Crop Sensor Based Non-destructive Estimation of Nitrogen Nutritional Status, Yield, and Grain Protein Content in Wheat
Abstract
1. Introduction
2. Materials and Methods
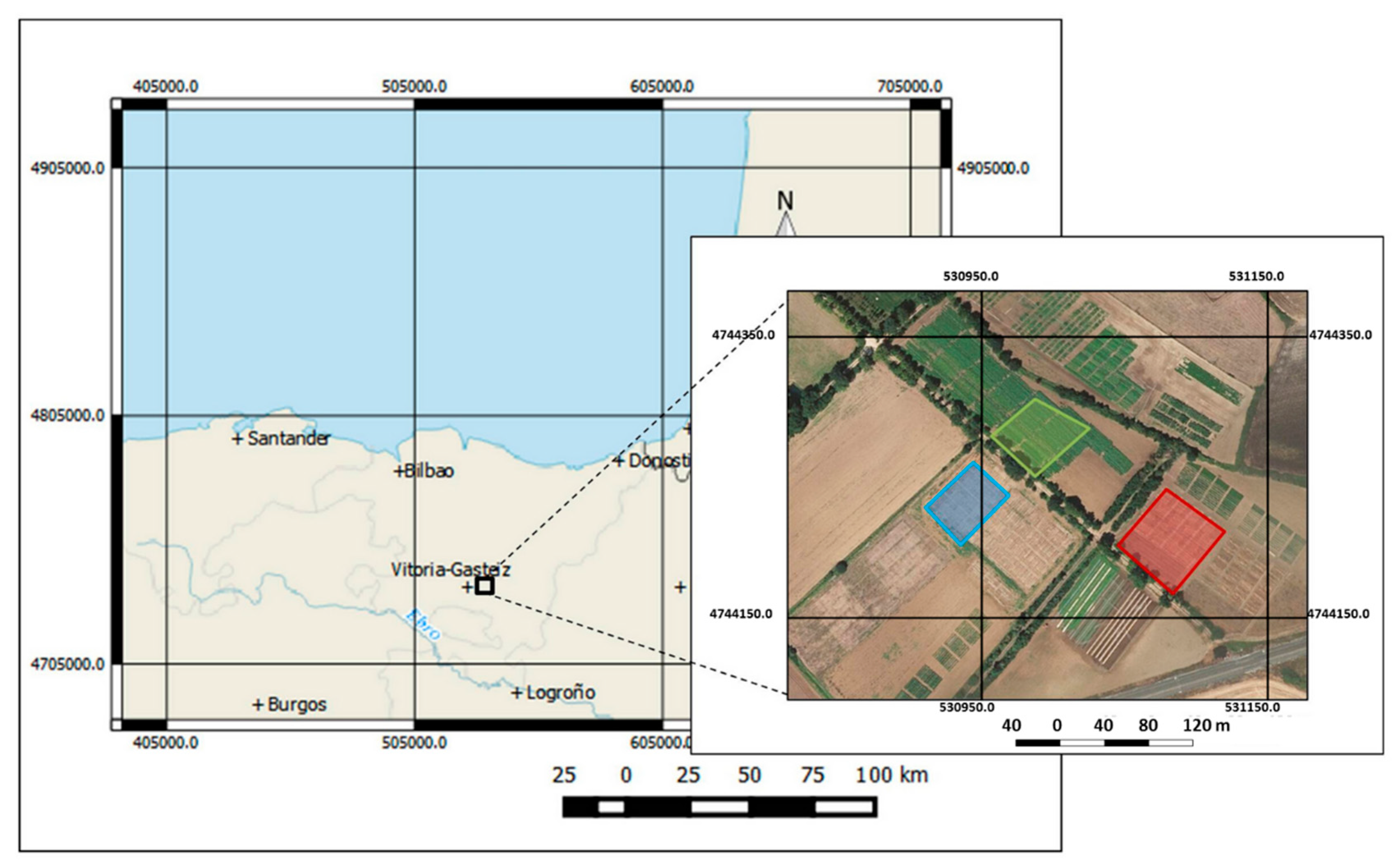
2.1. Study Site
2.2. Treatments
2.3. Plant Biomass and Nitrogen Nutrition Index (NNI)
2.4. Crop Sensors for Following Crop N Status
2.5. Models to be Fitted
2.5.1. The Linear Model
2.5.2. The Exponential Model
3. Results
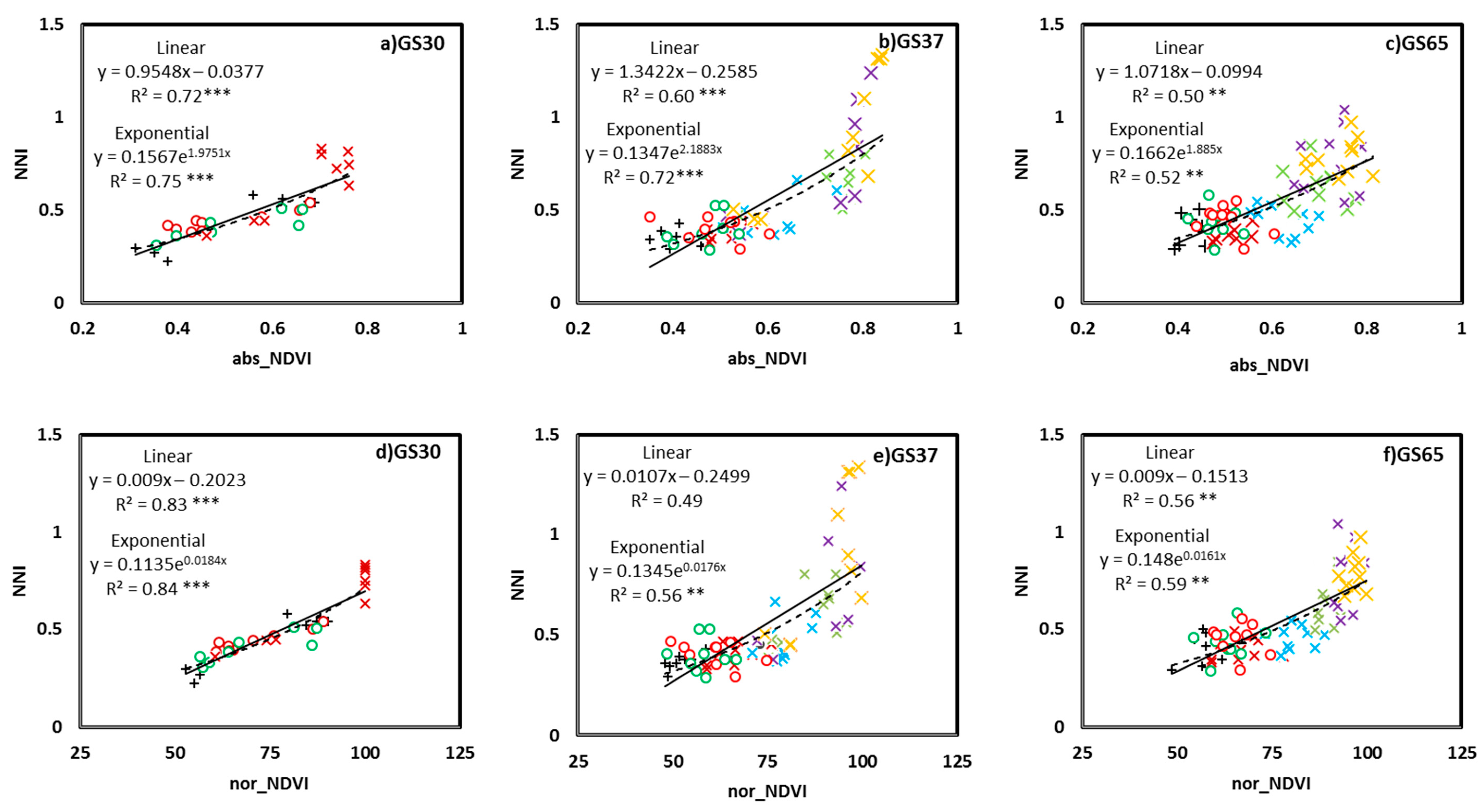
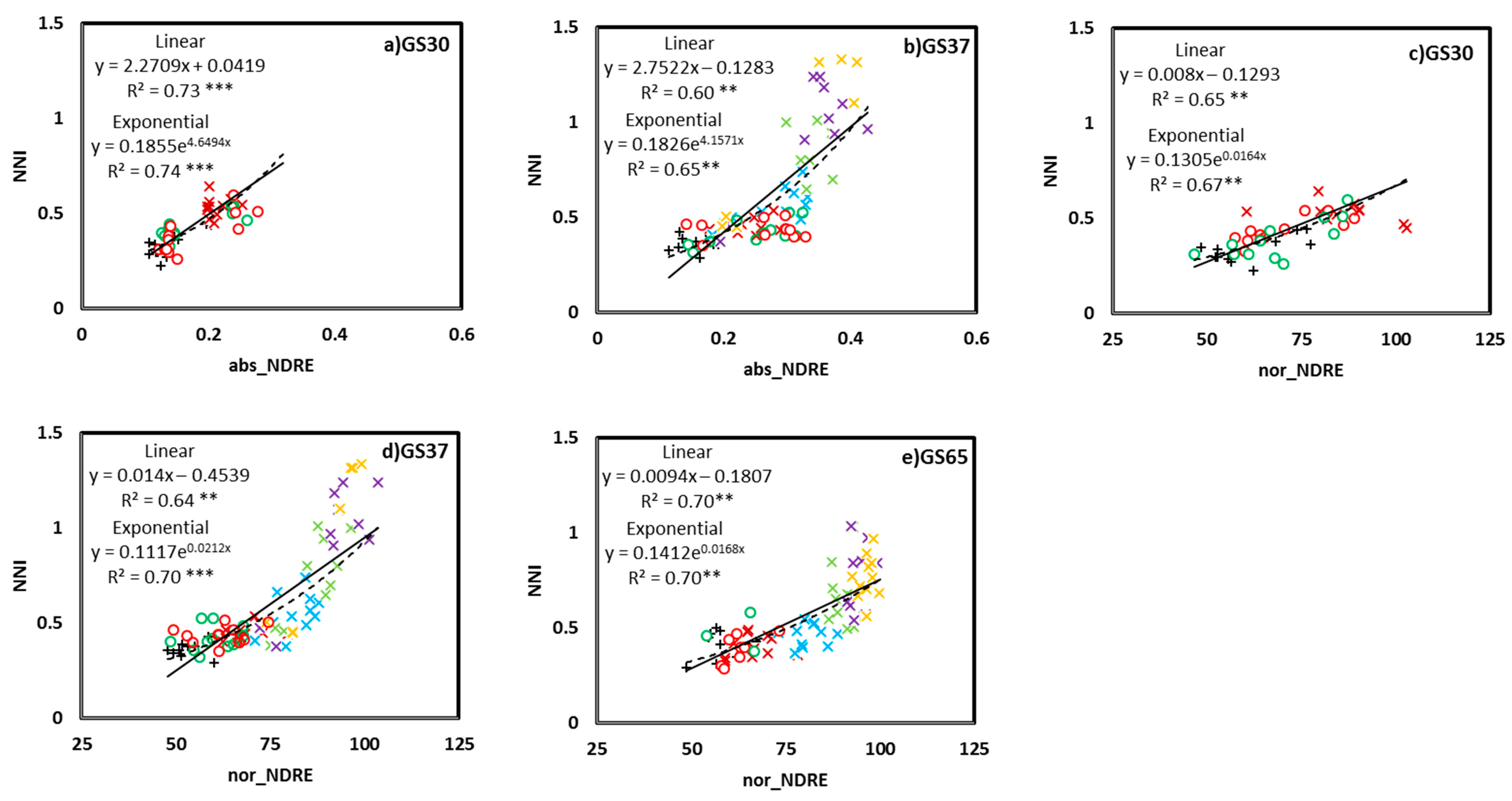
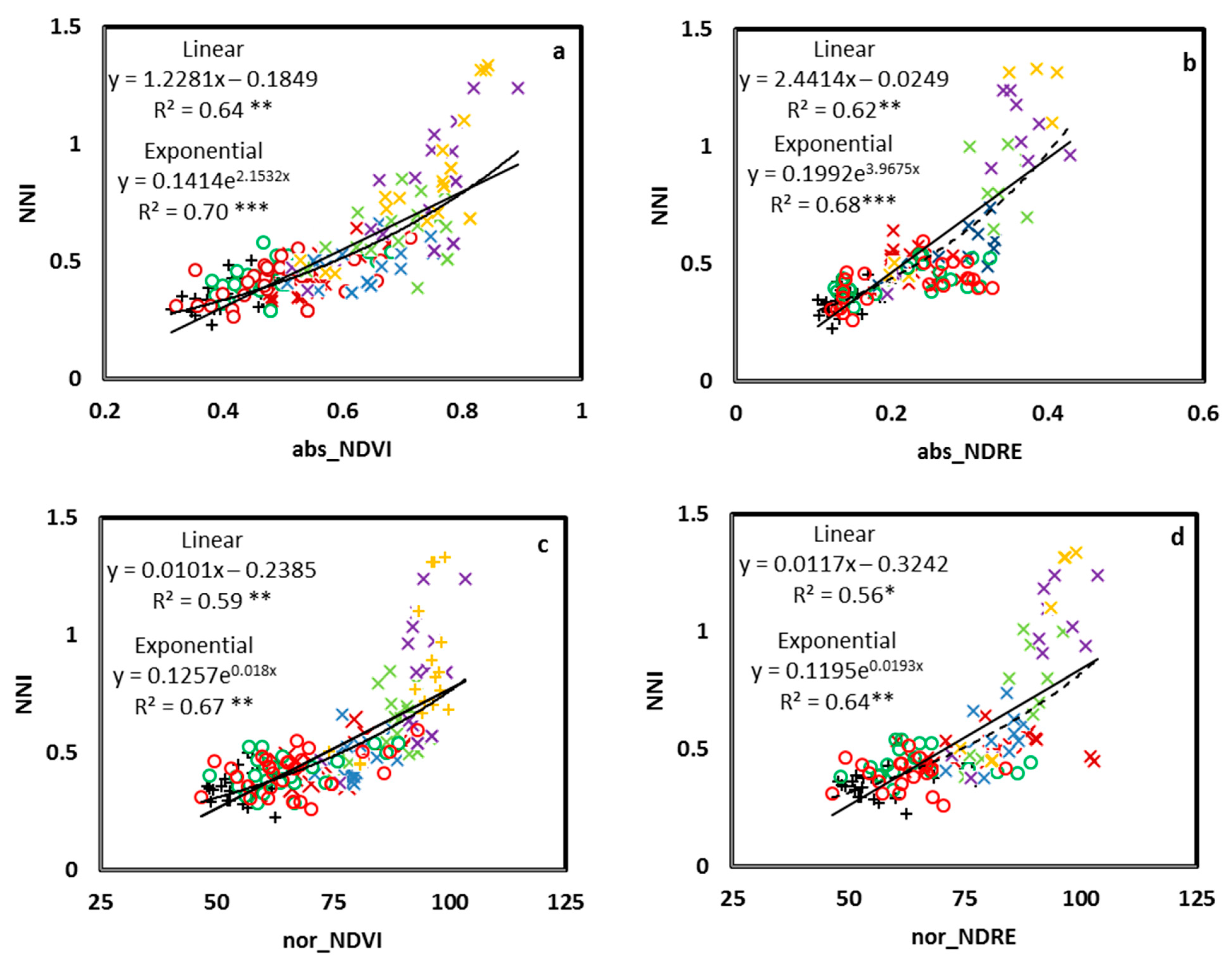
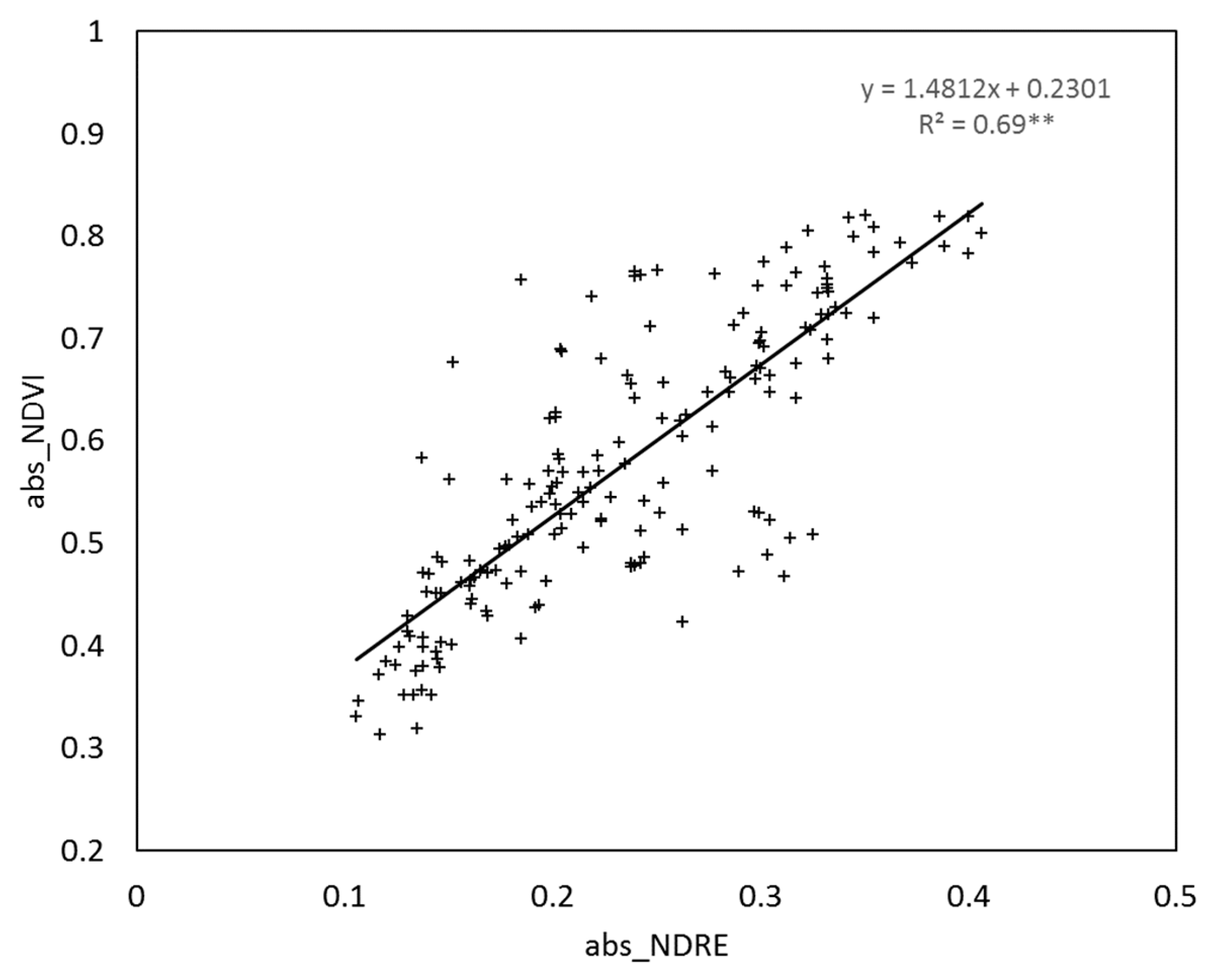
3.1. Relationship between the NNI and Crop Sensor Readings
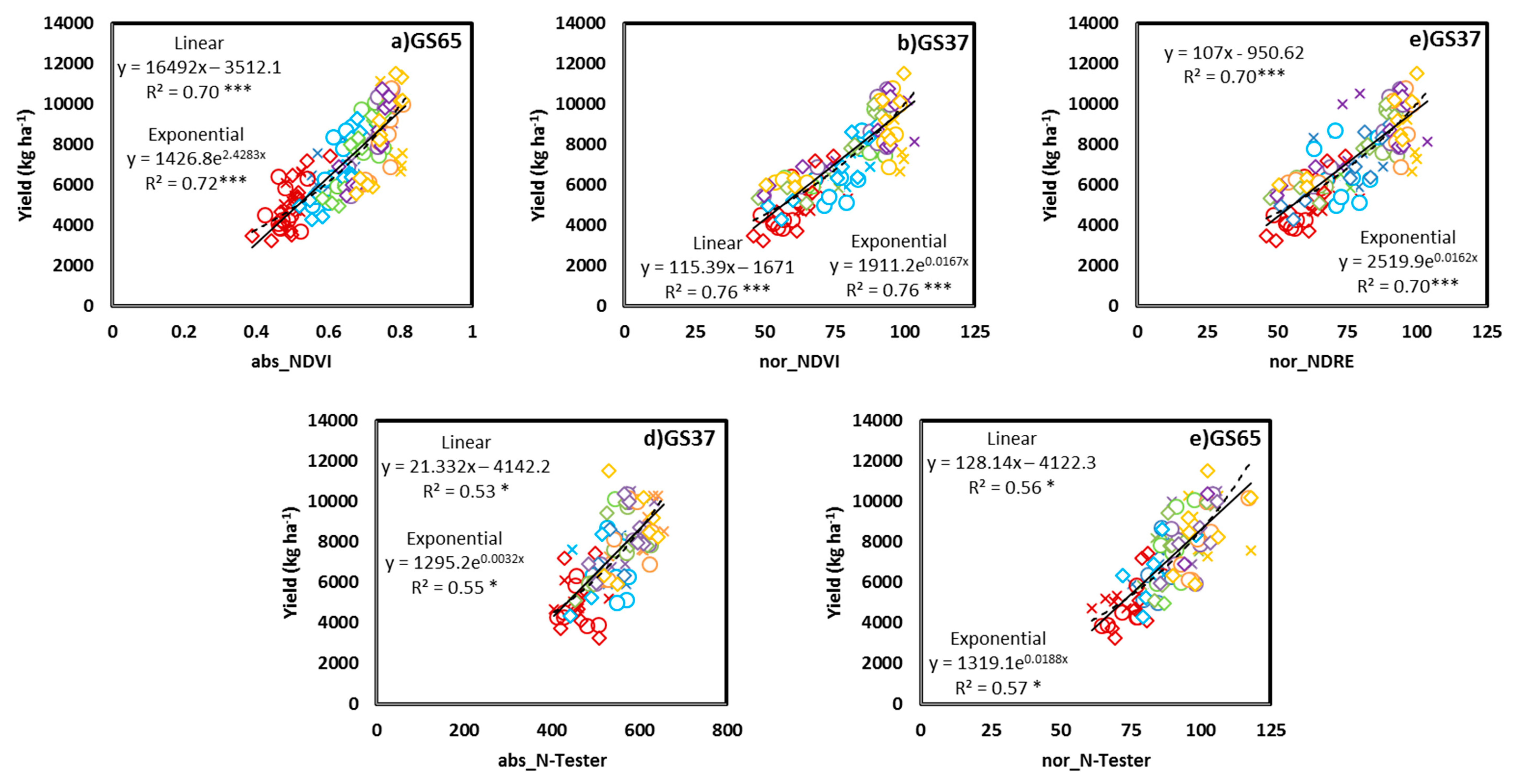
3.2. Relationship between the Yield and GPC and Crop Sensor Readings
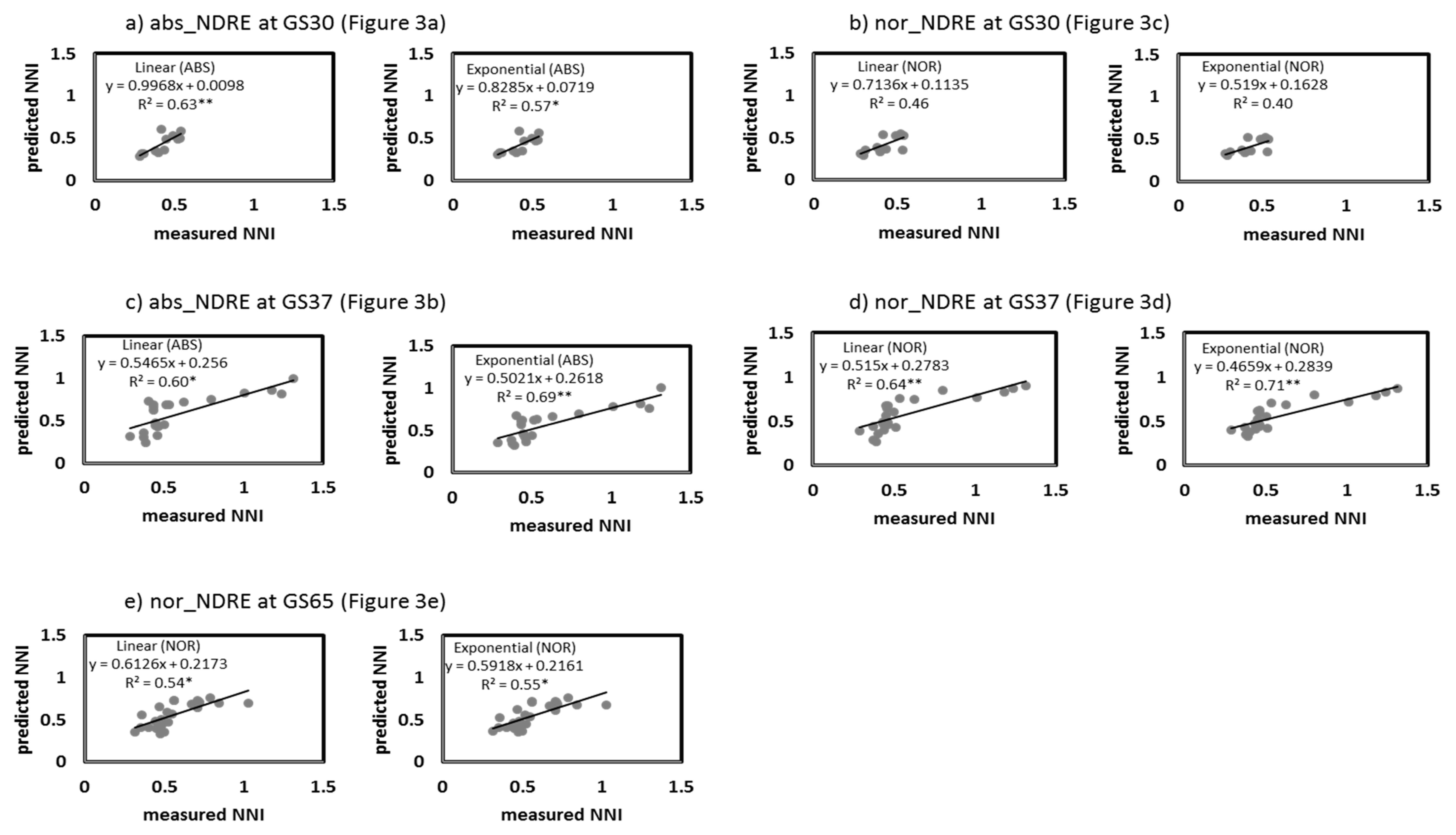
3.3. NNI Estimation Perfomances from Proximal Sensing Tools
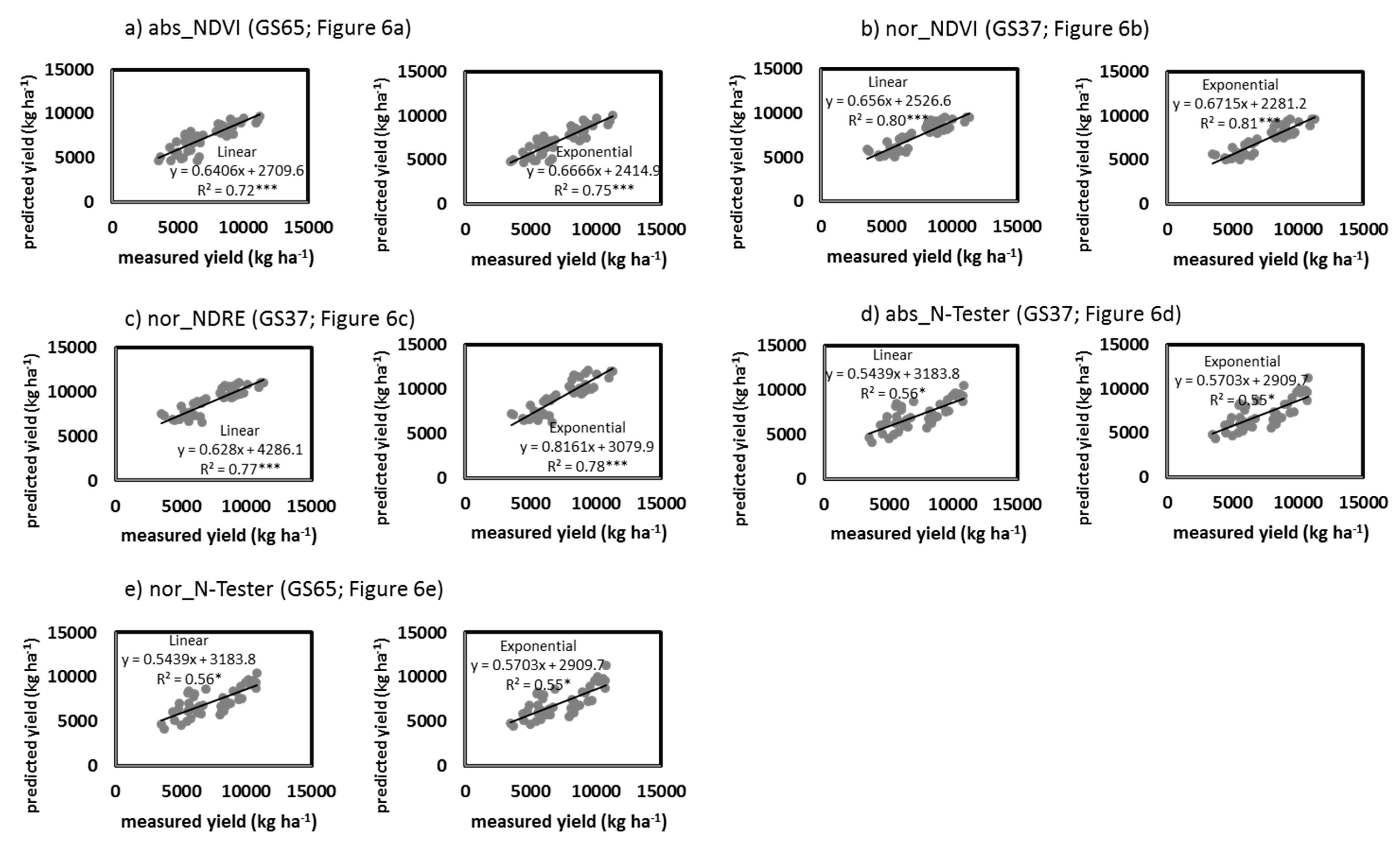
3.4. Grain Yield and GPC Estimation Perfomance from Proximal Sensing Tools
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ravier, C.; Meynard, J.M.; Cohan, J.P.; Gate, P.; Jeuffroy, M.H. Early nitrogen deficiencies favor high yield, grain protein content and N use efficiency in wheat. Eur. J. Agron. 2017, 89, 16–24. [Google Scholar] [CrossRef]
- Raun, W.R.; Johnson, G.V.; Stone, M.L.; Solie, J.B.; Lukina, E.V.; Thomason, W.E.; . Schepers, J.S. In-season prediction of potential grain yield in winter wheat using canopy reflectance. Agron. J. 2001, 93, 131–138. [Google Scholar] [CrossRef]
- Hansen, P.M.; Jørgensen, J.R.; Thomsen, A. Predicting grain yield and protein content in winter wheat and spring barley using repeated canopy reflectance measurements and partial least squares regression. J. Agric. Sci. 2003, 139, 307–318. [Google Scholar] [CrossRef]
- Zhao, B.; Duan, A.; Ata-Ul-Karim, S.T.; Liu, Z.; Chen, Z.; Gong, Z.; Zhang, J.; Xiao, J.; Liu, Z.; Anzhen Qin, A.; et al. Exploring new spectral bands and vegetation indices for estimating nitrogen nutrition index of summer maize. Eur. J. Agron. 2018, 93, 113–125. [Google Scholar] [CrossRef]
- Lemaire, G.; Gastal, F. Quantifying Crop Responses to Nitrogen Deficiency and Avenues to Improve Nitrogen Use Efficiency. In Crop. Physiology: Applications for Genetic Improvement and Agronomy; Sadras, V.O., Calderini, D.F., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 171–211. [Google Scholar]
- Ravier, C.; Jeuffroy, M.H.; Gate, P.; Cohan, J.P.; Meynard, J.M. Combining user involvement with innovative design to develop a radical new method for managing N fertilization. Nutr. Cycl. Agroecosyst. 2018, 110, 117. [Google Scholar] [CrossRef]
- Denuit, J.P.; Olivier, M.; Goffaux, M.J.; Herman, J.L.; Goffart, J.P.; Destain, J.P.; Frankinet, M. Management of nitrogen fertilization of winter wheat and potato crops using the chlorophyll meter for crop nitrogen status assessment. Agronomie 2002, 22, 847–854. [Google Scholar] [CrossRef]
- López-Bellido, R.J.; Shepherd, C.E.; Barraclough, P.B. Pedicting post-anthesis N requeriments of bread wheat with Minolta SPAD meter. Eur. J. Agron. 2004, 20, 313–320. [Google Scholar] [CrossRef]
- Arregui, L.M.; Lasa, B.; Lafarga, A.; Irañeta, I.; Baroja, E.; Quemada, M. Evaluation of chlorophyll meters as tools for N fertilization in winter wheat under humid Mediterranean conditions. Eur. J. Agron. 2006, 24, 140–148. [Google Scholar] [CrossRef]
- Aranguren, M.; Castellon, A.; Aizpurua, A. Topdressing nitrogen recommendation in wheat after applying organic manures: The use of field diagnostic tools. Nutr. Cycl. Agroecosys. 2018, 110, 89–103. [Google Scholar] [CrossRef]
- Aranguren, M.; Castellón, A.; Aizpurua, A. Crop Sensor-Based In-Season Nitrogen Management of Wheat with Manure Application. Remote Sens. 2019, 11, 1094. [Google Scholar] [CrossRef]
- Ortuzar-Iragorri, M.A.; Aizpurua, A.; Castellón, A.; Alonso, A.; Estavillo, J.M.; Besga, G. Use of an N-tester chlorophyll meter to tune a late third nitrogen application to wheat under humid Mediterranean conditions. J. Plant. Nutr. 2017, 41, 627–635. [Google Scholar] [CrossRef]
- Debaeke, P.; Rouet, P.; Justes, E. Relationship between normalized SPAD index and the nitrogen nutrition index: Application to durum wheat. J. Plant. Nutr. 2006, 29, 275–286. [Google Scholar] [CrossRef]
- Ravier, C.; Quemada, M.; Jeuffroy, M.H. Use of a chlorophyll meter to assess nitrogen nutrition index during the growth cycle in winter wheat. Fields Crops Res. 2017, 214, 73–82. [Google Scholar] [CrossRef]
- Ortuzar-Iragorri, M.A.; Alonso, A.; Castellón, A.; Besga, G.; Estavillo, J.M.; Aizpurua, A. N-tester use in soft winter wheat: Evaluation of nitrogen status and grain yield prediction. Agron. J. 2005, 97, 1380–1389. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens. of Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Padilla, F.M.; de Souza, R.; Peña-Fleitas, M.T.; Grasso, R.; Gallardo, M.; Thompson, R.B. Influence of time of day on measurement with chlorophyll meters and canopy reflectance sensors of different crop N status. Precision Agric. 2019, 20, 1087–1106. [Google Scholar] [CrossRef]
- Mistele, B.; Schmidhalter, U. Estimating the nitrogen nutrition index using spectral canopy reflectance measurements. Eur. J. Agron. 2008, 29, 184–190. [Google Scholar] [CrossRef]
- Marti, J.; Bort, J.; Slafer, G.A.; Araus, J.L. Can wheat yield be assessed by early measurements of Normalized Difference Vegetation Index? Ann. Appl. Biol. 2007, 150, 253–257. [Google Scholar] [CrossRef]
- Lu, J.; Miao, Y.; Shi, W.; Li, J.; Yuan, F. Evaluating different approaches to non-destructive nitrogen status diagnosis of rice using portable RapidSCAN active canopy sensor. Sci. Rep. 2017, 7, 14073. [Google Scholar] [CrossRef]
- Bonfil, D.J. Monitoring Wheat Fields by RapidScan: Accuracy and Limitations. In Proceedings of the Conference on Precision Agriculture 2017, John McIntyre Centre, Edinburgh, UK, 16–20 July 2017. [Google Scholar]
- Cao, Q.; Miao, Y.; Wang, H.; Huang, S.; Cheng, S.; Khosla, R.; Jiang, R. Non-destructive estimation of rice plant nitrogen status with CropCircle multispectral active canopy sensor. Fields Crops Res 2013, 154, 133–144. [Google Scholar] [CrossRef]
- Magney, T.S.; Vierling, L.A.; Eitel, J.U.H.; Huggins, D.R.; Garrity, S.R. Response of high frequency Photochemical Reflectance Index (PRI) measurements to environmental conditions in wheat. Remote Sens. Environ. 2016, 173, 84–97. [Google Scholar] [CrossRef]
- Zhang, K.; Ge, X.; Shen, P.; Li, W.; Liu, X.; Cao, Q.; Zhu, Y.; Cao, W.; Tian, Y. Predicting rice grain yield based on dynamic changes in vegetation indexes during early to mid-growth stages. Remote Sens. 2019, 11, 387. [Google Scholar] [CrossRef]
- Bijay-Singh, S.R.K.; Jaspreet-Kaur, J.M.L.; Martin, K.L.; Yadvinder-Singh, V.S.; Chandna, P.; Choudhary, O.P.; Gupta, R.K.; Thind, H.S.; Jagmohan-Singh, U.H.S.; Khurana, H.S.; et al. Assessment of the nitrogen management strategy using an optical sensor for irrigated wheat. Agron. Sustain. Dev. 2011, 31, 589–603. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for growth stages of cereals. Weed Res. 1974, 4, 415–421. [Google Scholar] [CrossRef]
- Climates of the World and Their Agricultural Potentialities; J. Papadakis: Buenos Aires, Argentina, 1966.
- Gee, G.W.; Bauder, J.W. Particle-Size Analysis. In Methods of Soil Analysis: Part 1. Physical and Mineralogical Methods; Klute, A., Ed.; SSSA: Madison, AL, USA, 1986; pp. 383–411. [Google Scholar]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; Natural Resources Conservation Service; U.S. Department of Agriculture Handbook 436: Washington, DC, USA, 1999.
- MAPA. Métodos Oficiales de Análisis. Tomo III; Ministerio de Agricultura; Pesca y Alimentación: Madrid, Spain, 1994.
- Walkey, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic and titration method. Soil Sci. 1934, 37, 29–37. [Google Scholar] [CrossRef]
- Soil Survey Staff. Illustrated Guide to Soil Taxonomy; U.S. Department of Agriculture, Natural Resources Conservation Service, National Soil Survey Center: Lincoln, Nebraska, 2015.
- AOAC, Association of Official Analytical Chemists International. Plants, 24, 127. In Official Methods of AOAC International, 16th ed.; Patricia, C., Ed.; AOAC International: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Teller, G.L. Non-protein nitrogen compounds in cereals and their relation to the nitrogen factor for protein in cereals and bread. Cereal Chem. 1932, 9, 261–274. [Google Scholar]
- Justes, E.; Mary, B.; Meynard, J.M.; Machet, J.M.; Thelier-Huché, L. Determination of a critical nitrogen dilution curve for winter wheat crops. Ann. Bot. 1994, 74, 397–407. [Google Scholar] [CrossRef]
- Wang, G.; Bronson, K.F.; Thorp, K.R.; Mon, J.; Badaruddin, M. Multiple leaf measurements improve effectiveness of chlorophyll meter for durum wheat nitrogen management. Crop. Sci. 2014, 54, 817. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Proceedings of the Second International Symposium on Information Theory, Tsahkadsor, Armenia, September 2–8, 1971; Petrov, B.N., Csaki, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations monitoring vegetation. J. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Feng, G.; Gao, X.; Li, F.; Liu, B.; Yue, S.; Cheng, S.; Ustin, L.U.; Khosla, R. Active canopy sensing of winter wheat nitrogen status: An evaluation of two sensor systems. Comput. Electron. Agric. 2015, 112, 54–67. [Google Scholar] [CrossRef]
- Xue, L.; Li, G.; Qin, X.; Yang, L.; Zhang, L. Topdressing nitrogen recommendation for early rice with an active sensor in south China. Precision Agric. 2014, 15, 95–110. [Google Scholar] [CrossRef]
- Craigie, R.; Yule, I.; McVeagh, P. Crop sensing for nitrogen management; Foundation for Arable Research: Christchurch, New Zealand, 2013. [Google Scholar]
- Sembiring, H.; Lees, H.L.; Raun, W.R.; Johnson, G.V.; Solie, J.B.; Stone, M.L.; DeLeon, M.J.; Lukina, E.V.; Cossey, D.A.; LaRuffa, J.M.; et al. Effect of growth stage and variety on spectral radiance in winter wheat. J. Plant. Nutr. 2000, 23, 141–149. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Gao, X.; Liu, B.; Feng, G.; Yue, S. Estimating the Nitrogen Nutrition Index of Winter Wheat Using an Active Canopy Sensor in the NorthChina Plain. In Proceedings of the 1st International Conference Agro-Geoinformatics, Agro-Geoinformatics 2012, Shanghai, China, 2–4 August 2012; IEEE Computer Society: Shanghai, China, 2012; pp. 178–182. [Google Scholar]
- Cao, Q.; Miao, Y.; Shen, J.; Yuan, F.; Cheng, S.; Cui, Z. Evaluating Two Crop Circle Active Canopy Sensors for In-Season Diagnosis of Winter Wheat Nitrogen Status. Agronomy 2018, 8, 201. [Google Scholar] [CrossRef]
- Erdle, K.; Mistele, B.; Schmidhalter, U. Comparison of active and passive spectral sensors in discriminating biomass parameters and nitrogen status in wheat cultivars. Field Crops Res. 2011, 124, 74–84. [Google Scholar] [CrossRef]
- Gnyp, M.L.; Miao, Y.; Yuan, F.; Ustin, S.L.; Yu, K.; Yao, Y.; Huang, S.; Bareth, G. Hyperspectral canopy sensing of paddy rice aboveground biomass at different growth stages. Field Crops Res. 2014, 155, 42–55. [Google Scholar] [CrossRef]
- Moriondo, M.; Maselli, F.; Bindi, M. A simple model of regional wheat yield based on NDVI data. Eur. J. Agron. 2007, 26, 266–274. [Google Scholar] [CrossRef]
- Sharma, L.K.; Bu, H.; Denton, A.; Franzen, W.F. Active-Optical Sensors Using Red NDVI Compared to Red Edge NDVI for Prediction of Corn Grain Yield in North Dakota, U.S.A. Sensors 2015, 15, 27832–27853. [Google Scholar] [CrossRef]
- Royo, C.; Aparicio, N.; Villegas, D.; Casadesus, J.; Monneveux, P.; Araus, J.L. Usefulness of spectral reflectance indices as durum wheat yield predictors under contrasting Mediterranean conditions. Int. J. Remote Sens. 2003, 24, 4403–4419. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Dupont, F.M.; Altenbach, S.B. Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis. J. Cereal Sci. 2003, 38, 133–146. [Google Scholar] [CrossRef]
- Montemurro, M.; Convertini, G.; Ferri, D. Nitrogen Application in Winter Wheat Grown in Mediterranean Conditions: Effects on Nitrogen Uptake, Utilization Efficiency, and Soil Nitrogen Deficit. J. Plant. Nutr. 2007, 30, 1681–1703. [Google Scholar] [CrossRef]
- Colaço, A.F.; Bramley, R.G.V. Do crop sensors promote improved nitrogen management in grain crops? Field Crops Res. 2018, 218, 126–140. [Google Scholar]


| Initial Fertilization | 2015-2016-2017 | Treatment Identification | |
|---|---|---|---|
| Topdressing at GS21 (kg N ha−1) | Topdressing at GS30 (kg N ha−1) | ||
| Conventional (--) | 40 | 0 | 40N + 0N |
| 40 | 40N + 40N | ||
| 80 | 40N + 80N | ||
| 120 | 40N + 120N | ||
| 160 | 40N + 160N | ||
| Dairy Slurry (DS) (40 t ha−1) | -- | 0 | DS + 0N |
| 40 | DS + 40N | ||
| 80 | DS + 80N | ||
| 120 | DS + 120N | ||
| 160 | DS + 160N | ||
| Sheep manure (SM) (40 t ha−1) | -- | 0 | SM + 0N |
| 40 | SM + 40N | ||
| 80 | SM + 80N | ||
| 120 | SM + 120N | ||
| 160 | SM + 160N | ||
| Control (--) | -- | -- | 0N |
| Overfertilized (--) | 80 | 200 | 280N |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranguren, M.; Castellón, A.; Aizpurua, A. Crop Sensor Based Non-destructive Estimation of Nitrogen Nutritional Status, Yield, and Grain Protein Content in Wheat. Agriculture 2020, 10, 148. https://doi.org/10.3390/agriculture10050148
Aranguren M, Castellón A, Aizpurua A. Crop Sensor Based Non-destructive Estimation of Nitrogen Nutritional Status, Yield, and Grain Protein Content in Wheat. Agriculture. 2020; 10(5):148. https://doi.org/10.3390/agriculture10050148
Chicago/Turabian StyleAranguren, Marta, Ander Castellón, and Ana Aizpurua. 2020. "Crop Sensor Based Non-destructive Estimation of Nitrogen Nutritional Status, Yield, and Grain Protein Content in Wheat" Agriculture 10, no. 5: 148. https://doi.org/10.3390/agriculture10050148
APA StyleAranguren, M., Castellón, A., & Aizpurua, A. (2020). Crop Sensor Based Non-destructive Estimation of Nitrogen Nutritional Status, Yield, and Grain Protein Content in Wheat. Agriculture, 10(5), 148. https://doi.org/10.3390/agriculture10050148





