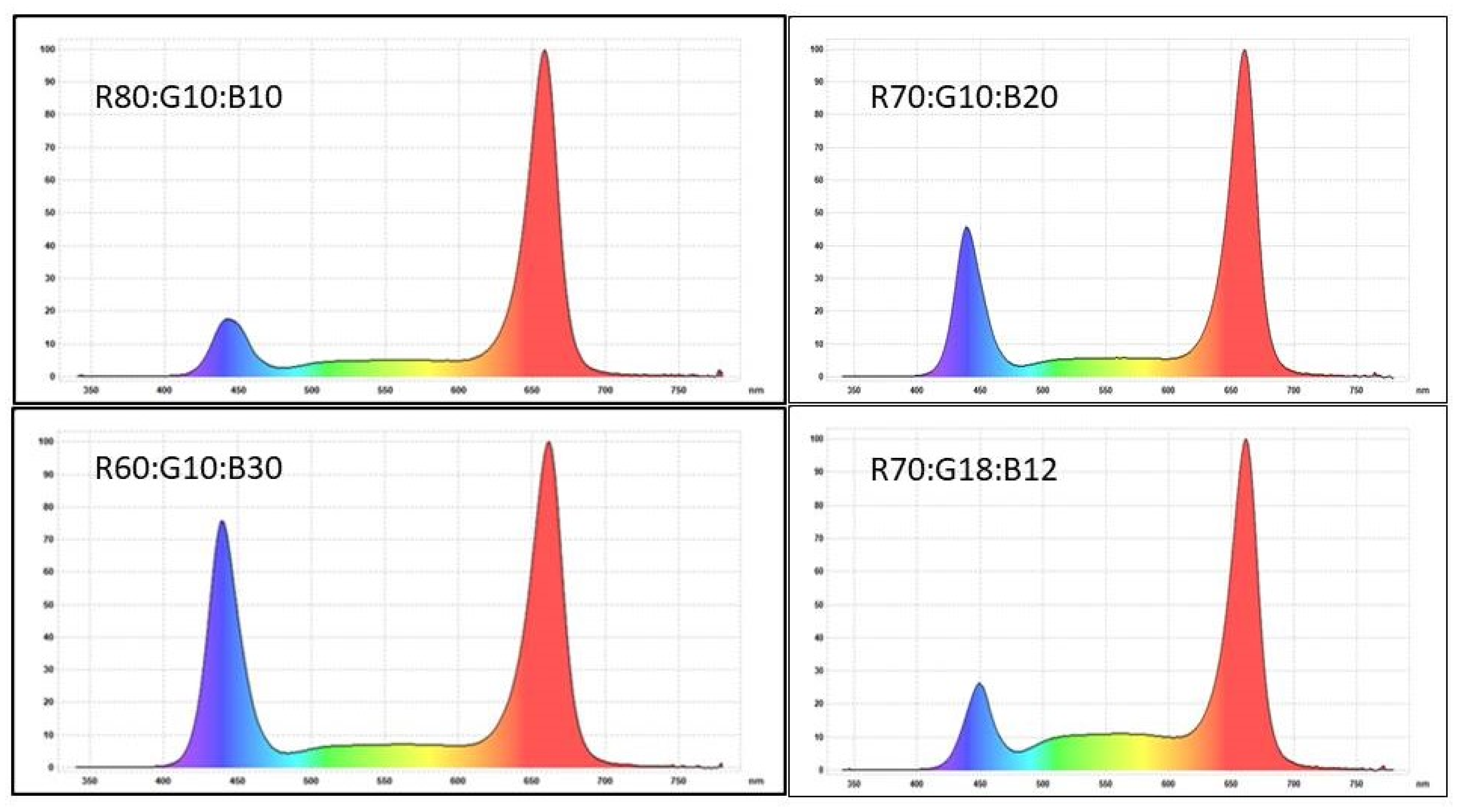
| Light Spectrum R:G:B (Red:Green:Blue) | UV-A 340–399 nm | Blue 400–499 nm | Green 500–599 nm | Red 600–699 nm | Far-Red 700–780 nm | R:B Ratio | Total nm |
|---|---|---|---|---|---|---|---|
| 80:10:10 | 0 | 45.2 | 45.0 | 349.5 | 1.3 | 7.7 | 441.0 |
| 70:10:20 | 0 | 89.6 | 42.0 | 306.6 | 1.0 | 3.4 | 439.2 |
| 60:10:30 | 0 | 127.4 | 46.5 | 267.2 | 1.1 | 2.1 | 442.2 |
| 70:18:12 | 0 | 52.0 | 80.3 | 304.1 | 1.8 | 5.8 | 438.2 |
| Growth and Morphological Trait | Light Spectrum | Nutrient Solution | Light Spectrum × Nutrient Solution |
|---|---|---|---|
| Fresh weight of leaves | 0.0005 | 0.9529 | 0.2375 |
| Plant height | 0.0119 | 0.0001 | 0.3899 |
| Plant diameter | 0.0001 | 0.1018 | 0.3182 |
| Head circumference | 0.0035 | 0.3910 | 0.3258 |
| Number of leaves per plant | 0.0009 | 0.9673 | 0.0734 |
| Percentage tipburn | 0.0001 | 0.0016 | 0.7371 |
| Chlorophyll index | 0.0001 | 0.0019 | 0.0651 |
| Flavonol index | 0.0001 | 0.0001 | 0.0720 |
| NBI | 0.0001 | 0.0001 | 0.5550 |
| Treatment | N-NO3 | N | P | K | Ca | Mg | Fe | Mn | Cu | Zn | B |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 f.w. | % | mg kg−1 d.w. | |||||||||
| Light spectrum R:G:B | |||||||||||
| 80:10:10 | 3538 a | 4.40 a | 0.66 a | 8.38 b | 1.20 a | 0.41 b | 124 a | 149 a | 6.3 a | 51 a | 72 c |
| 70:10:20 | 3909 a | 4.34 a | 0.66 a | 8.37 b | 1.24 a | 0.41 b | 135 a | 126 a | 6.0 a | 45 a | 63 b |
| 60:10:30 | 3538 a | 4.36 a | 0.65 a | 8.10 ab | 1.18 a | 0.42 b | 135 a | 131 a | 5.8 a | 48 a | 58 ab |
| 70:18:12 | 3128 a | 4.24 a | 0.64 a | 7.77 a | 1.18 a | 0.34 a | 146 a | 126 a | 5.6 a | 43 a | 57 a |
| Nutrient solution | |||||||||||
| A | 3218 a | 4.22 a | 0.64 a | 8.52 b | 1.20 a | 0.38 a | 141 a | 134 a | 6.5 b | 43 a | 69 b |
| B | 3899 b | 4.40 b | 0.67 b | 7.79 a | 1.20 a | 0.41 b | 130 a | 133 a | 5.4 a | 50 b | 55 a |
| Light spectrum × nutrient solution | n.s. | n.s. | n.s. | n.s | n.s. | n.s. | n.s. | n.s. | * | n.s. | * |
| Sufficient range (%) * | - | 2.1–5.6 | 0.5–0.9 | 4.0–8.0 | 0.9–2.0 | 0.4–0.8 | 50–200 | 25–200 | 5–18 | 30–200 | 25–65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matysiak, B.; Kaniszewski, S.; Dyśko, J.; Kowalczyk, W.; Kowalski, A.; Grzegorzewska, M. The Impact of LED Light Spectrum on the Growth, Morphological Traits, and Nutritional Status of ‘Elizium’ Romaine Lettuce Grown in an Indoor Controlled Environment. Agriculture 2021, 11, 1133. https://doi.org/10.3390/agriculture11111133
Matysiak B, Kaniszewski S, Dyśko J, Kowalczyk W, Kowalski A, Grzegorzewska M. The Impact of LED Light Spectrum on the Growth, Morphological Traits, and Nutritional Status of ‘Elizium’ Romaine Lettuce Grown in an Indoor Controlled Environment. Agriculture. 2021; 11(11):1133. https://doi.org/10.3390/agriculture11111133
Chicago/Turabian StyleMatysiak, Bożena, Stanisław Kaniszewski, Jacek Dyśko, Waldemar Kowalczyk, Artur Kowalski, and Maria Grzegorzewska. 2021. "The Impact of LED Light Spectrum on the Growth, Morphological Traits, and Nutritional Status of ‘Elizium’ Romaine Lettuce Grown in an Indoor Controlled Environment" Agriculture 11, no. 11: 1133. https://doi.org/10.3390/agriculture11111133
APA StyleMatysiak, B., Kaniszewski, S., Dyśko, J., Kowalczyk, W., Kowalski, A., & Grzegorzewska, M. (2021). The Impact of LED Light Spectrum on the Growth, Morphological Traits, and Nutritional Status of ‘Elizium’ Romaine Lettuce Grown in an Indoor Controlled Environment. Agriculture, 11(11), 1133. https://doi.org/10.3390/agriculture11111133






