Abstract
Chili, one of the most cultivated plants in the world, from the genus Capsicum sp., has great importance both in human nutrition and in the pharmaceutical industry. This study provides detailed information on the impact of chili crop fertilization on the production and accumulation of capsaicin and dihydrocapsaicin. During the vegetation period, 235 kg∙ha−1 NPK (chemical—Ch), 270 kg∙ha−1 NPK (organic—O) and 250 kg∙ha−1 NPK (mixed—Ch + O) fertilizers were applied on six varieties of chili pepper (De Cayenne, Traian 2, Turkish, Sigaretta di Bergamo, Jovial and Chorbadjiiski); all versions were compared with the control (Ct). The determination of capsaicinoid compounds from chili pepper samples was done using high-performance liquid chromatography, HPLC-UV/VIS. The chili pepper plants reacted differently according to the fertilizers used, both in terms of the production and accumulation of capsaicinoids. The highest production was obtained for the case of mixed treatments in all cultivars, with the highest production being found for Sigaretta di Bergamo (40.61 t∙ha−1). The capsaicin and dihydrocapsaicin content was influenced by both the type of fertilizer used and the variety of chili pepper. The accumulation of capsaicinoids in the chili fruits was found to be dependent on cultivar and fertilization management; higher amounts of capsaicinoids were found to accumulate in the fruits of the Chorbadjiiski variety treated with chemicals (0.83 mg∙g−1 capsaicin and 0.53 mg∙g−1 dihydrocapsaicin) compared with the amounts found for untreated De Cayenne (0.52 mg∙g−1 capsaicin and 0.33 mg∙g−1 dihydrocapsaicin).
1. Introduction
Chili (paprika), Capsicum annuum L., is one of the most cultivated species of the genus Capsicum worldwide [1,2]. In Romania, in 2019, the cultivated area for chili peppers was 10,780 ha, from which was obtained a production of 162,345 tons. In terms of cultivated area and production in Europe, in first place is Spain, with 21,430 ha and a total production of 1,402,380 tons of chili peppers. Globally, in the year 2019, the area cultivated with chili peppers was 1,990,926 hectares, with a total production of 38,027,164 tons [3]. Chili peppers belong to the Solanaceae family and they are grown in open fields in temperate regions for direct consumption (fresh) or to be used as a raw material in the food and pharmaceutical industries [4,5,6]. Unlike other species of the genus Capsicum, chili peppers are characterized by fruits that are relatively small in size—about 0.5–2 cm in diameter and with a length between 1 cm and 25 cm—and have a hot taste, which varies in intensity according to the variety [7,8,9]. This pungent taste and the sharpness of the peppers has led these fruits to find a place in many international cuisines as a spice, and they are loved by many consumers [10,11,12,13].
Capsaicinoids are the compounds responsible for the pungency of pepper fruits and their products [14]. The two most abundant capsaicinoids are capsaicin (C) (8-methyl-N-vanillyl-trans-6-nonenamide) and dihydrocapsaicin (DhC) (8-methyl-N-vanillylnonanamide) [15,16,17]. The chemical structures of these compounds are shown in Figure 1.
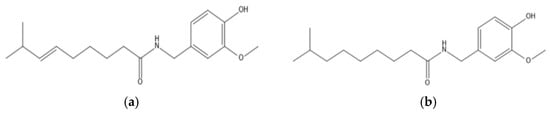
Figure 1.
Chemical structure of capsaicin (a) and dihydrocapsaicin (b).
The content of capsaicinoids in peppers is one of the major parameters determining their commercial quality. The structural characteristics of capsaicinoids that are responsible for their spicy flavor are associated with the presence of an amide bond connecting a vanillyl ring and an acyl chain [18,19]. The biological activities of these compounds and pepper fruits are also associated with these structural characteristics. They have been used as an analgesic against arthritis and inflammation. Furthermore, they have been reported to show an anticancer effect and to be active against neurogenic inflammation, high cholesterol levels and obesity [20,21,22]. However, high levels of capsaicinoids also have negative health impacts, leading to a greater risk of gastric cancer [23].
The amounts of capsaicinoids found in pepper fruits can vary in accordance with the light intensity, temperature and mineral elements with which the plant is grown; the age of the fruit; and the position of the fruit on the plant [8,24]. In commercial pepper fruits, capsaicin content is generally 33–59%, while dihydrocapsaicin content is generally 30–51% and nondihydrocapsaicin 7–15%, with the remainder being <5% capsaicinoids [25,26,27].
The aim of this study was to obtain further answers regarding the types of fertilizers used during phenophases in order to highlight their effect on production and their influence on fruit accumulation of capsaicin and dihydrocapsaicin in six varieties of chili peppers.
2. Materials and Methods
2.1. Experimental Site
The experiment was performed during the years 2018–2019 in the experimental field of the University of Agricultural Sciences and Veterinary Medicine of Iasi.
To achieve the goal of this research, six chili varieties were studied (De Cayenne, Traian 2, Turkish, Sigaretta di Bergamo, Jovial and Chorbadjiiski). The chili pepper seeds were purchased at the market from Romanian seed traders.
The seedlings destined for cropping were produced in the greenhouse. At the age of 55 days, the chili seedlings were planted in a vegetable field at density of 48,000 plants∙ha−1. The crop technology applied for the chili peppers was that recommended by the specialized literature [28].
The experiment was carried out using a split-plot design, with three replicates per treatment for each variety. During the vegetation period, five fertilizations in vegetation were applied using chemical fertilizers (Ch), organic fertilizers (O) and mixed fertilizers (Ch + O) on the six varieties of chili peppers, which were then compared with an unfertilized variant (Ct). In the case of the control variant, no fertilizers were applied. All treatments were applied to the soil. The irrigation regime was identical for all treatments.
The treatments were applied during the following growing and development phenophases: first flower bud visible (BBCH 501), first flower open (BBCH 601), 10th flower open (BBCH 610), first fruit reached typical size and form (BBCH 701) and 10th fruit reached typical form and size (BBCH 710).
Three fertilization types consisted of the application of 235 kg∙ha−1 chemical fertilizer (Nutrispore, NPK 24-5-16; 30.10.10; 15.10.30; 8.24.24 by MsBiotech, Termoli, Italy), 270 kg∙ha−1 organic fertilizer (Orgevit® by SolarLegume Ltd., Matca, Galati, Romania) and 250 kg∙ha−1 mixed treatment consisting of 108 kg∙ha−1 NPK from organic fertilizer and 142 kg∙ha−1 NPK 15.10.30; 8.24.24 (Table 1).

Table 1.
Experimental design of the treatments according to phenophases.
Chemical fertilization consisted of 235 kg∙ha−1 NPK from Nutrispore®. The organic fertilizer used was a product based on chicken manure with the following characteristics: pH 7, 6% N, 4.5% P2 O5, 3% K2O, 8% CaO, 1% MgO, 0.03% Fe, 0.01% Mn, 0.01% B, 0.01% Zn, 0.001% Cu and 0.001% Mo. In the case of the O version, the chicken manure was complexed with biological products based on Bacillus sp. and Glomus sp. and was used at 35 kg∙ha−1 three times applied.
The doses of fertilizers were calculated by taking into account the following: the chemical composition of each formulation; assuming that 75–80% of N, P2O5 and K2O contents of the O (organic) fertilizer was available for plant assimilation in the year of application [29].
Harvesting of chili pepper fruits, in order to determine the production, was done in phenological phase 809—fully ripe: fruits have typical fully ripe color (BBCH scale) [30]. From the beginning of the fruit harvest, measurements were made on the height of the plants in each repetition and the fruits harvested on each plant were counted. The total yield according with fertilization schemes was carried out by weighing the fruits for each harvest.
2.2. Dry Matter Content
After harvesting, 10 ripe fruits from each replication were dried in a laboratory oven with ventilation. The fruits were dried without the placenta and seeds. Drying was carried at 55 ± 5 °C until the weight of the samples at 12 h intervals remained constant after each sample was separately ground with a laboratory mill [31].
The percentage of dry matter of chili pepper fruits was calculated using the formula:
D.W.% = 100 – ((m1 − m2) / m) × 100 [6], where: m1 = weight of the sample with the laboratory tray before drying; m2 = weight of the sample with the laboratory tray after drying; m = weight of the analyzed sample before drying.
2.3. Chili Pepper Analyses
2.3.1. Analysis by HPLC-UV
The determination of compounds C and DhC, both quantitatively and qualitatively, in chili pepper samples was performed using high-performance liquid chromatography (HPLC-UV/VIS) at the analytical laboratory of Van Hall Larenstein University in Leeuwarden (the Netherlands) [32,33,34]. For all analyses, a Shimadzu liquid chromatograph (LC-20 AT) coupled with an auto sampler (SIL-20AC ht), a UV-VIS detector (SPD-10A) and a column oven (CTO-10A) were used. Furthermore, separation of C and DhC was achieved using a Luna 5 µm C18(2) 100A 250—4.6 mm column. During the analysis, the mobile phase consisted of 20% Milli-Q water and 80% methanol (HPLC-grade) with a total run time of 20 min at a flow rate of 1.0 mL/minute and an oven temperature of 40 °C. For this, a 5 µL sample was injected and both compounds were detected at 284 nm.
2.3.2. Sample Preparation
All chili samples (0.4 g) were dissolved in 10 mL methanol (VWR, CAS: 67-56-1) and placed in a 60 °C, ultrasonic water bath for 20 min for extraction. Subsequently, the dissolved sample was centrifuged at 2500 rpm for five minutes, and subsequently filtered over a 0.45 µm nylon filter [35,36].
2.3.3. Quantification and Validation
Quantification was achieved by injecting known concentrations of C and DhC, respectively: 0.5–60 ppm and 0.5–40 ppm [37,38]. These standard solutions were measured 3 times on HLPC-UV/VIS using the autosampler and processed into a calibration curve (external standard method). With this data, the linearity of the method was determined. The precision of the method was expressed in term of repeatability and reproducibility of peak area/gram, using available chili flakes and powder. Initial repeatability was performed on the same day and reproducibility performance was spread over 3 days [39,40,41], and both compounds were prepared and measured 10 times [42].
2.4. Capsaicinoids Ratio
The ratio of capsaicinoids is calculated by dividing the content of capsaicin by dihydrocapsaicin, and it is usually 2:1 to 1:1 [13].
2.5. Scoville Heat Units (SHU)
Two of the most important capsaicinoids in this regard are C and DhC. The pungency of the chili peppers is measured on the Scoville scale in Scoville Heat Units (SHU). This scale was created by Wilbur Scoville in 1912 and it is also known as the Organoleptic Test. The hot taste (pungency) of peppers, weaker or stronger, depends on the fruit content of capsaicinoids.
In order to express the degree of pungency in SHU, depending on the content of C and DhC in chili fruit, the following formula [10] was used:
2.6. Statistical Analysis
The data is expressed as the mean ± standard deviation (SD). Two-way ANOVA was used to see the influence of the treatments on the biochemical and yield parameters of De Cayenne, Traian2, Turkish, Sigaretta, Jovial and Chorbadjiiski chili pepper cultivars. The significant differences between treatments were established by using Tukey’s post hoc test with a degree of confidence of 95% (p < 0.05).
3. Results and Discussions
As shown in Figure 2a, regarding the influence of the cultivar on the dry matter content of pepper fruits, it varied from 11.90%, in the case of the Chorbadjiiski variety, to 16.50%, in the case of Trajan 2. A low content in dry matter was registered in the Sigaretta (12.70%) and Jovial (13.40%) cultivars. The varieties Traian 2, Turkish and De Cayenne demostrated better adaptation to the ecological conditions of the continental temperate climate (Figure 2b).
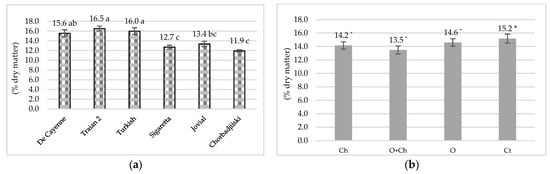
Figure 2.
Dry matter content of samples influenced by cultivar (a) and fertilization (b). Ch—Chemical; O + Ch—Organic + Chemical; O—Organic; Ct—Control. Along each line, values followed by different letters are significantly different according to the Tukey’s test at p ≤ 0.05; *—non-significant.
Dry matter content varied from 13.5%, in the case of O + Ch, to 15.2%, in the case of the control. The fertilized variants Ch and O registered intermediate values of 14.20% and 14.60%, respectively, but the differences between the varieties were insignificant at p ≤ 0.05.
The influence of the interaction between cultivar and treatment is presented in Figure 2. The dry matter of the hot pepper fruits varied from 11.21%, in the case of the Sigaretta variety fertilized with O + Ch, up to 17.46%, in the case of the unfertilized Traian 2 cultivar. From a statistical point of view, the differences between the variants were insignificant, which means that the interaction between the two factors blurs the significance between the varieties. Low levels of dry matter content were obtained for the Chorbadjiiski variety fertilized with O + Ch (11.48%), Ch (11.75%), O (12.31%) and Ct (12.33%). Statistically high values were obtained for the unfertilized (17.20%) and O-fertilized Turkish cultivar (16.86%). The values obtained are in accordance with those from the scientifically literature obtained for chili [25,43,44] and sweet peppers [45].
The dry matter from the hot pepper fruits in the case of the interaction of the factors is presented in Figure 3 and varied from 11.21% per 100 g dry matter, in the case of Sigaretta fertilized with O + Ch, up to 17.46% per 100 g dry matter, in the case of the unfertilized Traian 2 cultivar. From a statistical point of view, the results were insignificant. Low values of dry matter content were obtained for the Chorbadjiiski and Sigaretta cultivars regardless of treatment, which suggests a lower adaptation to growing conditions and a lower sensitivity to storage, as it is known that vegetables with higher water content are more perishable [46].
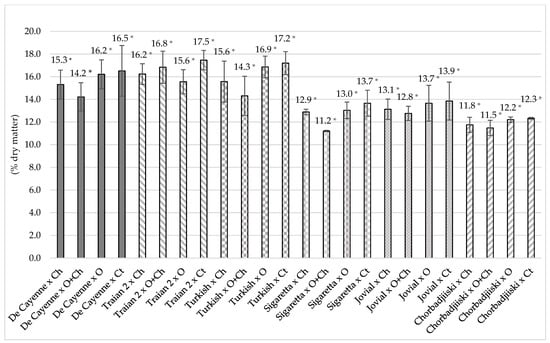
Figure 3.
Dry matter content of samples influenced by interaction between cultivar and fertilization. Ch—Chemical; O + Ch—Organic + Chemical; O—Organic; Ct—Control. Along each line, values followed by different letters are significantly different according to the Tukey’s test at p ≤ 0.05; *—non-significant.
Statistically high values were obtained for the unfertilized (17.20%) and O-fertilized Turkish cultivar (16.86%). The values obtained are in accordance with those in the literature obtained for hot peppers [43] and sweet peppers [45].
Regarding the influence of the cultivar on the height of the hot pepper plants, it varied widely from 53.71 cm, in the case of the Turkish cultivar, up to 83.28 cm, in the case of the Jovial cultivar (Table 2). Under the type of fertilizer applied, the plant height was insignificant according to the Tukey test for p ≤ 0.05. However, it ranged from 67.38 cm in the case of the control to 74.99 cm in the case of treatment with O + Ch. In the case of Keunsarang plant height, it increased by 27.4% compared with the untreated control version, when the variety was fertilized using an amount of 265.4 kg·ha−1 NPK from manure [30]. Similar data were obtained by Meena et. al. for 60 chili pepper hybrids under chemical fertilization with 122.5 kg∙ha−1 NPK; plant height increased from 69.60 cm for the MS463D13A F1 hybrid to 132.82 cm for CMS4626A F1 [47].

Table 2.
Biometric indicators and yield affected by cultivar and fertilization.
Regarding the effect of the cultivar on the number of fruits per plant, it varied from 26.82 in the case of De Cayenne to 47.09 in the case of Sigaretta. Chorbadjiiski (36.62) and Turkish (34.66) cultivars also obtained statistically positive results. The type of fertilizer applied positively influenced the number of fruits per plant according to the Tukey test, which varied from 30.46 in the case of the control to 38.24 in the case of O + Ch.
Regarding the influence of the studied factors on the average weight of hot peppers fruits, it varied widely from 13.01 g, in the case of the Sigaretta cv. to 19.08 g in the case of the Jovial cv.; the difference between the cultivars being 46.6%. Differences between varieties in most cases can be genetically influenced [48]. Significant results between varieties were also obtained for the Turkish (17.51 g) and Traian 2 (16.55 g) cultivars. Regarding the effect of the influence of the type of fertilizer on the average weight of hot peppers fruits, it varied from 14.51 g, in the case of the non-fertilized variants, up to 17.31 g, in the case of the O + Ch-treated variants. Under organic fertilization, the average weight of the fruit was 15.82 g, and in the case of chemical fertilization, the value of 15.98 g was recorded. From a statistical point of view, the favorable effect of the combination of the two types of treatments could be observed on the average weight of the fruit. Data from the literature highlight the favorable effect on Chichen Itza chili peppers under chemical fertilization, where the fruit weight increased by 26.87% compared to the control [49].
The type of cultivar used significantly influenced the total production obtained from the hot pepper culture for p ≤ 0.05. This varied from 17.75 t∙ha−1, in the case of the De Cayenne cultivar, to 29.71 t∙ha−1, in the case of the Sigaretta cultivar. Statistically positive results could be observed for the Turkish (29.32 t∙ha−1), Jovial (27.77 t∙ha−1) and Chorbadjiiski (27.28 t∙ha−1) cultivars. In an experiment conducted in 2012–2013 in Central Chile, the total production of hot peppers in the field varied from 15.90 t∙ha−1, in the case of the local population “Cacho de Cabra”, to 26.50 t∙ha−1, in the case of Chilean negro [27,36].
The yield varied widely with fertilization type from 20.82 t∙ha−1, in the case of the control variant, up to 31.58 t∙ha−1, in the case of O + Ch, which highlights the favorable effect of the combination between chemical and organic treatment, through the synergistic effect of mineral ions from these two types of fertilizers.
The height of hot pepper plants varied widely depending on the interaction between the cultivar and the treatment, from 49.87 cm, in the case of the unfertilized Turkish cultivar, to 89.34 cm, in the case of Jovial × O + Ch (Table 3). Positive differences according to the Tukey test for p ≤ 0.05 were also recorded by the combinations Jovial × Ch (85.61 cm), and Sigaretta × O + Ch (85.02 cm). The results obtained for the height of the plants highlight the positive effect of the relationship between mixed fertilization and variety and on the growth phenophase. The combined effect of chemical and organic fertilization at 8 cultivars of chili peppers increased plant height from 55 cm to 70.26 cm, under 102.6 kg∙ha−1 NPK applied [50].

Table 3.
Biometric parameters and yield of chili peppers under interaction of cultivar and fertilization.
The lowest heights of pepper plants were recorded in the Turkish variety regardless of the fertilizer used. Insignificant results between variants were recorded in the case of the cultivars De Cayenne, Traian 2, Sigaretta, Jovial and Chorbadjiiski.
Regarding the combined influence of the cultivar and fertilizer on the number of fruits per plant, it varied from 21.50, in the case of the unfertilized variant of the Traian 2 variety, to 56.36, in the case of Sigaretta × O + Ch. Significant results compared to the unfertilized Traian 2 cultivar were registered for the combinations Jovial × O + Ch, Chorbadjiiski × Ch, Turkish × O + Ch, Chorbadjiiski × O + Ch and Sigaretta × O. Further, regarding the number of fruits, it was observed that the interaction of cultivars with the mixed treatment favored the stage of floral differentiation in a much more accentuated way compared to the control variants.
Regarding the influence of cultivar and fertilizer on the average weight of hot pepper fruits, weights ranged from 11.74 g in the case of De Cayenne × O to 20.43 g in the case of Jovial × O + Ch. Significant differences from the Jovial × O + Ch variant were registered in the case of the Sigaretta cultivar regardless of the type of fertilization.
Regarding the combined influence of cultivar and fertilizer on the total production, it varied widely from 14.78 t∙ha−1, in the case of the unfertilized Trajan 2 cultivar, up to 40.61 t∙ha−1, in the case of Sigaretta × O + Ch. Significant differences compared to the unfertilized Traian 2 variant were registered for the Chorbadjiiski and Turkish cultivars fertilized with O + Ch and for Chorbadjiiski × Ch. Recent studies pointed out that under 120 kg∙ha−1 NPK from manure, for Grande cultivar hot peppers, the yield was increased by 38.8% [51].
Although the average mass of fruit was the lowest, production was offset by the higher number of fruits per plant.
Regarding the effect of the cultivar on the total capsaicin content, it varied from 0.30 mg∙g−1 d.w., in the case of the Turkish cultivar, to 0.65 mg∙g−1 d.w., in the case of the Jovial cultivar. The positive results of the total capsaicin content from a statistical point of view were also obtained for the cultivars De Cayenne (0.52 mg∙g−1 d.w.) and Chorbadjiisk (0.47 mg∙g−1 d.w.) (Table 4).

Table 4.
Influence of cultivar and fertilization on capsaicinoid content and Scoville Heat Units.
The capsaicin content varied from 0.40 mg∙g−1 d.w., in the case of the O variant, to 0.54 mg∙g−1 d.w., in the case of the Ch variant.
Differences between C and DhC content can be attributed to the genotype, as revealed by other studies in northeast India, showing that the capsaicin content of various cultivars of Capsicum ranged from 0.02 mg∙g−1 d.w. up to 72.05 mg∙g−1 d.w. [52,53].
Regarding the influence of the cultivar on the dihydrocapsaicin content of hot peppers, it varied widely from 0.14 mg∙g−1 d.w., in the case of the Turkish cultivar, to 0.43 mg∙g−1 d.w., in the case of the Jovial cultivar. The statistically negative results were also obtained for the cultivars De Cayenne (0.23 mg∙g−1 d.w.) and Traian 2 (0.28 mg∙g−1 d.w.).
Under fertilizer type, the dihydrocapsaicin content ranged from 0.23 mg∙g−1 d.w., in the case of O, to 0.33 mg∙g−1 d.w., in the case of Ch. The results are in accordance with the scientific literature [54,55].
The ratio between the main compounds (capsaicin and dyhidrocapsaicin) that give the pungency of pepper fruits [13].
The influence of the type of cultivar and fertilizer used on the total content of capsaicinoids was in direct correlation with the results obtained for capsaicin and dihydrocapsaicin. The highest capsaicin content was found in the Jovial cultivar, and the differences for the other five varieties were significant for p < 0.05.
The type of treatment used determines the different accumulation of total capsaicin, the highest values being registered with the chemical treatments and control compared to the organic variants. Higher results in control variants can also be attributed to the mechanisms of adaptation of chili pepper plants to the conditions of nutritional stress.
The cultivar used significantly influenced the SHU, ranging from 7124.24 SHU in the case of the Turkish variety to 17347.75 SHU in the case of the Jovial variety. According to the scientific literature, according to their SHU, the cultivars used in the experiment are moderately pungent [54]. The fertilization type attenuated the degree of spiciness; the SHU varied from 10,169.83 with organic treatment to 13,953.33 in the case of chemical treatment.
Regarding the combination between cultivar and the type of fertilizer used, the capsaicin content of hot pepper fruits varied widely from 0.27 mg∙g−1 d.w., in the case of the Turkish variety fertilized with O + Ch, to 0.83 mg∙g−1 d.w., in the case of Jovial × Ch (Table 5). All combinations showed significant differences compared to the maximum capsaicin content obtained by Jovial × Ch. Statistically significant differences from the variant with the lowest capsaicin content (Turkish × O + Ch) were not recorded for the combinations Turkish × O, Sigaretta × Ch, Turkish × Control, Turkish × Ch or Traian 2 × O + Ch. These results are in agreement with scientific literature regarding the C and Dhc content; Morales-Soriano et al. mention values for C between 0.1418 mg∙g−1 d.w., in the case of the Panca cultivar and 2.01 mg∙g−1 d.w., in the case of the Arnaucho cultivar [5]

Table 5.
Interaction between cultivar and fertilization on capsaicinoid content and Scoville scale.
Regarding the effect of the cultivar and the type of fertilizer used on the dihydrocapsaicin content, the highest value was obtained for the same variant as capsaicin (Jovial × Ch), with a content of 0.53 mg∙g−1 d.w., while the lowest value was recorded by the Turkish variety fertilized with O (0.11 mg∙g−1 d.w.). Significant results compared to the highest value were obtained by all variants, except for the combination of Jovial × O + Ch (0.48 mg∙g−1 d.w.). Compared to the combination that recorded the lowest dihydrocapsaicin content (Turkish × O), the combinations of Turkish × O + Ch, Turkish × Ch and Traian 2 × O + Ch were the only ones that did not show significant differences.
As for the influence of cultivar × fertilization on the total content of capsaicinoids, the lowest content (0.40 mg∙g−1 d.w.) was recorded for the Turkish variety fertilized by O + Ch and O; while the highest content was obtained for Jovial × Ch (1.36 mg∙g−1 d.w.). Compared to Turkish × O, which obtained the highest total content of capsaicinoids, all combinations showed significant differences, with the exception of Turkish × Ch (2.55 mg∙g−1 d.w.).
Regarding the influence of the cultivar × fertilization combination on the Scoville Heat Units (SHU), they varied from 6440 SHU, in the case of Turkish × O + Ch and Turkish × O combinations, to 21,896 SHU, in the case of Jovial × Ch. In the Jovial × Ch combination, which obtained the highest SHU content, all experimental variants obtained statistically significant results according to the Tukey test for p ≤ 0.05. In a study on the SHU of nine varieties of C. chinense, values from 9792 SHU for the Mochero cultivar to 39,755 SHU for the Arnaucho cultivar were reported [5]. Data from Table 5 show increased SHU values for the Chorbadjiiski and Jovial cultivars under chemical treatment.
The combination of variety and fertilization type did not change the degree of pungency, which indicates that the variants obtain fruits that fall into the moderately pungent category (3000–25,000 SHU) as determined primarily by genotype and to a lesser extent by the treatment used [56].
4. Conclusions
The results presented in this study provide new data on the regulation of metabolism of capsaicinoids in the fruits and their production in response to different types of treatments of six chili pepper varieties.
The dry matter content was not influenced by the applied treatments, the results obtained being insignificant in the case of the combined influence of the two factors studied. Significant results were obtained in the case of the individual influence of the cultivar for the Traian 2 and Turkish varieties.
The applied treatments had a positive impact on the production parameters; from the measurements performed, it could be observed that the type of fertilizer used had different effects depending on the response of the cultivar. Thus, the plant height registered significant values in the case of the combinations of Jovial with O + Ch fertilization and of Turkish with chemical fertilization. The average weight of the fruits indicated significant values in the case of Jovial × O + Ch and De Cayenne × Ch.
The best cultivar regarding yield was Sigaretta under O + Ch, and Jovial treated with Ch obtained the highest content of capsaicinoids (over 135 mg∙g−1).
The effect of genotype and fertilizers interaction is a complex phenomenon; genotype plays a major role in the accumulation and content of capsaicinoids.
The chili peppers responded well in terms of the yield results for the organic + chemical-treated variants, and the chemical-treated variants in terms of the capsaicinoid contents.
Farmers can produce chili peppers with different types of pungency and with high productivity using appropriate cultivars and fertilizers.
Author Contributions
T.S., G.-C.T. and A.C. conducted the field experiments; V.S. and G.-C.T. contributed to statistical processing and interpretation of data; T.S., V.S. and N.M. conceived and planned the experimental protocol and performed the research supervision; A.C. and G.-C.T. were involved in the bibliographic search; N.M., V.S. and T.S. wrote the draft and final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Authors ensure that data shared are in accordance with participants consent.
Acknowledgments
The authors wish to thank “Ion Ionescu de la Brad” University of Agricultural Sciences and Veterinary Medicine for the financial support of this experiment and Gabriela Leusink-Ionescu for the supervision of analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- García, C.C.; Barfuss, M.H.J.; Sehr, E.M.; Barboza, G.E.; Samuel, R.; Moscone, E.A.; Ehrendorfer, F. Phylogenetic relationships, diversification and expansion of chili peppers (Capsicum, Solanaceae). Ann. Bot. 2016, 118, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Grozeva, S. Effect of copper levels in the culture medium on shoot regeneration in pepper. Banat. J. Biotechnol. 2015, 6, 86–91. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 1 February 2021).
- Reyes-Escogido, M.D.L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and Pharmacological Aspects of Capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef]
- Morales-Soriano, E.; Kebede, B.; Ugas, R.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Flavor characterization of native Peruvian chili peppers through integrated aroma fingerprinting and pungency profiling. Food Res. Int. 2018, 109, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Jeeatid, N.; Suriharn, B.; Chanthai, S.; Bosland, P.; Techawongstien, S. Influence of water stresses on capsaicinoid production in hot pepper (Capsicum chinense Jacq.) cultivars with different pungency levels. Food Chem. 2018, 245, 792–797. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, J.; Xie, H.; Ng, S.H. Capsaicin determination and chili sauce discrimination using low-cost and portable electrochemical sensors based on all graphite pencil electrodes. Anal. Methods 2016, 8, 7025–7029. [Google Scholar] [CrossRef]
- Mali, S.; Naik, S.; Jha, B.; Singh, A.; Bhatt, B. Planting geometry and growth stage linked fertigation patterns: Impact on yield, nutrient uptake and water productivity of Chilli pepper in hot and sub-humid climate. Sci. Hortic. 2019, 249, 289–298. [Google Scholar] [CrossRef]
- Bhutia, N.D.; Seth, T.; Shende, V.D.; Dutta, S.; Chattopadhyay, A. Estimation of Heterosis, dominance effect and genetic control of fresh fruit yield, quality and leaf curl disease severity traits of chilli pepper (Capsicum annuum L.). Sci. Hortic. 2015, 182, 47–55. [Google Scholar] [CrossRef]
- Al Othman, Z.A.; Ahmed, Y.B.H.; Habila, M.A.; Ghafar, A.A. Determination of Capsaicin and Dihydrocapsaicin in Capsicum Fruit Samples using High Performance Liquid Chromatography. Molecules 2011, 16, 8919–8929. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Biological Activities of Red Pepper (Capsicum annuum) and Its Pungent Principle Capsaicin: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1488–1500. [Google Scholar] [CrossRef]
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chem. 2013, 140, 794–802. [Google Scholar] [CrossRef]
- González-Zamora, A.; Sierra-Campos, E.; Luna-Ortega, J.G.; Pérez-Morales, R.; Ortiz, J.C.R.; García-Hernández, J.L. Characterization of Different Capsicum Varieties by Evaluation of Their Capsaicinoids Content by High Performance Liquid Chromatography, Determination of Pungency and Effect of High Temperature. Molecules 2013, 18, 13471–13486. [Google Scholar] [CrossRef]
- Arabaci, B.; Gulcin, I.; Alwasel, S. Capsaicin: A Potent Inhibitor of Carbonic Anhydrase Isoenzymes. Molecules 2014, 19, 10103–10114. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Luo, X.-J.; Peng, J.; Li, Y.-J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum-Titze, P.; Hiepler, C.; Mueller-Seitz, E.; Petz, M. Pungency in Paprika (Capsicum annuum). 1. Decrease of Capsaicinoid Content Following Cellular Disruption. J. Agric. Food Chem. 2002, 50, 1260–1263. [Google Scholar] [CrossRef]
- Zhao, Z.-D.; Zan, L.-S.; Li, A.-N.; Cheng, G.; Li, S.-J.; Zhang, Y.-R.; Wang, X.-Y.; Zhang, Y.-Y. Characterization of the promoter region of the bovine long-chain acyl-CoA synthetase 1 gene: Roles of E2F1, Sp1, KLF15 and E2F4. Sci. Rep. 2016, 6, 19661. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Wang, C.-Y.; Hung, Y.-H.; Weng, T.-Y.; Yen, M.-C.; Lai, M.-D. Systematic Analysis of Gene Expression Alterations and Clinical Outcomes for Long-Chain Acyl-Coenzyme A Synthetase Family in Cancer. PLoS ONE 2016, 11, e0155660. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, Y.; Li, Y.; Xu, D.-P.; Li, S.; Li, H.-B. Spices for Prevention and Treatment of Cancers. Nutrients 2016, 8, 495. [Google Scholar] [CrossRef]
- Thoennissen, N.H.; O’Kelly, J.; Lu, D.; Iwanski, G.B.; La, D.T.; Abbassi, S.; Leiter, A.; Karlan, B.; Mehta, R.; Koeffler, H.P. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 2009, 29, 285–296. [Google Scholar] [CrossRef]
- Lee, S.-H.; Richardson, R.L.; Dashwood, R.H.; Baek, S.J. Capsaicin represses transcriptional activity of β-catenin in human colorectal cancer cells. J. Nutr. Biochem. 2012, 23, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Chapa-Oliver, A.M.; Mejía-Teniente, L. Capsaicin: From Plants to a Cancer-Suppressing Agent. Molecules 2016, 21, 931. [Google Scholar] [CrossRef] [PubMed]
- Marincaş, O.; Feher, I.; Magdas, D.A.; Puşcaş, R. Optimized and validated method for simultaneous extraction, identification and quantification of flavonoids and capsaicin, along with isotopic composition, in hot peppers from different regions. Food Chem. 2018, 267, 255–262. [Google Scholar] [CrossRef]
- Olguin-Rojas, J.A.; Vazquez-Leon, L.A.; Salgado-Cervantes, M.A.; Barbero, G.F.; Diaz-Pacheco, A.; Garcia-Alvarado, M.A.; Rodriguez-Jimenes, G.C. Water and phytochemicals dynamic during drying of red habanero chili pepper (Capsicum chinense) slices. Rev. Mex. Ing. Química 2019, 18, 851–864. [Google Scholar] [CrossRef]
- Baytak, A.K.; Aslanoglu, M. Sensitive determination of capsaicin in pepper samples using a voltammetric platform based on carbon nanotubes and ruthenium nanoparticles. Food Chem. 2017, 228, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Concha, D.; Quiñones, X.; Hernández, J.P.; Romero, S. Chili Pepper Landrace Survival and Family Farmers in Central Chile. Agronomy 2020, 10, 1541. [Google Scholar] [CrossRef]
- Zamljen, T.; Zupanc, V.; Slatnar, A. Influence of irrigation on yield and primary and secondary metabolites in two chilies species, Capsicum annuum L. and Capsicum chinense Jacq. Agric. Water Manag. 2020, 234, 106104. [Google Scholar] [CrossRef]
- Rippy, J.F.; Peet, M.M.; Louws, F.J.; Nelson, P.V.; Orr, D.B.; Sorensen, K.A. Plant Development and Harvest Yields of Greenhouse Tomatoes in Six Organic Growing Systems. Hort. Sci. 2004, 39, 223–229. [Google Scholar] [CrossRef]
- Khaitov, B.; Yun, H.J.; Lee, Y.; Ruziev, F.; Le, T.H.; Umurzokov, M.; Bo, A.B.; Cho, K.M.; Park, K.W. Impact of Organic Manure on Growth, Nutrient Content and Yield of Chilli Pepper under Various Temperature Environments. Int. J. Environ. Res. Public Health 2019, 16, 3031. [Google Scholar] [CrossRef]
- Sellitto, V.M.; Golubkina, N.A.; Pietrantonio, L.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Florin, I.; Caruso, G. Tomato Yield, Quality, Mineral Composition and Antioxidants as Affected by Beneficial Microorganisms Under Soil Salinity Induced by Balanced Nutrient Solutions. Agriculture 2019, 9, 110. [Google Scholar] [CrossRef]
- Butnariu, M.; Caunii, A.; Putnoky, S. Reverse phase chromatographic behaviour of major components in Capsicum Annuumextract. Chem. Central J. 2012, 6, 146. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Menichini, F.; Tundis, R. Evaluation of chemical profile and antioxidant activity of twenty cultivars from Capsicum annuum, Capsicum baccatum, Capsicum chacoense and Capsicum chinense: A comparison between fresh and processed peppers. LWT 2015, 64, 623–631. [Google Scholar] [CrossRef]
- Gómez-García, M.D.R.; Ochoa-Alejo, N. Biochemistry and Molecular Biology of Carotenoid Biosynthesis in Chili Peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.; Zheng, J.; Huang, Q. Effects of regulated deficit irrigation on yield and water productivity of chili pepper (Capsicum annuum L.) in the arid environment of Northwest China. Irrig. Sci. 2018, 36, 61–74. [Google Scholar] [CrossRef]
- Rêgo, E.R.D.; Rêgo, M.M.D.; Finger, F.L.; Cruz, C.D.; Casali, V.W.D. A diallel study of yield components and fruit quality in chilli pepper (Capsicum baccatum). Euphytica 2009, 168, 275–287. [Google Scholar] [CrossRef]
- Antonious, G.F.; Berke, T.; Jarret, R.L. Pungency inCapsicum chinense: Variation among countries of origin. J. Environ. Sci. Health Part B 2009, 44, 179–184. [Google Scholar] [CrossRef]
- Naves, E.R.; Silva, L.D.Á.; Sulpice, R.; Araújo, W.L.; Nunes-Nesi, A.; Peres, L.E.; Zsögön, A. Capsaicinoids: Pungency beyond Capsicum. Trends Plant Sci. 2019, 24, 109–120. [Google Scholar] [CrossRef]
- Frias, B.; Merighi, A. Capsaicin, Nociception and Pain. Molecules 2016, 21, 797. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Wang, H.-C.; Hsu, Y.-C.; Cho, C.-L.; Yang, M.-Y.; Chien, C.-Y. Capsaicin Induces Autophagy and Apoptosis in Human Nasopharyngeal Carcinoma Cells by Downregulating the PI3K/AKT/mTOR Pathway. Int. J. Mol. Sci. 2017, 18, 1343. [Google Scholar] [CrossRef]
- Fayos, O.; Ochoa-Alejo, N.; De La Vega, O.M.; Savirón, M.; Orduna, J.; Mallor, C.; Barbero, G.F.; Garcés-Claver, A. Assessment of Capsaicinoid and Capsinoid Accumulation Patterns during Fruit Development in Three Chili Pepper Genotypes (Capsicum spp.) Carrying Pun1 and pAMT Alleles Related to Pungency. J. Agric. Food Chem. 2019, 67, 12219–12227. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, X.; Ding, L.; Cui, F.; Tang, Z.; Liu, Z. Stage extraction of capsaicinoids and red pigments from fresh red pepper (Capsicum) fruits with ethanol as solvent. LWT 2014, 59, 396–402. [Google Scholar] [CrossRef]
- Olatunji, T.L.; Afolayan, A.J. The suitability of chili pepper (Capsicum annuum L.) for alleviating human micronutrient dietary deficiencies: A review. Food Sci. Nutr. 2018, 6, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Getahun, E.; Gabbiye, N.; Delele, M.A.; Fanta, S.W.; Gebrehiwot, M.G.; Vanierschot, M. Effect of maturity on the moisture sorption isotherm of chili pepper (Mareko Fana variety). Heliyon 2020, 6, e04608. [Google Scholar] [CrossRef]
- Sobczak, A.; Kowalczyk, K.; Gajc-Wolska, J.; Kowalczyk, W.; Niedzińska, M. Growth, Yield and Quality of Sweet Pepper Fruits Fertilized with Polyphosphates in Hydroponic Cultivation with LED Lighting. Agronomy 2020, 10, 1560. [Google Scholar] [CrossRef]
- Sharafi, Y.; Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Razavi, F.; Fard, J.R.; Farmani, B. Melatonin treatment promotes endogenous melatonin accumulation and triggers GABA shunt pathway activity in tomato fruits during cold storage. Sci. Hortic. 2019, 254, 222–227. [Google Scholar] [CrossRef]
- Meena, O.P.; Dhaliwal, M.S.; Jindal, S.K. Heterosis breeding in chilli pepper by using cytoplasmic male sterile lines for high-yield production with special reference to seed and bioactive compound content under temperature stress regimes. Sci. Hortic. 2020, 262, 109036. [Google Scholar] [CrossRef]
- Caruso, G.; Stoleru, V.V.; Munteanu, N.C.; Sellitto, V.M.; Teliban, G.C.; Burducea, M.; Tenu, I.; Morano, G.; Butnariu, M. Quality Performances of Sweet Pepper under Farming Management. Not. Bot. Horti Agrobot. 2018, 47, 458–464. [Google Scholar] [CrossRef][Green Version]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Grau, F.; Drechsel, N.; Haering, V.; Trautz, D.; Weerakkody, W.J.S.K.; Drechsel, P.; Marschner, B.; Dissanayake, D.M.P.S.; Sinnathamby, V. Impact of Fecal Sludge and Municipal Solid Waste Co-Compost on Crop Growth of Raphanus Sativus L. and Capsicum Anuum L. under Stress Conditions. Resources 2017, 6, 26. [Google Scholar] [CrossRef]
- Valenzuela-García, A.A.; Figueroa-Viramontes, U.; Salazar-Sosa, E.; Orona-Castillo, I.; Gallegos-Robles, M.Á.; García-Hernández, J.L.; Troyo-Diéguez, E. Effect of Organic and Inorganic Fertilizers on the Yield and Quality of Jalapeño Pepper Fruit (Capsicum annuum L.). Agriculture 2019, 9, 208. [Google Scholar] [CrossRef]
- Islam, A.; Sharma, S.S.; Sinha, P.; Negi, M.S.; Neog, B.; Tripathi, S.B. Variability in capsaicinoid content in different landraces of Capsicum cultivated in north-eastern India. Sci. Hortic. 2015, 183, 66–71. [Google Scholar] [CrossRef]
- Andrade, N.J.P.; Monteros-Altamirano, A.; Bastidas, C.G.T.; Sørensen, M. Morphological, Sensorial and Chemical Characterization of Chilli Peppers (Capsicum spp.) from the CATIE Genebank. Agronomy 2020, 10, 1732. [Google Scholar] [CrossRef]
- Liu, H.; Yang, H.; Zheng, J.; Jia, D.; Wang, J.; Li, Y.; Huang, G. Irrigation scheduling strategies based on soil matric potential on yield and fruit quality of mulched-drip irrigated chili pepper in Northwest China. Agric. Water Manag. 2012, 115, 232–241. [Google Scholar] [CrossRef]
- De Farias, V.L.; Araújo Ídila, M.D.S.; Da Rocha, R.F.J.; Garruti, D.D.S.; Pinto, G.A.S. Enzymatic maceration of Tabasco pepper: Effect on the yield, chemical and sensory aspects of the sauce. LWT 2020, 127, 109311. [Google Scholar] [CrossRef]
- Weiss, E.A. Spice Crops; CABI Publishing International: New York, NY, USA, 2002; p. 411. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).