Endophytism of Lecanicillium and Akanthomyces
Abstract
:1. Introduction
2. Taxonomic Background
3. Occurrence
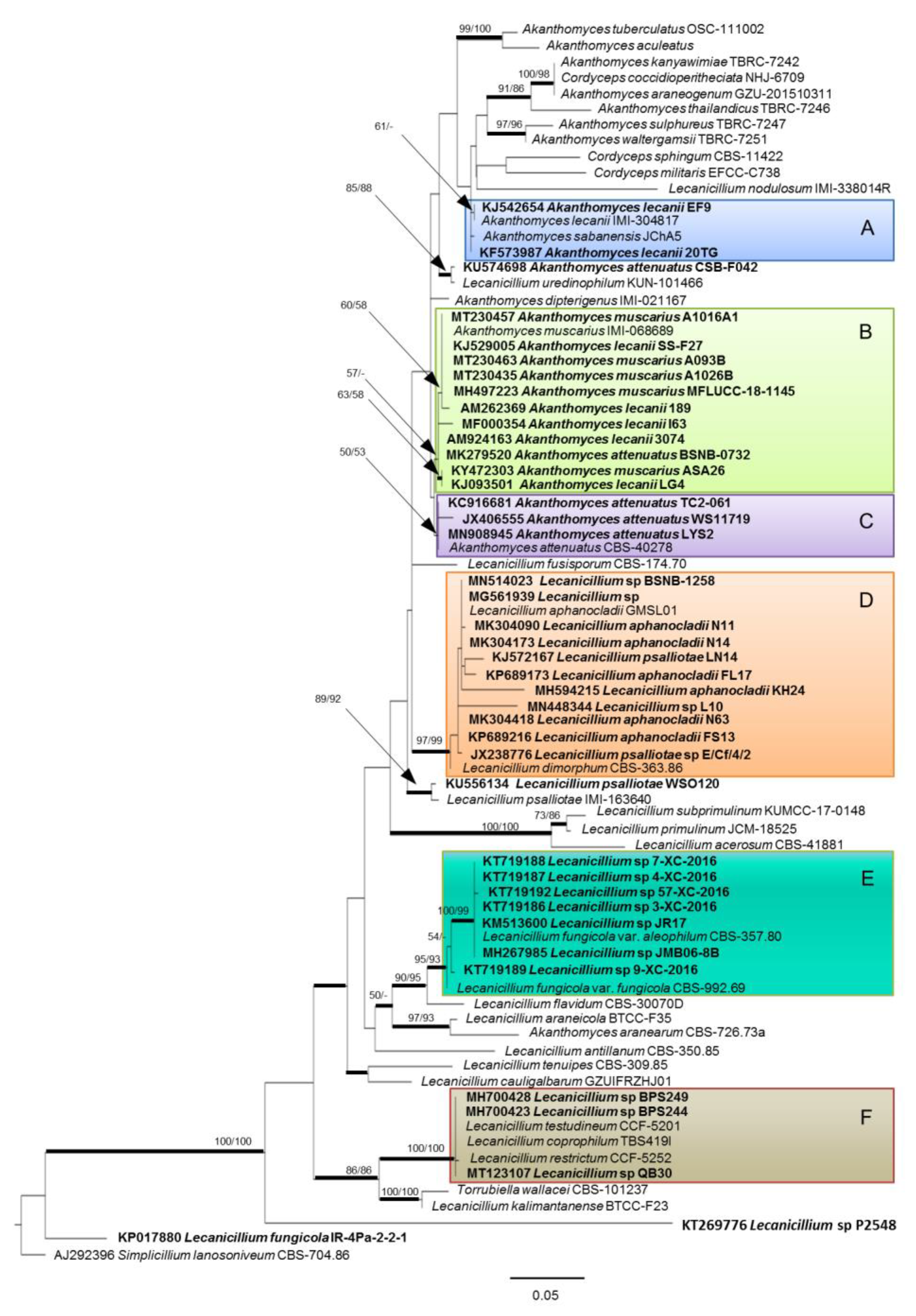
Phylogenetic Relationships of Endophytic Strains
4. Implications in Crop Protection
5. Biochemical Factors Involved in the Tritrophic Interaction with Plants and Pests
6. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Busby, P.E.; Ridout, M.; Newcombe, G. Fungal endophytes: Modifiers of plant disease. Plant. Mol. Biol. 2016, 90, 645–655. [Google Scholar] [CrossRef]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- McKinnon, A.C.; Saari, S.; Moran-Diez, M.E.; Meyling, N.V.; Raad, M.; Glare, T.R. Beauveria bassiana as an endophyte: A critical review on associated methodology and biocontrol potential. BioControl 2017, 62, 1–17. [Google Scholar] [CrossRef]
- Vega, F.E. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef]
- Gams, W.; Van Zaayen, A. Contribution to the taxonomy and pathogenicity of fungicolous Verticillium species. I. Taxonomy. Neth. J. Plant. Pathol. 1982, 88, 57–78. [Google Scholar] [CrossRef]
- Zare, R.; Gams, W. A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium gen. nov. Nova Hedwig. 2001, 73, 1–50. [Google Scholar]
- Zare, R.; Gams, W. A revision of the Verticillium fungicola species complex and its affinity with the genus Lecanicillium. Mycol. Res. 2008, 112, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.H.; Hywel-Jones, N.J.; Sung, J.M.; Luangsa-ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef] [Green Version]
- Mains, E.B. Entomogenous species of Akanthomyces, Hymenostilbe and Insecticola in North America. Mycologia 1950, 42, 566–589. [Google Scholar] [CrossRef]
- Mongkolsamrit, S.; Noisripoom, W.; Thanakitpipattana, D.; Wutikhun, T.; Spatafora, J.W.; Luangsa-ard, J. Disentangling cryptic species with Isaria-like morphs in Cordycipitaceae. Mycologia 2018, 110, 230–257. [Google Scholar] [CrossRef]
- Su, L.; Zhu, H.; Guo, Y.; Du, X.; Guo, J.; Zhang, L.; Qin, C. Lecanicillium coprophilum (Cordycipitaceae, Hypocreales), a new species of fungus from the feces of Marmota monax in China. Phytotaxa 2019, 387, 55–62. [Google Scholar] [CrossRef]
- Wei, D.P.; Wanasinghe, D.N.; Chaiwat, T.A.; Hyde, K.D. Lecanicillium uredinophilium known from rusts, also occurs on animal hosts with chitinous bodies. Asian J. Mycol. 2018, 1, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kepler, R.M.; Luangsa-ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Mycobank Database. Available online: http://www.mycobank.org/ (accessed on 9 April 2020).
- Barthélemy, M.; Elie, N.; Pellissier, L.; Wolfender, J.L.; Stien, D.; Touboul, D.; Eparvier, V. Structural identification of antibacterial lipids from Amazonian palm tree endophytes through the molecular network approach. Int. J. Mol. Sci. 2019, 20, 2006. [Google Scholar] [CrossRef] [Green Version]
- Ellsworth, K.T.; Clark, T.N.; Gray, C.A.; Johnson, J.A. Isolation and bioassay screening of medicinal plant endophytes from eastern Canada. Can. J. Microbiol. 2013, 59, 761–765. [Google Scholar] [CrossRef]
- Sánchez-Márquez, S.; Bills, G.F.; Zabalgogeazcoa, I. Diversity and structure of the fungal endophytic assemblages from two sympatric coastal grasses. Fungal Divers. 2008, 33, 87–100. [Google Scholar]
- Sánchez Márquez, M.; Bills, G.F.; Zabalgogeazcoa, I. The endophytic mycobiota of the grass Dactylis Glomerata. Fungal Divers. 2007, 27, 171–195. [Google Scholar]
- Poosakkannu, A.; Nissinen, R.; Kytöviita, M.M. Culturable endophytic microbial communities in the circumpolar grass, Deschampsia flexuosa in a sub-Arctic inland primary succession are habitat and growth stage specific. Environ. Microbiol. Rep. 2015, 7, 111–122. [Google Scholar] [CrossRef]
- Molina-Montenegro, M.A.; Oses, R.; Torres-Díaz, C.; Atala, C.; Núñez, M.A.; Armas, C. Fungal endophytes associated with roots of nurse cushion species have positive effects on native and invasive beneficiary plants in an alpine ecosystem. Perspect. Plant. Ecol. Evol. Syst. 2015, 17, 218–226. [Google Scholar] [CrossRef]
- Giordano, L.; Gonthier, P.; Varese, G.C.; Miserere, L.; Nicolotti, G. Mycobiota inhabiting sapwood of healthy and declining Scots pine (Pinus sylvestris L.) trees in the Alps. Fungal Divers. 2009, 38, e83. [Google Scholar]
- Behnke-Borowczyk, J.; Kwaśna, H.; Kulawinek, B. Fungi associated with Cyclaneusma needle cast in Scots pine in the west of Poland. For. Pathol. 2019, 49, e12487. [Google Scholar] [CrossRef]
- Ashkezari, S.J.; Fotouhifar, K.B. Diversity of endophytic fungi of common yew (Taxus baccata L.) in Iran. Mycol. Progr. 2017, 16, 247–256. [Google Scholar] [CrossRef]
- Nicoletti, R.; De Filippis, A.; Buommino, E. Antagonistic aptitude and antiproliferative properties on tumor cells of fungal endophytes from the Astroni Nature Reserve, Italy. Afr. J. Microbiol. Res. 2013, 7, 4073–4083. [Google Scholar]
- Vinit, K.; Doilom, M.; Wanasinghe, D.N.; Bhat, D.J.; Brahmanage, R.S.; Jeewon, R.; Xiao, Y.; Hyde, K.D. Phylogenetic placement of Akanthomyces muscarius, a new endophyte record from Nypa fruticans in Thailand. Curr. Res. Environ. Appl. Mycol. 2018, 8, 404–417. [Google Scholar] [CrossRef]
- Widler, B.; Müller, E. Untersuchungen über endophytische pilze von Arctostaphylos uva-ursi (L.) Sprengel (Ericaceae). Bot. Helv. 1984, 94, 307–337. [Google Scholar]
- Bills, G.F.; Polishook, J.D. Microfungi from Carpinus caroliniana. Can. J. Bot. 1991, 69, 1477–1482. [Google Scholar] [CrossRef]
- Fang, K.; Miao, Y.F.; Chen, L.; Zhou, J.; Yang, Z.P.; Dong, X.F.; Zhang, H.B. Tissue-specific and geographical variation in endophytic fungi of Ageratina adenophora and fungal associations with the environment. Front. Microbiol. 2019, 10, 2919. [Google Scholar] [CrossRef] [Green Version]
- Shobha, M.; Bharathi, T.R.; Sampath Kumara, K.K.; Prakash, H.S. Diversity and biological activities of fungal root endophytes of Hemidesmus indicus (L.) R.Br. J. Pharmacogn. Phytochem. 2019, 8, 273–280. [Google Scholar]
- Wang, Y.; Lai, Z.; Li, X.X.; Yan, R.M.; Zhang, Z.B.; Yang, H.L.; Zhu, D. Isolation, diversity and acetylcholinesterase inhibitory activity of the culturable endophytic fungi harboured in Huperzia serrata from Jinggang Mountain, China. World J. Microbiol. Biotechnol. 2016, 32, 20. [Google Scholar] [CrossRef]
- Summerbell, R.C. Root endophyte and mycorrhizosphere fungi of black spruce, Picea mariana, in a boreal forest habitat: Influence of site factors on fungal distributions. Stud. Mycol. 2005, 53, 121–145. [Google Scholar] [CrossRef] [Green Version]
- Khalmuratova, I.; Kim, H.; Nam, Y.J.; Oh, Y.; Jeong, M.J.; Choi, H.R.; You, Y.H.; Choo, Y.S.; Lee, I.J.; Shin, J.H.; et al. Diversity and plant growth promoting capacity of endophytic fungi associated with halophytic plants from the west coast of Korea. Mycobiology 2015, 43, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginting, R.C.B.; Sukarno, N.; Widyastuti, U.; Darusman, L.K.; Kanaya, S. Diversity of endophytic fungi from red ginger (Zingiber officinale Rosc.) plant and their inhibitory effect to Fusarium oxysporum plant pathogenic fungi. HAYATI J. Biosci. 2013, 20, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; You, Y.H.; Yoon, H.; Seo, Y.; Kim, Y.E.; Choo, Y.S.; Lee, I.J.; Shin, J.H.; Kim, J.G. Culturable fungal endophytes isolated from the roots of coastal plants inhabiting Korean east coast. Mycobiology 2014, 42, 100–108. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, L.M.; Almeida, A.R.; Salgado, M.; Pfenning, L.H. Fungal endophytes associated with the mistletoe Phoradendron perrottettii and its host tree Tapirira guianensis. Mycol. Progr. 2010, 9, 559–566. [Google Scholar] [CrossRef]
- Brownbridge, M.; Reay, S.D.; Nelson, T.L.; Glare, T.R. Persistence of Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte following inoculation of radiata pine seed and seedlings. Biol. Control. 2012, 61, 194–200. [Google Scholar] [CrossRef]
- You, Y.H.; Park, J.M.; Seo, Y.G.; Lee, W.; Kang, M.S.; Kim, J.G. Distribution, characterization, and diversity of the endophytic fungal communities on Korean seacoasts showing contrasting geographic conditions. Mycobiology 2017, 45, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Ofek-Lalzar, M.; Gur, Y.; Ben-Moshe, S.; Sharon, O.; Kosman, E.; Mochli, E.; Sharon, A. Diversity of fungal endophytes in recent and ancient wheat ancestors Triticum dicoccoides and Aegilops sharonensis. FEMS Microbiol. Ecol. 2016, 92, fiw152. [Google Scholar] [CrossRef] [Green Version]
- Skaltsas, D.N.; Badotti, F.; Vaz, A.B.M.; da Silva, F.F.; Gazis, R.; Wurdack, K.; Castlebury, L.; Góes-Neto, A.; Chaverri, P. Exploration of stem endophytic communities revealed developmental stage as one of the drivers of fungal endophytic community assemblages in two Amazonian hardwood genera. Sci. Rep. 2019, 9, 12685. [Google Scholar] [CrossRef] [Green Version]
- Glynou, K.; Ali, T.; Buch, A.K.; Haghi Kia, S.; Ploch, S.; Xia, X.; Çelik, A.; Thines, M.; Maciá-Vicente, J.G. The local environment determines the assembly of root endophytic fungi at a continental scale. Environ. Microbiol. 2016, 18, 2418–2434. [Google Scholar] [CrossRef]
- Mai, P.Y.; Levasseur, M.; Buisson, D.; Touboul, D.; Eparvier, V. Identification of antimicrobial compounds from Sandwithia guyanensis-associated endophyte using molecular network approach. Plants 2020, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Evans, H.C.; Holmes, K.A.; Thomas, S.E. Endophytes and mycoparasites associated with an indigenous forest tree, Theobroma gileri, in Ecuador and a preliminary assessment of their potential as biocontrol agents of cocoa diseases. Mycol. Progr. 2003, 2, 149–160. [Google Scholar] [CrossRef]
- Kago, L.; Njuguna, J.; Njarui, D.M.G.; Ghimire, S.R. Fungal endophyte communities of Brachiaria grass (Brachiaria spp.) in Kenya. In Climate Smart Brachiaria Grasses for Improving Livestock Production in East Africa—Kenya Experience: Proceedings of the Workshop, Naivasha, Kenya, 14–15 September 2016; Njarui, D.M.G., Gichangi, E.M., Ghimire, S.R., Muinga, R.W., Eds.; Kenya Agricultural and Livestock Research Organization: Nairobi, Kenya, 2016; pp. 150–162. [Google Scholar]
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol. Control. 2010, 55, 34–41. [Google Scholar] [CrossRef]
- McGee, P.A. Reduced growth and deterrence from feeding of the insect pest Helicoverpa armigera associated with fungal endophytes from cotton. Aus. J. Experim. Agric. 2002, 42, 995–999. [Google Scholar] [CrossRef]
- de Souza Vieira, P.D.; de Souza Motta, C.M.; Lima, D.; Torres, J.B.; Quecine, M.C.; Azevedo, J.L.; de Oliveira, N.T. Endophytic fungi associated with transgenic and non-transgenic cotton. Mycology 2011, 2, 91–97. [Google Scholar] [CrossRef]
- Castillo-Lopez, D. Ecological roles of two entomopathogenic endophytes: Beauveria bassiana and Purpureocillium lilacinum in cultivated cotton. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 11 May 2015. [Google Scholar]
- Dash, C.K.; Bamisile, B.S.; Keppanan, R.; Qasim, M.; Lin, Y.; Islam, S.U.; Hussain, M.; Wang, L. Endophytic entomopathogenic fungi enhance the growth of Phaseolus vulgaris L. (Fabaceae) and negatively affect the development and reproduction of Tetranychus urticae Koch (Acari: Tetranychidae). Microb. Pathog. 2018, 125, 385–392. [Google Scholar] [CrossRef]
- Dolatabad, H.K.; Javan-Nikkhah, M.; Shier, W.T. Evaluation of antifungal, phosphate solubilisation, and siderophore and chitinase release activities of endophytic fungi from Pistacia vera. Mycol. Progr. 2017, 16, 777–790. [Google Scholar] [CrossRef]
- González, V.; Tello, M.L. The endophytic mycota associated with Vitis vinifera in central Spain. Fungal Divers. 2011, 47, 29–42. [Google Scholar] [CrossRef]
- Kuchár, M.; Glare, T.R.; Hampton, J.G.; Dickie, I.A.; Christey, M.C. Virulence of the plant-associated endophytic fungus Lecanicillium muscarium to diamondback moth larvae. New Zeal. Plant. Prot. 2019, 72, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Benhamou, N.; Brodeur, J. Pre-inoculation of Ri T-DNA transformed cucumber roots with the mycoparasite, Verticillium lecanii, induces host defense reactions against Pythium ultimum infection. Physiol. Mol. Plant. Pathol. 2001, 58, 133–146. [Google Scholar] [CrossRef]
- Hirano, E.; Koike, M.; Aiuchi, D.; Tani, M. Pre-inoculation of cucumber roots with Verticillium lecanii (Lecanicillium muscarium) induces resistance to powdery mildew. Res. Bull. Obihiro Univ. 2008, 29, 82–94. [Google Scholar]
- Aghdam, S.A.; Fotouhifar, K.B. Introduction of some endophytic fungi of sour cherry trees (Prunus cerasus) in Iran. Rostaniha 2017, 18, 77–94. [Google Scholar]
- Rijavec, T.; Lapanje, A.; Dermastia, M.; Rupnik, M. Isolation of bacterial endophytes from germinated maize kernels. Can. J. Microbiol. 2007, 53, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Vidal, S.; Lopez-Llorca, L.V.; Jansson, H.B.; Salinas, J. Endophytic colonization of date palm (Phoenix dactylifera L.) leaves by entomopathogenic fungi. Micron 2006, 37, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Bhagyasree, S.; Ghosh, S.; Thippaiah, M.; Rajgopal, N. Survey on natural occurrence of endophytes in maize (Zea mays L.) ecosystem. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2526–2533. [Google Scholar] [CrossRef] [Green Version]
- Zimowska, B.; Okoń, S.; Becchimanzi, A.; Krol, E.D.; Nicoletti, R. Phylogenetic characterization of Botryosphaeria strains associated with Asphondylia galls on species of Lamiaceae. Diversity 2020, 12, 41. [Google Scholar] [CrossRef] [Green Version]
- Mitina, G.; Kazartsev, I.; Vasileva, A.; Yli-Mattila, T. Multilocus genotyping based species identification of entomopathogenic fungi of the genus Lecanicillium (=Verticillium lecanii sl). J. Basic Microbiol. 2017, 57, 950–961. [Google Scholar] [CrossRef]
- Kouvelis, V.N.; Sialakouma, A.; Typas, M.A. Mitochondrial gene sequences alone or combined with ITS region sequences provide firm molecular criteria for the classification of Lecanicillium species. Mycol. Res. 2008, 112, 829–844. [Google Scholar] [CrossRef]
- Nonaka, K.; Kaifuchi, S.; Ōmura, S.; Masuma, R. Five new Simplicillium species (Cordycipitaceae) from soils in Tokyo, Japan. Mycoscience 2013, 54, 42–53. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.S.J.; Gené, J.; Guarro, J.; Baseia, I.G.; García, D.; Gusmão, L.F.P.; Souza-Motta, C.M.; et al. Fungal planet description sheets: 716–784. Persoonia 2018, 40, 240–393. [Google Scholar] [CrossRef]
- Anderson, C.M.; McGee, P.A.; Nehl, D.B.; Mensah, R.K. The fungus Lecanicillium lecanii colonises the plant Gossypium hirsutum and the aphid Aphis gossypii. Australas. Mycol. 2007, 26, 65–70. [Google Scholar]
- Gurulingappa, P.; McGee, P.A.; Sword, G. Endophytic Lecanicillium lecanii and Beauveria bassiana reduce the survival and fecundity of Aphis gossypii following contact with conidia and secondary metabolites. Crop. Prot. 2011, 30, 349–353. [Google Scholar] [CrossRef]
- Lin, Y.; Hussain, M.; Avery, P.B.; Qasim, M.; Fang, D.; Wang, L. Volatiles from plants induced by multiple aphid attacks promote conidial performance of Lecanicillium lecanii. PLoS ONE 2016, 11, e0151844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Qasim, M.; Hussain, M.; Akutse, K.S.; Avery, P.B.; Dash, C.K.; Wang, L. The herbivore-induced plant volatiles methyl salicylate and menthol positively affect growth and pathogenicity of entomopathogenic fungi. Sci. Rep. 2017, 7, 40494. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Skillman, J.; Vandermeer, J. Indirect biological control of the coffee leaf rust, Hemileia vastatrix, by the entomogenous fungus Lecanicillium lecanii in a complex coffee agroecosystem. Biol. Control. 2012, 61, 89–97. [Google Scholar] [CrossRef]
- Romero, D.; De Vicente, A.; Zeriouh, H.; Cazorla, F.M.; Fernández-Ortuño, D.; Torés, J.A.; Pérez-García, A. Evaluation of biological control agents for managing cucurbit powdery mildew on greenhouse-grown melon. Plant. Pathol. 2007, 56, 976–986. [Google Scholar] [CrossRef] [Green Version]
- Ownley, B.H.; Gwinn, K.D.; Vega, F.E. Endophytic fungal entomopathogens with activity against plant pathogens: Ecology and evolution. BioControl 2010, 55, 113–128. [Google Scholar] [CrossRef]
- Gómez-Vidal, S.; Salinas, J.; Tena, M.; Lopez-Llorca, L.V. Proteomic analysis of date palm (Phoenix dactylifera L.) responses to endophytic colonization by entomopathogenic fungi. Electrophoresis 2009, 30, 2996–3005. [Google Scholar] [CrossRef]
- Kim, J.J.; Goettel, M.S.; Gillespie, D.R. Potential of Lecanicillium species for dual microbial control of aphids and the cucumber powdery mildew fungus, Sphaerotheca Fuliginea. Biol. Control 2007, 40, 327–332. [Google Scholar] [CrossRef]
- Goettel, M.S.; Koike, M.; Kim, J.J.; Aiuchi, D.; Shinya, R.; Brodeur, J. Potential of Lecanicillium spp. for management of insects, nematodes and plant diseases. J. Invertebr. Pathol. 2008, 98, 256–261. [Google Scholar] [CrossRef]
- Gurulingappa, P.; Mc Gee, P.; Sword, G.A. In vitro and in planta compatibility of insecticides and the endophytic entomopathogen, Lecanicillium lecanii. Mycopathologia 2011, 172, 161–168. [Google Scholar] [CrossRef]
- Ondráčková, E.; Seidenglanz, M.; Šafář, J. Effect of seventeen pesticides on mycelial growth of Akanthomyces, Beauveria, Cordyceps and Purpureocillium strains. Czech. Mycol. 2019, 7, 123–135. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Blackburn, L.F.; Northing, P.; Luo, W.; Cannon, R.J.C.; Walters, K.F.A. Chemical compatibility testing of the entomopathogenic fungus Lecanicillium muscarium to control Bemisia tabaci in glasshouse environment. Int. J. Environ. Sci. Technol. 2010, 7, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Zhang, C.; Wang, Z.; Wang, X.M.; Wu, J.H.; Cuthbertson, A.G.; Shao, Z.; Qiu, B.L. Toxicological and biochemical basis of synergism between the entomopathogenic fungus Lecanicillium muscarium and the insecticide matrine against Bemisia tabaci (Gennadius). Sci. Rep. 2017, 7, 46558. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.; Jacob, T.K.; Devasahayam, S.; Thomas, S.; Geethu, C. Multifarious plant growth promotion by an entomopathogenic fungus Lecanicillium psalliotae. Microbiol. Res. 2018, 207, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Fiorentino, A. Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta. Agriculture 2015, 5, 918–970. [Google Scholar] [CrossRef] [Green Version]
- Claydon, N.; Grove, J.F. Insecticidal secondary metabolic products from the entomogenous fungus Verticillium lecanii. J. Invertebr. Pathol. 1982, 40, 413–418. [Google Scholar] [CrossRef]
- Claydon, N.; Grove, J.F.; Pople, M.; Begley, M.J. New metabolic products of Verticillium lecanii. Part 1. 3β-Hydroxy-4,4,14α-trimethyl-5α-pregna-7,9(11)-diene-20S-carboxylic acid and the isolation and characterisation of some minor metabolites. J. Chem. Soc. Perkin Trans. 1 1984, 497–502. [Google Scholar] [CrossRef]
- Gindin, G.; Barash, I.; Harari, N.; Raccah, B. Effect of endotoxic compounds isolated from Verticillium lecani on the sweet potato whitefly, Bemisia tabaci. Phytoparasitica 1994, 22, 189–196. [Google Scholar] [CrossRef]
- Soman, A.G.; Gloer, J.B.; Angawi, R.F.; Wicklow, D.T.; Dowd, P.F. Vertilecanins: New phenopicolinic acid analogues from Verticillium lecanii. J. Nat. Prod. 2001, 64, 189–192. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; You, M.; Guan, X.; Liu, B. Toxicity and feeding deterrence of crude toxin extracts of Lecanicillium (Verticillium) lecanii (Hyphomycetes) against sweet potato whitefly, Bemisia tabaci (Homoptera: Aleyrodidae). Pest. Manag. Sci. 2007, 63, 381–387. [Google Scholar] [CrossRef]
- Roll, D.M.; Barbieri, L.R.; Bigelis, R.; McDonald, L.A.; Arias, D.A.; Chang, L.P.; Singh, M.P.; Luckman, S.W.; Berrodin, T.J.; Yudt, M.R. The lecanindoles, nonsteroidal progestins from the terrestrial fungus Verticillium lecanii 6144. J. Nat. Prod. 2009, 72, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.; Dang, L.Z.; Zhao, P.J.; Zhang, K.Q.; Li, G.H. Two new aromadendrane sesquiterpenes from Verticillium psalliotae. Nat. Prod. Res. 2019, 33, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, S.; Sang, X.; Pan, H.; Li, Z.; Hua, H.; Han, A.; Bai, J. Lecanicillolide, an α-pyrone substituted spiciferone from the fungus Lecanicillium sp. PR-M-3. Tetrahedron Lett. 2017, 58, 740–743. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Sang, X.N.; Sun, K.; Huang, S.D.; Chen, S.S.; Xue, C.M.; Ban, L.F.; Li, Z.L.; Hua, H.M.; Pei, Y.H.; et al. Lecanicillones A–C, three dimeric isomers of spiciferone A with a cyclobutane ring from an entomopathogenic fungus Lecanicillium sp. PR-M-3. RSC Advan. 2016, 6, 82348–82351. [Google Scholar] [CrossRef]
- Li, C.Y.; Lo, I.W.; Wang, S.W.; Hwang, T.L.; Chung, Y.M.; Cheng, Y.B.; Tseng, S.P.; Liu, Y.H.; Hsu, Y.M.; Chen, S.R.; et al. Novel 11-norbetaenone isolated from an entomopathogenic fungus Lecanicillium antillanum. Bioorg. Med. Chem. Lett. 2017, 27, 1978–1982. [Google Scholar] [CrossRef]
- Nagaoka, T.; Nakata, K.; Kouno, K. Antifungal activity of oosporein from an antagonistic fungus against Phytophthora infestans. Z. Naturforsch. C. Biosci. 2004, 59, 302–304. [Google Scholar] [CrossRef] [Green Version]
- Butt, T.M.; Hadj, N.B.E.; Skrobek, A.; Ravensberg, W.J.; Wang, C.; Lange, C.M.; Vey, A.; Umi-Kulsoom, S.; Dudley, E. Mass spectrometry as a tool for the selective profiling of destruxins; their first identification in Lecanicillium longisporum. Rapid Commun. Mass Spectrom. 2009, 23, 1426–1434. [Google Scholar] [CrossRef]
- Ravindran, K.; Sivaramakrishnan, S.; Hussain, M.; Dash, C.K.; Bamisile, B.S.; Qasim, M.; Wang, L. Investigation and molecular docking studies of bassianolide from Lecanicillium lecanii against Plutella xylostella (Lepidoptera: Plutellidae). Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2018, 206–207, 65–72. [Google Scholar] [CrossRef]
- Ishidoh, K.I.; Kinoshita, H.; Igarashi, Y.; Ihara, F.; Nihira, T. Cyclic lipodepsipeptides verlamelin A and B, isolated from entomopathogenic fungus Lecanicillium sp. J. Antibiot. 2014, 67, 459–463. [Google Scholar] [CrossRef] [Green Version]
- Wagenaar, M.M.; Gibson, D.M.; Clardy, J. Akanthomycin, a new antibiotic pyridone from the entomopathogenic fungus Akanthomyces gracilis. Org. Lett. 2002, 4, 671–673. [Google Scholar] [CrossRef]
- Kuephadungphan, W.; Helaly, S.E.; Daengrot, C.; Phongpaichit, S.; Luangsa-ard, J.J.; Rukachaisirikul, V.; Stadler, M. Akanthopyrones A–D, α-pyrones bearing a 4-O-methyl-β-D-glucopyranose moiety from the spider-associated ascomycete Akanthomyces novoguineensis. Molecules 2017, 22, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helaly, S.E.; Kuephadungphan, W.; Phongpaichit, S.; Luangsa-ard, J.J.; Rukachaisirikul, V.; Stadler, M. Five unprecedented secondary metabolites from the spider parasitic fungus Akanthomyces novoguineensis. Molecules 2017, 22, 991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, N.; Hattori, M.; Yokoyama, E.; Isomura, S.; Ujita, M.; Hara, A. Entomogenous fungi that produce 2,6-pyridine dicarboxylicacid (dipicolinic acid). J. Biosci. Bioeng. 2006, 102, 365–368. [Google Scholar] [CrossRef]
- Gan, Z.; Yang, J.; Tao, N.; Liang, L.; Mi, Q.; Li, J.; Zhang, K.Q. Cloning of the gene Lecanicillium psalliotae chitinase Lpchi1 and identification of its potential role in the biocontrol of root-knot nematode Meloidogyne incognita. Appl. Microbiol. Biotechnol. 2007, 76, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Pino, Z.; Vigueras, G.; Shirai, K. Production and activities of chitinases and hydrophobins from Lecanicillium lecanii. Bioproc. Biosyst. Eng. 2011, 34, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.Q.; Quyen, D.T.; Nguyen, S.L.T.; Vu, V.H. An extracellular antifungal chitinase from Lecanicillium lecanii: Purification, properties, and application in biocontrol against plant pathogenic fungi. Turk. J. Biol. 2015, 39, 6–14. [Google Scholar] [CrossRef]
- Saksirirat, W.; Hoppe, H.H. Degradation of uredospores of the soybean rust fungus (Phakopsora pachyrhizi Syd.) by cell-free culture filtrates of the mycoparasite Verticillium psalliotae Treschow. J. Phytopathol. 1991, 132, 33–45. [Google Scholar] [CrossRef]
- Bye, N.J.; Charnley, A.K. Regulation of cuticle-degrading subtilisin proteases from the entomopathogenic fungi, Lecanicillium spp: Implications for host specificity. Arch. Microbiol. 2008, 189, 81–92. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef]
- Hanan, A.; Basit, A.; Nazir, T.; Majeed, M.Z.; Qiu, D. Anti-insect activity of a partially purified protein derived from the entomopathogenic fungus Lecanicillium lecanii (Zimmermann) and its putative role in a tomato defense mechanism against green peach aphid. J. Invertebr. Pathol. 2020, 170, 107282. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Afolabi, O.G.; Hussain, M.; Qasim, M.; Wang, L. Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: An insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiol. Res. 2018, 217, 34–50. [Google Scholar] [CrossRef] [PubMed]
| Species Names * | ITS Sequence Used in | ||
|---|---|---|---|
| Lecanicillium | Akanthomyces | Cordyceps/Torrubiella | Phylogenetic Analysis |
| L. acerosum | NR11268 | ||
| L. antillanum | AJ292392 | ||
| L. aphanocladii | LT220701 | ||
| L. aranearum | A. aranearum | T. alba | AJ292464 |
| L. araneicola | AB378506 | ||
| L. araneogenum | A. neoaraneogenus | NR161115 | |
| L. attenuatum | A. attenuatus | AJ292434 | |
| L. cauligalbarum | MH730663 | ||
| L. coprophilum | MH177615 | ||
| L. dimorphum | AJ292429 | ||
| L. flavidum | EF641877 | ||
| L. fungicola var.aleophilum | NR111064 | ||
| L. fungicola var.fungicola | NR119653 | ||
| L. fusisporum | AJ292428 | ||
| L. kalimantanense | AB360356 | ||
| L. lecanii | A. lecanii | C. confragosa | AJ292383 |
| L. longisporum | A. dipterigenus | AJ292385 | |
| L. muscarium | A. muscarius | NR111096 | |
| L. nodulosum | Akanthomyces sp. | EF513012 | |
| L. primulinum | NR119418 | ||
| L. psalliotae | AJ292389 | ||
| L. restrictum | LT548279 | ||
| L. sabanense | A. sabanensis | KC633232 | |
| L. subprimulinum | MG585314 | ||
| L. tenuipes | AJ292391 | ||
| L. testudineum | LT548278 | ||
| L. uredinophilum | Akanthomyces sp. | MG948305 | |
| L. wallacei | T. wallacei | NR111267 | |
| Lecanicillium sp. | C. militaris | AF153264 | |
| A. aculeatus | KC519371 | ||
| A. coccidioperitheciatus | C. coccidioperitheciata | JN049865 | |
| A. kanyawimiae | MF140751 | ||
| A. sphingum | C. sphingum | AY245641 | |
| A. sulphureus | Torrubiella sp. | MF140756 | |
| A. thailandicus | Torrubiella sp. | MF140755 | |
| A. tuberculatus | C. tuberculata | JN049830 | |
| A. waltergamsii | MF140747 | ||
| Species | Host Plant | Country | ITS Sequence √ | Reference |
|---|---|---|---|---|
| A. attenuatus | Astrocaryum sciophilum | French Guyana | MK279520 | [15] |
| Conifer plant | China | MN908945 | GenBank | |
| Symplocarpus foetidus | Canada | KC916681 | [16] | |
| A. lecanii | Ammophila arenaria | Spain | - | [17] |
| Dactylis glomerata | Spain | AM262369 | [18] | |
| Deschampsia flexuosa | Finland | KJ529005 | [19] | |
| Elymus farctus | Spain | AM924163 | [17] | |
| Laretia acaulis | Chile | - | [20] | |
| Pinus sylvestris | Italy | KJ093501 | [21] | |
| Pinus sylvestris | Poland | - | [22] | |
| Shorea thumbuggaia | India | KJ542654 | GenBank | |
| Taxus baccata | Iran | KF573987 | [23] | |
| A. muscarius | Acer campestre | Italy | MT230457 | This paper |
| Laurus nobilis | Italy | - | [24] | |
| Myrtus communis | Italy | MT230435 | This paper | |
| Nypa fruticans | Thailand | MH497223 | [25] | |
| Quercus robur | Italy | MT230463 | This paper | |
| Akanthomyces sp. * | Arctostaphylos uva-ursi | Switzerland | - | [26] |
| Carpinus caroliniana | USA | - | [27] | |
| L. aphanocladii | Ageratina adenophora | China | MK304090 MK304173 MK304418 | [28] |
| Hemidesmus indicus | India | MH594215 | [29] | |
| Huperzia serrata | China | KP689216 KP689173 | [30] | |
| Picea mariana | Canada | - | [31] | |
| L. fungicola | Phragmites australis | Korea | KP017880 | [32] |
| L. kalimantanense | Zingiber officinale | Indonesia | - | [33] |
| L. psalliotae | Cerastium fischerianum | Korea | JX238776 | [34] |
| Coix lachryma-jobi | China | KJ572167 | GenBank | |
| Magnolia officinalis | China | GenBank | ||
| Phoradendron perrottettii | Brazil | - | [35] | |
| Pinus radiata | New Zealand | - | [36] | |
| Sedum oryzifolium | Korea | KU556134 | [37] | |
| Tapirira guianensis | Brazil | - | [35] | |
| Triticum dicoccoides | Israel | - | [38] | |
| Lecanicillium sp. | Artocarpus lacucha | India | MH700423 MH700428 | GenBank |
| Bupleurum chinense | China | MG561939 | GenBank | |
| Huperzia serrata | China | KM513600 | [30] | |
| Liparis japonica | China | KT719186 KT719187 KT719188 KT719189 KT719192 | GenBank | |
| Micrandra spruceana | Peru | MH267985 | [39] | |
| Microthlaspi perfoliatum | Greece | KT269776 | [40] | |
| Quassia indica | India | MH910098 | GenBank | |
| Sandwithia guyanensis | French Guyana | MN514023 | [41] | |
| Theobroma gileri | Ecuador | - | [42] |
| Species | Host Plant | Country | ITS Sequence √ | Reference |
|---|---|---|---|---|
| A. attenuatus | Brachiaria sp. | Kenya | KU574698 | [43] |
| Salvia miltiorrhiza | China | JX406555 | GenBank | |
| A. lecanii | Cucurbita maxima | Australia | - | [44] |
| Gossypium hirsutum | Australia | - | [45] | |
| Gossypium hirsutum | Brazil | - | [46] | |
| Gossypium hirsutum | Texas, USA | KP407570 | [47] | |
| Solanum lycopersicum | Australia | - | [44] | |
| Phaseolus vulgaris | Australia | - | [44] | |
| Phaseolus vulgaris | China | - | [48] | |
| Pistacia vera | Iran | MF000354 | [49] | |
| Triticum aestivum | Australia | - | [44] | |
| Vitis vinifera | Spain | - | [50] | |
| Zea mays | Australia | - | [44] | |
| A. muscarius | Brassica oleracea | New Zealand | - | [51] |
| Cucumis sativus | Canada | - | [52] | |
| Cucumis sativus | Japan | - | [53] | |
| Prunus cerasus | Iran | KY472303 | [54] | |
| L. aphanocladii | Zea mays | Slovenia | - | [55] |
| L. dimorphum | Phoenix dactylifera | Spain | - | [56] |
| L. psalliotae | Phoenix dactylifera | Spain | - | [56] |
| Lecanicillium sp. | Citrus limon | Iran | MN448344 | GenBank |
| Vitis vinifera | China | MT123107 | GenBank | |
| Zea mays | India | - | [57] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoletti, R.; Becchimanzi, A. Endophytism of Lecanicillium and Akanthomyces. Agriculture 2020, 10, 205. https://doi.org/10.3390/agriculture10060205
Nicoletti R, Becchimanzi A. Endophytism of Lecanicillium and Akanthomyces. Agriculture. 2020; 10(6):205. https://doi.org/10.3390/agriculture10060205
Chicago/Turabian StyleNicoletti, Rosario, and Andrea Becchimanzi. 2020. "Endophytism of Lecanicillium and Akanthomyces" Agriculture 10, no. 6: 205. https://doi.org/10.3390/agriculture10060205
APA StyleNicoletti, R., & Becchimanzi, A. (2020). Endophytism of Lecanicillium and Akanthomyces. Agriculture, 10(6), 205. https://doi.org/10.3390/agriculture10060205






