Abstract
Microgreens, considered “superfood”, are easy to cultivate and very rich in health-promoting compounds, as antioxidants. White cabbage (Brassica oleracea var. capitata) microgreens contain high quantities of phenolics, which contribute together with other bioactive compounds to their important antioxidant properties. The present study analyses the effects of development stage (5-, 7- and 9-days) and two sodium salts, NaCl and Na2SO4, in two concentrations (0.01 M and 0.1 M), on the antioxidant properties of white cabbage microgreens. Among the three development levels, the 5-day microgreens revealed the highest total phenolic content, DPPH radical scavenging and total reducing capacities. Concerning the effects of sodium salts, 0.01 M NaCl and 0.1 M Na2SO4 determined higher total reducing capacity. Additionally, 0.01 M NaCl induced the highest DPPH radical scavenging capacity, while the most important total phenolics and anthocyanins contents were obtained in case of 0.1 M Na2SO4. In conclusion, from the developmental point of view, the 5-day old microgreens present, globally, the best characteristics. Considering the elicitor effects of sodium salts, 0.01 M NaCl and 0.1 M Na2SO4 generally determined the strongest antioxidant properties. The results could be used to develop new production technologies for antioxidant-enriched microgreens.
1. Introduction
Nowadays, world population is subjected to severe trials in the context of COVID-19 pandemic threat. Today’s people, generally, have low immunity due to stress, pollution, sedentary lifestyle and unhealthy diet. Now, more than ever, it is to be taken into account the possibility of improving humans’ immunity and general condition through health-promoting food. Microgreens and sprouts are considered such functional foods (products that offer health benefits beyond their nutritional value) due to their high content in antioxidants and other bioactive compounds [1,2]. Both microgreens and sprouts are early stages of the plant development. Sprouts are germinated seeds, the very first stage of a plant; are grown basically on water and harvested at 2- to 7-days old; the whole being edible: seed, root, and stem [3,4]. Microgreens are the immediate superior stage of development, the “cotyledon growth stage”, when the first couple of leaves appears; are grown in soil, peat moss or similar substrate and harvested at 5- to 21-days old, by cutting the above-soil part, which is edible: the stem and developing leaves [3,4]. This differentiation among the two types of products was clearly formulated the latest years. Previously, most of the studies were treating both sprouts and microgreens, as sprouts. For example, there are publications presenting results concerning 10-day “sprouts” of white and red cabbage [5], 11-day broccoli “sprouts” [6] or 12-day tronchuda cabbage “sprouts” [7]. More than that, 4 varieties of Brassica oleracea “sprouts” were grown 9 days on vermiculite, in growth chamber, and were harvested by cutting above the substrate [8]. However, no matter if they are named microgreens or sprouts, it was repetitively proved by numerous studies that these edible seedlings contain superior quantities of antioxidants and other bioactive compounds then their seeds and mature plants [1,6,9]. Some authors even name microgreens and sprouts as “superfood” [3,10].
Among edible seedlings of various families, those of cruciferous (including white cabbage) have the advantage of different classes of bioactive compounds, e.g., phenolics (the main antioxidants), vitamins, and the specific Brassicaceae constituents-glucosinolates and isothiocyanates. They have antioxidant, antibacterial, antiviral, antiinflammatory properties, improve the immunity and offer protection against cancers, cardio-vascular and degenerative diseases [11,12,13,14,15,16,17,18]. The phenolic compounds are very active in human body protection at the cellular level against the negative effects of free radicals [19]. More than that, it was recently noted that phenolics are “the most promising small molecules identified as coronavirus inhibitors” [20].
Microgreens and sprouts are easy to produce in a short period of time and the content in bioactive compounds depends on their development (number of days since sowing) [21] and can be affected by different treatments or technologies [22,23,24], e.g., a salt-enriched growth substrate. Hassini et al. (2017) observed that NaCl treatments slightly influenced the total phenolics, anthocyanins and flavonols of white and red cabbage 10-days “sprouts” [5]. Yuan et al., 2010 noticed also the change of antioxidant capacity and total phenolics of radish sprouts in presence of NaCl, and depending on the salt concentration, it was registered an improvement or, contrary, a diminution [25]. The general conclusion was that “adequate salt stress could be one useful way to enhance health-promoting compounds of plant food” [25].
The present study aims to investigate the possibilities to improve the health-promoting potential of white cabbage microgreens by harvesting at the most appropriate moment of their growth, or by using non-conventional conditions for germination in order to stimulate the biosynthesis of phenolic compounds, the main antioxidants of Brassicaceaes. It explores the effects of germination duration (5, 7 and 9 days) and two sodium salts—sodium chloride and sodium sulphate—administrated in two concentrations (0.01 M and 0.1 M), on the phenolic content and antioxidant properties reflected by the Folin-Ciocalteu total reducing capacity and DPPH radical scavenging capacity of white cabbage microgreens. The results can be used for the elaboration of new production technologies for antioxidant-enriched microgreens.
2. Materials and Methods
2.1. Production of Microgreens
The experiment used white cabbage seeds of cultivar Mercado de Copenhague 2, from Amia Company (Otopeni, Romania), sterilized with 0.07% sodium hypochlorite and soaked 10 h in distilled water. Sprouting was performed in trays filled with a peat-based germination substrate (DSM 2w, from Kekkilä Garden Professional Substrate, Vantaa, Finland), in a climatic germination room (MRC PGI-500H, Holon, Israel). n = 200 seeds were sown in ditches of 7.5 ± 2.5 mm deep and 25 mm distance between rows. Sprouting conditions: first 60 h at 24 °C, humidity (RH) 80%, lighting 80 W with fluorescent lamps, and for the rest of time till harvest, at 22 °C, RH 80% and lighting 360 W. Microgreens were watered with distilled water and harvested at 5-, 7- and 9-day old. The effects of two sodium salts at 0.01 M and 0.1 M: sodium chloride (NaCl), and sodium sulphate (Na2SO4) were tested by replacing water with the same quantity of saline solution (110 ± 0.5 mL/tray/day) and by the direct administration on the substrate during 9 days, in 4 parallel assays, one for each concentration and each of them performed in triplicate and compared to untreated control. Treated microgreens were harvested at 9 days old.
2.2. Soluble Dry Matter (SDM)
It was determined in the juice resulted from squeezing the whole microgreens at 19 ± 1 °C, using a Zeiss portable refractometer, and was expressed as Brix degrees (°Bx).
2.3. Extraction
Hydroethanolic extracts were obtained from whole microgreens, using 70% ethanol acidified with 0.01% hydrochloric acid in a two-steps protocol described by Patras et al. (2017). Briefly, from each sample, 3.2 g of mixed microgreens were weighted to 4 decimal and grounded. Then, samples were extracted in presence of 15 mL ethanolic solvent for 30 min at 38 ± 1 °C on a water-bath under shaking. The obtained extract was centrifuged at 7000 rpm for 15 min. The remaining residue from centrifugation was re-extracted with 10 mL solvent for 15 min in the same conditions, followed by same centrifugation (15 min). The two supernatants, resulted from one sample, were mixed and stored at 4 °C prior to analysis [26].
2.4. Total Phenolic Content (TPC)
The direct reading of extract’s absorbance at 280 nm, after adequate dilution, according to the method described by Ribéreau-Gayon et al. (2006), was used for the total phenolic content determination. The results are expressed as gallic acid equivalents per fresh weight (mg GAE/100 g FW) using a calibration curve with gallic acid [27].
2.5. Total Anthocyanins Content (TAC)
The total content of anthocyanins was determined by pH differential method following the procedure described by Ribéreau-Gayon et al. (2006) with slight modifications. The pH of the extract was adjusted to 0.6 and 3.5, respectively, with adequate buffer solutions [27], as follows: 10 mL buffer was added to a mixture of 1 mL extract and 1 mL of 96% ethanol containing 0.1% HCl. Absorbances of both solutions were measured at 520 nm against a distilled water reference. The results were calculated from a calibration curve with cyanidin-3-glucoside, and reported as mg/100 g FW.
2.6. Total Reducing Capacity (TRC)
The Folin-Ciocalteu method is a measure of the total reducing capacity [28], but also reflects the phenolic content of a liquid sample. A mixture of 0.1 mL extract, 7.4 mL distilled water, 0.5 mL Folin-Ciocalteu reagent and 2 mL 20% Na2CO3 was kept in the dark for 30 min. The measurement of absorbance was done at 750 nm, compared to a blank (same mixture, but replacing the extract with 0.1 mL distilled water). The results were expressed as mg GAE/100 g FW, calculated from a calibration curve with gallic acid.
2.7. DPPH Radical Scavenging Capacity (RSC)
The Brand-Williams assay with slight modifications was used in order to analyze the antiradical activity of microgreens’ extracts [29]. The reduction of the absorbance at 515 nm of a DPPH• methanolic solution (0.12 mM) in the presence of studied extracts (ratio extract:DPPH• = 1:2) was measured against a methanol reference, after 5 min of incubation in the dark. The control sample contains 70% ethanol acidified with 0.01% hydrochloric acid, which replaces the microgreens extracts and DPPH• methanolic solution (0.12 mM), in same ratio (1:2). The results of DPPH RSC were expressed as mg ascorbic acid/100 g fresh weight (mg ascorbic acid/100 g FW) from a calibration curve with ascorbic acid.
2.8. Statistical Analysis
Three independent experiments were performed. The obtained data were represented as scatter plots (excepting the soluble dry matter), grouped by the growth stage factor (5, 7, and 9 days old) and treatment (9-day-old control/9-day-old treatment). Data were analyzed through the SPSS Statistics 21 (IBM) software, using one-way analysis of variance (ANOVA) and the post-hoc test Tukey Honestly Significance Difference (HSD). The significance level was α = 0.05. The factors are between-subjects. The degrees of freedom (df) for the between-groups estimate of variance, the F ratio and p-value are given in the legend of each graph. The between-groups estimate of variance forms the numerator of the F ratio. F represents the ratio between the mean square between-groups and the mean square within-groups. Each mean square was calculated by dividing the sum of square by its degrees of freedom. The p-value represents the significance of the F ratio. If the p-value is p ≤ α (p ≤ 0.05), the null hypothesis that all means are equal can be rejected and differences between some of the means are considered significant.
3. Results and Discussion
3.1. Soluble Dry Matter
During the growth of microgreens, the soluble dry matter (SDM) is significantly decreasing by 8.8% from 5 to 7 days and by 12.9% (from 3.41 to 2.97 °Bx) from 5 to 9 days (Table 1). The sodium chloride is slightly, but significantly decreasing the SDM of 9-days microgreens at 0.01 M, while at 0.1 M, the change is not significant. The sodium sulphate is increasing the SDM. At 0.01 M Na2SO4 the augmentation is 2%, insignificant statistically, while at 0.1 M Na2SO4 was registered a strong increase by 68.7% compared to untreated 9-days samples (from 2.97 to 5.01 °Bx).

Table 1.
Soluble dry matter (SDM), °Bx, of untreated (5-, 7- and 9-day) and 9-day treated (with NaCl or Na2SO4) white cabbage microgreens.
Similarly, a previous study found that 0.01 M NaCl decreased SDM in broccoli and cauliflower 9-day-old sprouts and 0.1 M Na2SO4 induced a powerful increase, comparable to present result [26]. Other previous studies found that salt stress enhanced the soluble dry matter and dry matter percent of cauliflower and broccoli mature florets [30,31]. Hassini et al. (2017) found that 0.15 M NaCl significantly increased the moisture percent of 10-days-old broccoli “sprouts”, which has the significance of a decrease of dry matter percent [22].
3.2. Total Phenolic Content
Some authors consider the direct reading of extract’s absorbance at 280 nm, a simple, fast and reliable method for the estimation of total phenolic content (TPC), although overestimation of the total phenolic content may occur due to the ability of other compounds to absorb the 280 nm UV-light, as proteins, or other compounds containing π conjugated systems with hydroxyl-phenolic groups [32,33]. Contrary, there are few phenolic compounds, such as cinnamic acids or chalcones, which do not show absorption features at 280 nm [33].
The total phenolic content (TPC) has considerable values in all evolution stages of cabbage microgreens (Figure 1A) and under all treatments (Figure 1B), being between 80 and 149 mg GAE/100 g FW. Its variation is similar to the evolution of soluble dry matter. TPC significantly decreases during the growth of white cabbage, by about 3% from 5-days to 7-days and by 18.36% from 5- to 9-days (from 108.63 to 88.69 mg GAE/100 g FW). This diminution must be the result of the use of phenolic compounds in different anabolic processes of the young plant’s metabolism. Sousa et al. found important loses in phenolics during the germination of tronchuda cabbage (Brassica oleracea var. costata DC) from 2 to 12 days [7]. Studying 3-, 4-, 5- and 6-days lentil sprouts, Fouad and Rehab (2015) stated the maximum concentration of TPC in 5-days sprouts (increasing from seeds to 3, 4 and 5-days) and a significant decrease in 6-days sprouts, which they considered to be due to the higher activity of polyphenol oxidase [21]. Similarly, Korus (2011) observed the increase of polyphenol oxidases’ activity in kale (also from Brassicaceae family) during seedlings maturation [34]. A possible similar increase may be an explanation for the observed decrease of TPC during the growth of microgreens in the present study.
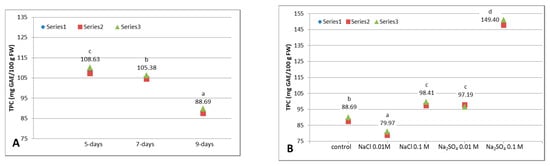
Figure 1.
Total phenolic content (TPC, mg GAE/100 g FW) of white cabbage microgreens. (A). Influence of growth: untreated 5-, 7- and 9-day-old microgreens (α = 0.05, between groups df = 2, F = 247.962, p = 0.000). (B). Influence of NaCl or Na2SO4 treatments on 9-day-old microgreens (α = 0.05, between groups df = 4, F = 1657.826, p = 0.000). Above the data points are displayed the mean values and a letter: different letters denote significant differences between values (Tukey HSD test). Series 1, 2 or 3 from the legend represent the 3 individual experiments.
The treatments, generally, increase the TPC, with only one exception: the natrium chloride at small concentration (0.01 M), which diminishes the TPC of 9-days untreated plants by 9.8% (which represents 8.72 mg GAE/100 g FW). The higher concentration of NaCl increases the 9-days control’s TPC by 11%. Similarly, Yuan et al. (2010) observed that 0.01 M NaCl decreased the TPC of 3- 5-, 7-day radish sprouts (another vegetable from Brassicaceae family), but did not affect its antioxidant activity [25]. Same authors found that 0.1 M NaCl increased the TPC in 3- and 5-day radish sprouts. Hassini et al. (2017) stated that 0.15 M NaCl decreased (non-significantly) the TPC of 10-days white cabbage edible seedlings [5]. These fluctuations of TPC in presence of different concentrations of salt can be explained by the complexity of metabolic processes in young plants. Phenolic compounds are generated by the phenylpropanoid pathway. The increase of TPC in presence of 0.1 M NaCl and both concentrations of Na2SO4 could be the result of the activation of this pathway. More than that, in present case, the decrease, in presence of 0.01 M NaCl, of TPC measured by absorbance at 280 nm, could be an artifact due, not to a real decrease of phenolic content, but to a destruction of some π conjugated systems with hydroxyl-phenolic groups which became unable to absorb the 280 nm UV-light. Other authors also noticed that “the phenolic compounds in plants can be changed by salt stress, but this is critically dependent on the salt sensitivity of plants” [25]. Further in-depth studies at molecular level should be performed in order to research the implications of various concentration of NaCl in the chain reactions of the phenylpropanoid pathway, or in any other plant processes.
While the impact of NaCl on sprouts and microgreens was intensively analyzed, only scarced information can be found concerning the Na2SO4 influence. In the present study, the natrium sulphate stimulates the augmentation of TPC at both concentrations, with an impressive increase, by more than 68% (from 88.69 to 149.40 mg GAE/100 g FW) in case of 0.1 M Na2SO4 and 9.6% in case of 0.01 M Na2SO4 (Figure 1B). Similar enhancement was registered in other Brassica edible seedlings (broccoli and cauliflower) in case of treatment with 0.1 M Na2SO4 [26]. Other study analyzed the elicitor effect of sulfur (administrated as K2SO4), not on phenolics, but on glucosinolates in broccoli sprouts [23].
3.3. Total Anthocyanins Content
Anthocyanin pigments belong to the class of phenolic compounds, subclass flavonoids. They can exist in very small quantities in some varieties of white cabbage [35], but they are characteristic for the red cabbage. Our research found very small amounts of anthocyanins (between 0.28–0.71 mg/100 g FW) in all untreated microgreens of the studied white cabbage cultivar (Mercado de Copenhague 2). The content of total anthocyanins (TAC) was analyzed due to their importance in a healthy diet and their contribution to the antioxidant capacity of the plant extract. During the growth of white cabbage microgreens was registered an insignificant increase of TAC from 0.28 mg/100 g FW (at 5-days microgreens), to 0.43 and to 0.71 mg/100 g FW at 7- and respectively, 9-days-old (Figure 2A). All the applied sodium salts increased the anthocyanins content compared to 9-days untreated control, especially the NaCl 0.01 M and the Na2SO4 0.1 M. But only in the case of the highest sodium sulphate concentration, the difference from the control is statistically significant. The TAC in treated samples was between 0.72 and 2.81 mg/100 g FW (Figure 2B). In other previous research, it was also found a similar influence of the same treatments on cauliflower sprouts [26]. Some other authors showed that saline excess and other osmotic stress, such as water deficiency, and flooding stress, may induce anthocyanins accumulations [36,37,38].
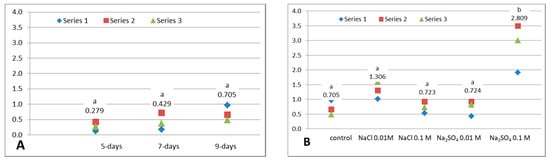
Figure 2.
Total anthocyanins content (TAC) of white cabbage microgreens. (A). Influence of growth: untreated 5-, 7- and 9-day-old microgreens (α = 0.05, between groups df = 2, F = 2.721, p = 0.144). (B). Influence of NaCl or Na2SO4 treatments on 9-day-old microgreens (α = 0.05, between groups df = 4, F = 13.586, p = 0.000). Above the data points are displayed the mean values and a letter: different letters denote significant differences between values (Tukey HSD test). Series 1, 2 or 3 from the legend represent the 3 individual experiments.
3.4. Total Reducing Capacity
The values of total reducing capacity (TRC) of white cabbage are summarized in Figure 3A,B. During the maturation of microgreens, it was noticed a continuous decrease of TRC from 5- to 7- and 9-days, with statistically significant differences. This evolution is similar to TPC (D280) variation. The most important diminution was registered from 5- to 7-day old plants, by 17.99% from 101.08 to 82.90 mg GAE/100 g FW, followed by a second diminution from 7- to 9-days, representing an additional 2.55% decrease, from 82.90 to 80.32 mg GAE/100 g FW.
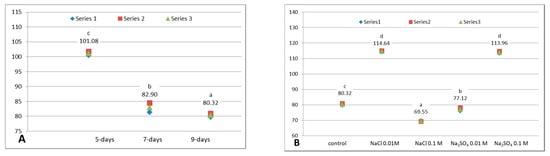
Figure 3.
Total reducing capacity (TRC, mg GAE/100 g FW) of white cabbage microgreens. (A). Influence of growth: untreated 5-, 7- and 9-day-old microgreens (α = 0.05, between groups df = 2, F = 363.932 p = 0.000). (B). Influence of NaCl or Na2SO4 treatments on 9-day-old microgreens (α = 0.05, between groups df = 4, F = 3816.965, p = 0.000). Above the data points are displayed the mean values and a letter: different letters denote significant differences between values (Tukey HSD test). Series 1, 2 or 3 from the legend represent the 3 individual experiments.
Concerning the effects of treatments, two of them determined strong augmentations of TRC: 0.01 M NaCl-by 42.73%, leading to the highest TRC value, which is 114.64 mg GAE/100 g FW, and 0.1 M Na2SO4-by 41.88%, up to a TRC of 113.96 mg GAE/100 g FW. Besides, the difference between these two highest values is not significant statistically. The other applied sodium salts treatments determined slight decreases of TRC, by 3.98% in case of 0.1 M Na2SO4, and by 13.4% in case of 0.1 M NaCl. Other authors found that NaCl in 3 concentrations (0.01, 0.05 and 0.1 M) diminished the reducing capacity measured by ferric reducing antioxidant power assay (FRAP) and the trolox equivalent antioxidant capacity (TEAC) of 5-days broccoli sprouts, while the Folin-Ciocalteu method revealed increases for all 3 NaCl treatments [39].
Comparing the results obtained for TPC (measuring the absorbance at 280 nm) with those for TRC (Folin-Ciocalteu assay), similar evolution is observed (through both methods) during the growth of microgreens and following the 0.1 M Na2SO4 treatment. This similarity is normal, as both methods can be used for the estimation of total phenolic content. However, the treatment with 0.01 M NaCl induced a completely different behavior: the increase of TRC and decrease of TPC. This may suggest the presence of antioxidants (e.g., ascorbic acid), or other compounds (even other phenolics, e.g., cinnamic acids and chalcones) which react with Folin-Ciocalteu reagent but do not absorb light at 280 nm and a concomitant depletion of some phenolics or other compounds containing π conjugated systems, as aromatic glucosinolates. Due to this type of situations, it is necessary to apply multiple assays: for the detection of phenolic compounds, reducing capacity and antioxidant activity and it is strongly recommended that based on the obtained results, to follow in-depth studies in order to elucidate which are the compounds generated/depleted in presence of salts and the exact mechanisms involved.
3.5. DPPH Radical Scavenging Capacity
The radical scavenging capacity (RSC) is one of the most important antioxidant properties. In case of white cabbage, it is mainly due to the phenolic compounds [35], even if other antioxidants as vitamins (especially vitamin C), glucosinolates and isothiocyanates or minerals, contribute to it. The results of the DPPH RSC analyses are summarized in Figure 4A,B.
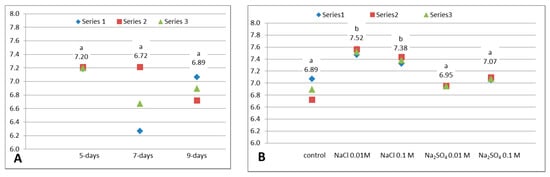
Figure 4.
DPPH radical scavenging capacity (DPPH RSC, mg ascorbic acid/100 g FW) of white cabbage microgreens. (A). Influence of growth: untreated 5-, 7- and 9-day-old microgreens (α = 0.05, between groups df = 2, F = 1.618, p = 0.274). (B). Influence of NaCl or Na2SO4 treatments on 9-day-old microgreens (α = 0.05, between groups df = 4, F = 31.515, p = 0.000). Above the data points are displayed the mean values and a letter: different letters denote significant differences between values (Tukey HSD test). Series 1, 2 or 3 from the legend represent the 3 individual experiments.
During the maturation of white cabbage microgreens no significant change was noticed. It was registered a slight decrease from 5-day to 7-day samples, similar to the results previously observed for broccoli sprouts [26]. This decrease is justified by the obtained diminution of the total phenolic content and total reducing capacity, and also, may be due to a diminution of vitamin C level, which was previously reported in broccoli sprouts by Pérez-Balibrea et al. [40,41]. From 7 days to 9 days, the RSC of cabbage microgreens registered an insignificant augmentation, but the obtained value was inferior to the DPPH RSC of 5-day microgreens (Figure 4A). As consequence, the 5-day-old microgreens presented the highest antioxidant characteristics among the studied developmental stages. However, the length of 5-day microgreens was smaller than of 9-day-old (data not shown), and was preferred to test the effects of sodium salts on bigger size microgreens.
All the 4 applied treatments determined increases of DPPH RSC of 9-day cabbage microgreens, but only in case of NaCl at both concentrations, the augmentations were statistically significant compared to untreated control. The highest antioxidant activity was obtained in case of 0.01 M NaCl treatment. Thus, 0.01 M NaCl induced an enhancement by 9.14% and 0.1 M NaCl, by 7.11%. The highest DPPH RSC under the treatment with 0.01 M NaCl is correlated with the highest TRC and can occur simultaneously with the decrease of TPC measured at 280 nm. The important antioxidant activity is due to the increased content of all antioxidants, including also vitamin C, glucosinolates, or even other phenolics, e.g., cinnamic acids and chalcones, which are not detected by reading the absorbance at 280 nm.
More than that, it was noticed that 0.01 M NaCl had no negative influences on the microgreens development, compared to untreated samples, and their sensorial properties are acceptable for consumers. The previous studies on NaCl influence are contradictory. Guo et al. studied the influence of NaCl on the sprouts of broccoli and found that 0.16 M NaCl significantly increased antioxidant activity and TPC, while 0.04 M and 0.08 M NaCl decreased them [42]. Hassini et al. (2017) discovered that DPPH RSC and TPC in 10-days broccoli sprouts strongly decreased in presence of 0.15 M NaCl [22]. Another study of sodium chloride effect, but on artichoke leaves, showed that DPPH RSC enhances continuously when salinity increases and the maximum was obtained at the highest level, which was 0.2 M NaCl [43].
The sodium sulphate at tested concentrations increased insignificantly the DPPH RSC (Figure 4B). More than that, it inhibited the germination and the development of microgreens.
4. Conclusions
Concerning the development stage, the 5-day-old microgreens proved, globally, the best antioxidant properties. Comparing the effects of sodium salts, 0.01 M NaCl and 0.1 M Na2SO4, generally, determined the strongest antioxidant properties. Excepting the right choice of the harvest time, the germination under adequate sodium stress may become a way to increase the phenolic content and antioxidant capacityof microgreens, improving their health-promoting properties. The present results could be used to develop innovative technologies for the growth of antioxidant-enriched microgreens, but further complex studies are needed to elucidate all the changes induced in plants.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the author.
Acknowledgments
The author would like to thank the Horticultural Research Center of the University of Agricultural Sciences and Veterinary Medicine (UASVM) of Iaşi for research infrastructure opportunity and the Francophone University Agency (AUF) for the research equipment of the Laboratory for the Analysis of Antioxidant Bioactive Compounds of UASVM of Iaşi.
Conflicts of Interest
The author declares no conflict of interest.
References
- Šamec, D.; Pavlović, I.; Redovniković, I.R.; Salopek-Sondi, B. Comparative analysis of phytochemicals and activity of endogenous enzymes associated with their stability, bioavailability and food quality in five Brassicaceae sprouts. Food Chem. 2018, 269, 96–102. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Hill, F. Microgreens: How to Grow Nature’s Own Superfood, 2nd ed.; Firefly Books: Richmond Hill, ON, Canada, 2016; pp. 3–11. [Google Scholar]
- Urban Cultivator. Microgreens and Sprouts Are Not the Same Thing. Available online: https://www.urbancultivator.net/microgreens-vs-sprouts/ (accessed on 8 January 2020).
- Hassini, I.; Baenas, N.; Moreno, D.A.; Carvajal, M.; Boughanmi, N.; Martinez Ballesta, M.D.C. Effects of seed priming, salinity and methyl jasmonate treatment on bioactive composition of Brassica oleracea var. capitata (white and red varieties) sprouts. J. Sci. Food Agric. 2017, 97, 2291–2299. [Google Scholar] [CrossRef]
- López-Cervantes, J.; Tirado-Noriega, L.G.; Sánchez-Machado, D.I.; Campas-Baypoli, O.N.; Cantú-Soto, E.U.; Núñez-Gastélum, J.A. Biochemical composition of broccoli seeds and sprouts at different stages of seedling development. Int. J. Food Sci. Technol. 2013, 48, 2267–2275. [Google Scholar] [CrossRef]
- Sousa, C.; Valentão, P.; Pereira, D.M.; Taveira, M.; Ferreres, F.; Pereira, J.A.; Bento, A.; Seabra, R.M.; Andrade, P.B. Phytochemical and antioxidant characterization of Brassica oleraceae var. costata extracts. In Recent Progress on Medicinal Plants—Standardization of Herbal/Ayurvedic Formulations; Govil, J.N., Singh, V.K., Eds.; Studium Press LLC: Houston, TX, USA, 2009; Volume 24, pp. 311–339. [Google Scholar]
- Vale, A.P.; Santos, J.; Brito, N.V.; Marinho, C.; Amorim, V.; Rosa, E.; Oliveira, M.B.P. Effect of refrigerated storage on the bioactive compounds and microbial quality of Brassica oleraceae sprouts. Postharvest Biol. Technol. 2015, 109, 120–129. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef]
- Le, T.N.; Chiu, C.H.; Hsieh, P.C. Bioactive Compounds and Bioactivities of Brassica oleracea L. var. Italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yao, S.; You, Y.; Xiao, G.; You, Q. Antioxidant activity of isothiocyanate extracts from broccoli. Chin. J. Chem. Eng. 2010, 18, 312–321. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Velasco, P.; Lema, M.; Francisco, M.; Soengas, P.; Cartea, M.E. In vivo and in vitro effects of secondary metabolites against Xanthomonas campestris pv. campestris. Molecules 2013, 18, 11131–11143. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Basu, A. Phenolic compounds: Potential health benefits and toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Production Waste, 1st ed.; Vuong, Q., Ed.; CRC Press Taylor and Francis Group: London, UK, 2017; pp. 27–59. [Google Scholar]
- Traka, M.H. Health benefits of glucosinolates. In Advances in Botanical Research, 1st ed.; Popriva, S., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 80, pp. 247–279. [Google Scholar]
- Domínguez-Perles, R.; Mena, P.; Garcia-Viguera, C.; Moreno, D.A. Brassica foods as a dietary source of vitamin C: A review. Crit. Rev. Food Sci. 2014, 54, 1076–1091. [Google Scholar] [CrossRef]
- Butnariu, M.; Caunii, A. Design management of functional foods for quality of life improvement. Ann. Agric. Environ. Med. 2013, 20, 736–741. [Google Scholar]
- Bantis, F.; Fotelli, M.; Ilić, Z.S.; Koukounaras, A. Physiological and Phytochemical Responses of Spinach Baby Leaves Grown in a PFAL System with LEDs and Saline Nutrient Solution. Agriculture 2020, 10, 574. [Google Scholar] [CrossRef]
- Butnariu, M. Detection of the polyphenolic components in Ribes nigrum L. Ann. Agric. Environ. Med. 2014, 21, 11–14. [Google Scholar] [PubMed]
- Mani, J.S.; Johnson, J.B.; Steel, J.C.; Broszczak, D.A.; Neilsen, P.M.; Walsh, K.B.; Naiker, M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020, 284, 197989. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Rehab, F.M. Effect of germination time on proximate analysis, bioactive compounds and antioxidant activity of lentil (Lens culinaris Medik.) sprouts. Acta Scient. Pol. Technol. Alim. 2015, 14, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Hassini, I.; Martinez-Ballesta, M.C.; Boughanmi, N.; Moreno, D.A.; Carvajal, M. Improvement of broccoli sprouts (Brassica oleracea L. var. italica) growth and quality by KCl seed priming and methyl jasmonate under salinity stress. Sci. Hort. 2017, 226, 141–151. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Patras, A.; Stoleru, V.; Filimon, R.V.; Padureanu, S.; Chelariu, E.L.; Biliaderis, C.G. Influence of sodium and maturity stage on the antioxidant properties of cauliflower and broccoli sprouts. Not. Bot. Horti. Agrobo. 2017, 45, 458–465. [Google Scholar] [CrossRef][Green Version]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Phenolic compounds. In Handbook of Enology, the Chemistry of Wine Stabilization and Treatments, 1st ed.; Ribéreau-Gayon, P., Ed.; John Wiley Sons Ltd: Chichester, UK, 2006; Volume 2, pp. 141–203. [Google Scholar]
- Ragusa, L.; Picchi, V.; Tribulato, A.; Cavallaro, C.; Lo Scalzo, R.; Branca, F. The effect of the germination temperature on the phytochemical content of broccoli and rocket sprouts. Int. J. Food Sci. Nutr. 2016, 68, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Giuffrida, F.; Agnello, M.; Mauro, R.P.; Ferrante, A.; Leonardi, C. Cultivation under salt stress conditions influences postharvest quality and glucosinolates content of fresh-cut cauliflower. Sci. Hort. 2018, 236, 166–174. [Google Scholar] [CrossRef]
- Di Gioia, F.; Rosskopf, E.N.; Leonardi, C.; Giuffrida, F. Effects of application timing of saline irrigation water on broccoli production and quality. Agric. Water Manag. 2018, 203, 97–104. [Google Scholar] [CrossRef]
- Butu, M.; Rodino, S.; Butu, A.; Butnariu, M. Screening of bioflavonoid and antioxidant activity of Lens culinaris medikus. Dig. J. Nanomater. Biostruct. 2014, 9, 519–529. [Google Scholar]
- Aleixandre-Tudo, J.L.; Du Toit, W. The role of UV-visible spectroscopy for phenolic compounds quantification in winemaking. In Frontiers and New Trends in the Science of Fermented Food and Beverages, 1st ed.; Solís-Oviedo, R.L., De La Cruz Pech-Canul, A., Eds.; IntechOpen: London, UK, 2019; pp. 1–22. [Google Scholar]
- Korus, A. Level of vitamin C, polyphenols, and antioxidant and enzymatic activity in three varieties of kale (Brassica oleracea L. var Acephala) at different stages of maturity. Int. J. Food Prop. 2011, 14, 1069–1080. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Bartoszek, A.; Wolska, L.; Drzewiecki, J.; Gorinstein, S.; Namieśnik, J. Partial characterization of white cabbages (Brassica oleracea var. capitata f. alba) from different regions by glucosinolates, bioactive compounds, total antioxidant activities and proteins. LWT-Food Sci. Technol. 2008, 41, 1–9. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Li, H.; Li, X.; Cao, Y.; Zhang, H.; LI, S.; Zhang, L.; Qi, Y.; Ren, S.; et al. Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front. Plant. Sci. 2016, 7, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Hughes, N.M.; Carpenter, K.L.; Cannon, J.G. Estimating contribution of anthocyanin pigments to osmotic adjustment during winter leaf reddening. J. Plant. Physiol. 2013, 170, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Maldini, M.; Nardini, M.; Azzini, E.; Foddai, M.S.; Giusti, A.M.; Baima, S.; Morelli, G.; Scaccini, C. Improvement of the nutraceutical quality of broccoli sprouts by elicitation. Food Chem. 2016, 201, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Influence of light on health-promoting phytochemicals of broccoli sprouts. J. Sci. Food Agric. 2008, 88, 904–910. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Peñas, E.; Ciska, E.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C.; Frias, J. Time dependence of bioactive compounds and antioxidant capacity during germination of different cultivars of broccoli and radish seeds. Food Chem. 2010, 120, 710–716. [Google Scholar] [CrossRef]
- Guo, L.; Yang, R.; Wang, Z.; Guo, Q.; Gu, Z. Effect of NaCl stress on health-promoting compounds and antioxidant activity in the sprouts of three broccoli cultivars. Int. J. Food Sci. Nutr. 2014, 65, 476–481. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Ghasemnezhad, A.; Barani, M.; Telmadarrehei, T. Effect of salinity on phenolic composition and antioxidant activity of artichoke (Cynara scolymus L.) leaves. Res. J. Med. Plant. 2012, 6, 245–252. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).