1. Introduction
Sugar beet (
Beta vulgaris var.
saccharifera L.) is an economically viable crop produced mainly for white sugar. The world’s leading sugar beet producers (France, Germany and Poland) account for almost 50% of total world production (111.7 million tons in 2016). However, only 20% of the world’s sugar comes from sugar beet; 80% is produced from sugar cane [
1]. Given the production technology and the length of the growing season of almost 180 days, sugar beet is considered the most intensive agricultural crop [
2].
The economically important pests of South East Europe sugar beet include wireworms, pigmy mangold beetle, sugar beet and corn weevil, black beet weevil, alfalfa snout beetle, several species of noctuid moths, sugar beet flea beetle, aphids, and beet cyst nematode [
3,
4,
5,
6,
7,
8,
9,
10,
11]. Their appearance depends on the region and the year.
Since the introduction of neonicotinoid seed treatment in the 1990s, there has been a strong decrease in insecticide use in Croatia [
12]. Wireworms, aphids, and flea beetles were successfully controlled by neonicotinoid seed treatments [
7,
13,
14,
15] so additional treatment was only required in the case of severe infestation of some foliar pests that cannot be successfully controlled with neonicotinoids (e.g., sugar beet weevil) [
16]. In north-western Europe, only aphids require occasional control with foliar insecticides [
17].
Seed treatment is a method that has brought many advantages to modern agriculture [
18,
19,
20,
21,
22,
23,
24], although there are some negative effects as well. In heavy infestations the efficacy against wireworms and sugar beet weevil is weak, so additional protection measures are necessary [
7]. It is often applied at higher rates [
24] or when control is not even necessary.
The use of neonicotinoids has become a major controversy because of their negative effects on bees, other pollinators, and possibly other non-target organisms [
25,
26,
27]. According to the available evidence and a risk assessment carried out by EFSA, the use of neonicotinoid pesticides (clothianidin, imidacloprid, and thiamethoxam) was severely restricted by European Commission (EC) in 2013 by the implementation of Directive 485/2013 [
28]. The restriction applied to bee-friendly crops such as maize, oilseed rape, and sunflower, with the exception of greenhouse crops and the post-flowering treatment of certain crops, and to winter cereals. Based on the EFSA peer review of the pesticide risk assessment carried out for clothianidin [
25], imidacloprid [
26], and thiamethoxam [
27], the Commission adopted on 30 May 2018, regulations banning completely the outdoor use of imidacloprid, clothianidin, and thiamethoxam to protect domestic honey bees and wild pollinators [
29]. The only risk identified by EFSA for the treatment of sugar beet seeds with neonicotinoids was the risk of succeeding crop scenario [
25,
26,
27].
In the succeeding crop scenario, the residues of neonicotinoids are expected to remain in the soil and be absorbed by the succeeding crop or weeds in the same field. Thus, if the significant concentrations of neonicotinoids were to remain in the soil after the growing season, they could be adsorbed by the succeeding crop (or weeds) from the soil and then the neonicotinoids could be found in pollen or excreted in guttation fluid.
The Commission has not considered the possibility of proposing further options in addition to the total ban on the treatment of sugar beet seed with neonicotinoids. This decision could endanger sugar beet production. The ban was justified by the fact that some ecologically more acceptable substitute chemicals (diamides) are effective in controlling the most serious pests and that tools to control most pests are available under integrated pest management (IPM). However, the arguments do not fully apply to all economically important pests that damage sugar beet production in all production areas in the EU.
Hauer et al. [
17] discussed neonicotinoid seed treatments in European sugar beet cultivation with regard to their effectiveness against target pests and their impact on the environment. They proposed to develop monitoring systems and models to identify regions (and years) with a higher risk of occurrence of pests and to allow the use of insecticide seed treatments only when high pest pressure is likely. In their analysis, Hauer et al. [
17] only looked at sugar beet production in northwestern European countries and did not consider the different climatic conditions and the occurrence of pests in eastern and southeastern Europe, where problems in production are mainly caused by flea beetles and sugar beet weevils. This fact makes their proposal even more important.
The aim of this research was to determine the residue levels of imidacloprid and thiamethoxam used as a seed treatment in sugar beet plants in different agroclimatic regions in order to estimate environmental risk and possible transfer to other crops. Greenhouse trials have been established in order to provide insight to neonicotinoid behavior in controlled conditions.
4. Discussion
When the neonicotinoids were introduced to the market, they were considered safe to use because they are stable in soil and have low toxicity to mammals [
38]. However, recent studies have shown that neonicotinoids have adverse effects on bees, other pollinators, and possibly other non-target organisms [
25,
26,
27]. A complete EU Commission Regulation ban on the outdoor use of imidacloprid, thiamethoxam, and clothianidin could have a significant impact on the practice of sugar beet production in Europe, as 100% of all commercial sugar beet seeds have been treated with neonicotinoids. According to Ester et al. and Lanka et al. [
39,
40] spinosad and chlorantraniliprole applied as seed treatment were ineffective at controlling flea beetles and cabbage aphid [
39] as well as adult stages of rice water weevil [
40]. It is unlikely that they will become a good substitute of neonicotinoid seed treatment. Hauer et al. [
17] have pointed out the lack of effective alternatives for the control of
M. persicae on sugar beet in Central and North Europe. Moreover, Bažok et al. [
16] achieved the same conclusions for substituting control of sugar beet flea beetle in South and Eastern Europe. Therefore, the problems related to the control of the above mentioned pests could become a serious problem in the future if no alternatives are developed.
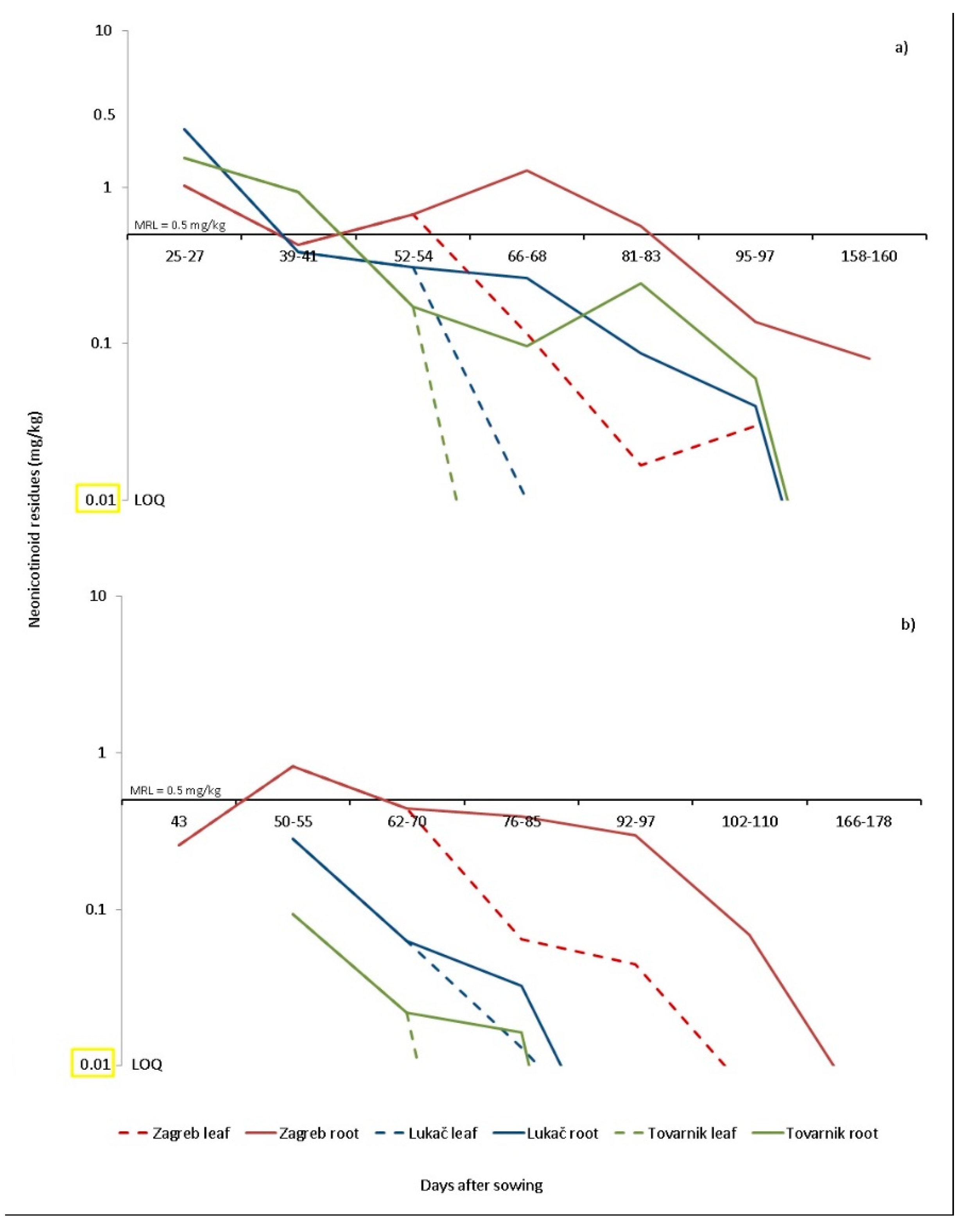
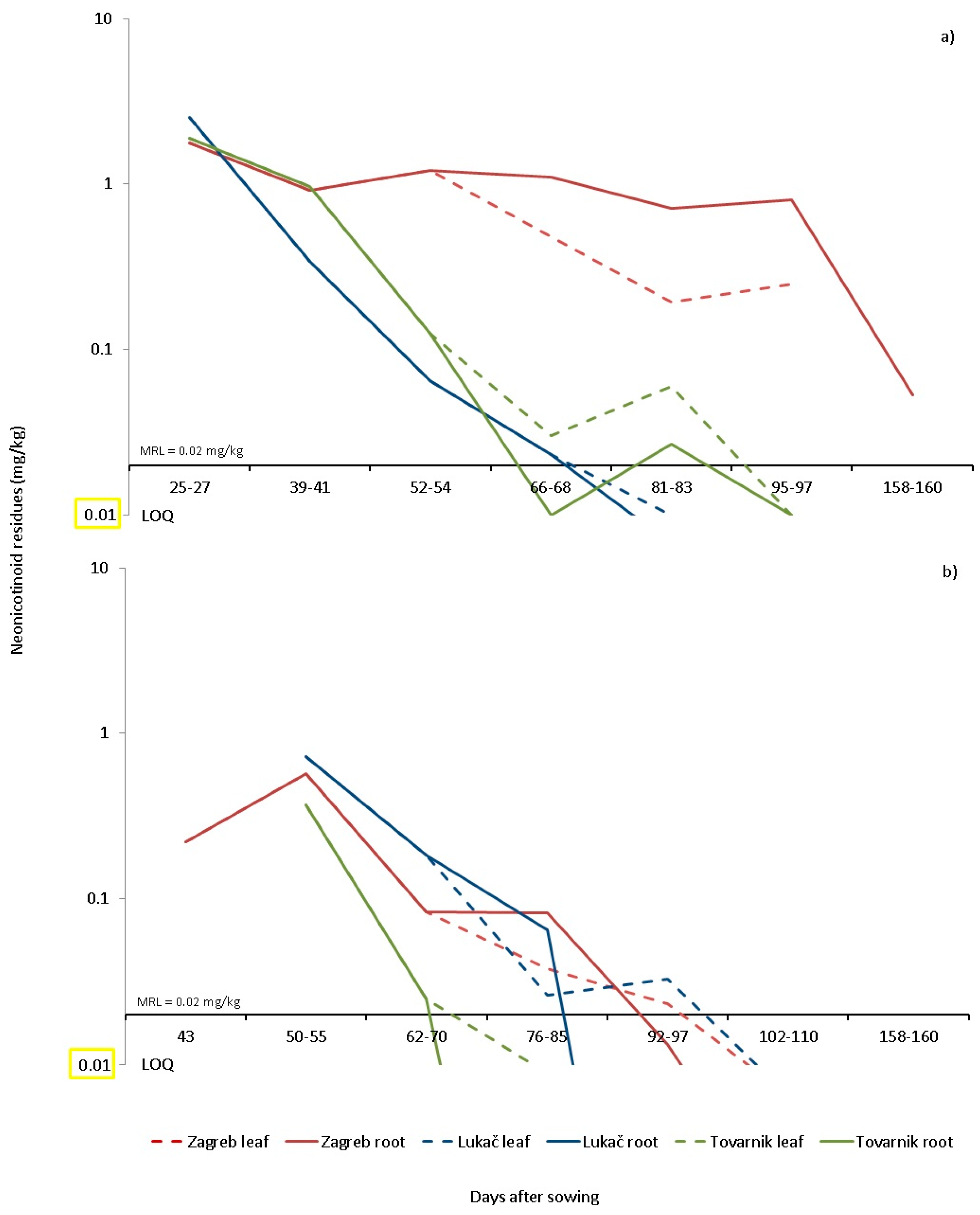
In our study, at the end of sugar beet cultivation (180 days after planting), imidacloprid residues at a concentration of 0.17 mg/kg and thiamethoxam residues at a concentration of 0.04 mg/kg were found in the soil of Tovarnik, while in Lukač all residues were below LOQ levels (
Table 4). Such a result is partially consistent with that of [
41] who randomly sampled 74 soils after the cultivation of maize, wheat, and barley grown from treated seeds. Imidacloprid was found in all samples, so the authors concluded that imidacloprid is always present in the soils after cultivation and is easily detectable if sampling is carried out in the year of treatment.
Alford and Krupke [
42] concluded that high water solubility of neonicotinoid seed treatment applications makes it unlikely that they will remain near the relatively confined rhizosphere of the target plant long enough to be absorbed by the plant when not on the seed. The loss of neonicotinoids from agricultural soils is thought to occur through degradation or leaching in soil water [
43]. EFSA’s risk assessment [
25,
26,
27] did not take into account the results of [
42] on the low probability of residues of neonicotinoids remaining in soil for a longer period of time. Their findings, together with those of [
44] on the recycling of neonicotinoid insecticides from contaminated groundwater back to crops, point to the possible risk scenario of irrigation, which will be further investigated. In our laboratory study, the sugar beet plants were sown at five times higher density than in the field, which means that the concentration of neonicotinoids is also significantly higher (40.95 mg imidacloprid and 32.76 + 1.62 mg thiamethoxam + teflutrin per container 100 l soil). Soil from greenhouse trials treated with imidacloprid contained the average value of 5.34 mg/kg a.i., while the thiamethoxam-treated variant of the sample form contained 2.65 mg/kg a.i. (
Table 4). This is much higher if we consider that in open field the application rate as seed coating is 112.2 g imidacloprid or 44.4 + 4.44 g thiamethoxam + tefluthrin to one ha, while one ha contains on average three million liters of soil (calculation of the average soil layer of 30 cm). This is the average concentration of 0.04 mg/kg a.i. imidacloprid or 0.015 + 0.0015 mg/kg thiamethoxam + tefluthrin. Our result confirms that high concentrations of neonicotinoids in soil are to be expected in case of dry conditions, leaching incapacity, or irregular flushing (bottom of the container) into ground water meaning that they can present potential risk for the succeeding crops. Concerning field trials, there is no systematic monitoring of the presence of pesticides in water in Croatia and no data on concentrations of neonicotinoids in the area of our study are available.
Studies on the degradation of neonicotinoids in soil depend on temperature, moisture, and soil type, in particular on texture and organic matter content, pH and UV radiation [
41]. According to Bonmatin [
41], persistence is highest under cool, dry conditions and in soils with high organic matter content. On average, Lukač has more precipitation (more humid soil), lower soil and air temperatures, while Tovarnik is drier with low precipitation and slightly higher air and soil temperatures (
Table 2).
Table 3 shows that in our investigations the pH of the soil at both locations was between 5 and 7, which means that the soils are slightly acidic to neutral and do not allow degradation in the moist soil or water. Guzsvány et al. [
45] found that imidacloprid and thiamethoxam degrade faster at 23 °C in alkaline media, while they remain relatively stable at pH 7 and 4. Regarding residues of neonicotinoids in soil after the vegetation period,
Table 3 shows that all residues were lower than LOQ in Lukač while in Tovarnik 0.17 mg/kg imidacloprid and 0.04 mg/kg thiamethoxam were detected. Such results can be explained by the dry conditions, low precipitation, and slightly higher air and soil temperatures prevailing in Tovarnik. The soils of Tovarnik also contain a large amount of soil organic matter as well as available phosphorus and potassium (
Table 3), which prevents the leaching of residues and allows higher sorption in soils with high organic matter content, which is also in line with the results of [
46]. Even though the results of the residues in soil are not statistically assessed, we may conclude that the faster reduction of residues in Lukač is most likely due to higher precipitation which is confirmed with the analyses of the residues in plants. The presence of a significant “treatment × location” (i.e., agroclimatic conditions) interaction for thiamethoxam in 2015 (when locations differ in temperature and precipitation) and the absence of a significant interaction for the same factors in 2016 (when locations differ only in temperature) implies that precipitation is an important factor in thiamethoxam leaching. The same logic could not be followed for the degradation of imidacloprid because there was a significant “treatment × location” (i.e., agroclimatic conditions) interaction for imidacloprid residues only in three out of seven samples in 2015 and in one out of six samples in 2016.
According to Bonmatin et al. [
41], the half-life of imidacloprid for seed treatment in France was about 270 days, while [
47] reported 83 to 124 days under field conditions and 174 days on bare soil. Under field conditions, thiamethoxam showed a moderate to fast degradation rate [
48]. The calculated half-life in soil was between 7 and 335 days for thiamethoxam [
49].
Uptake by the roots ranged from 1.6 to 20% for imidacloprid in aubergines and maize [
50]. Krupke et al. [
50] pointed out that the uptake of clothianidin by maize plants was relatively low and that plant-bound clothianidin concentrations followed an exponential decay pattern with initially high values, followed by a rapid decrease within the first ~20 days after planting. A maximum of 1.34% of the initial seed treatment rate (calculated as mg a.i./kg of seed) was successfully obtained from plant tissues (calculated as mg a.i./kg of plant tissue) and a maximum of 0.26% from root samples. Our study showed that 25 days to 27 days after planting in 2015, a maximum of 0.028% imidacloprid and 0.077% thiamethoxam was obtained from the raised plants (
Figure 1 and
Figure 2). In 2016, the recovery rate from the raised plants 40 days after planting was 0.003% for imidacloprid and 50 days after planting up to 0.022% for thiamethoxam. These data confirm that the degradation scenario of imidacloprid and thiamethoxam in sugar beet crops is similar to the scenario established for clothianidin by [
50].
Westwood et al. [
51] found that the concentration of imidacloprid in the leaves of sugar beet grown from treated seed was 15.2 mg/kg 21 days after planting and degradation to 0.5 mg/kg 97 days after planting (25-leaf stage). Bažok et al. [
52] found twice as high a concentration of 0.95 mg/kg imidacloprid in sugar beet leaves 42 days after planting using the HPLC method. Compared to HPLC, the LC -MS/MS method has a lower limit of determination (LOQ) and offers the possibility of a clear identification of the analyte [
53]. Therefore, our results show more precise results confirming that there are no residues of neonicotinoids in the roots of sugar beet during harvest time. Nevertheless, the risk is not negligible in dry climates or after a dry period since results showed higher soil concentrations of imidacloprid than expected in Tovarnik. Results have shown [
47] that field trials in Europe and the United States on the degradation of imidacloprid show that it does not accumulate in soil after repeated annual applications. Although sugar beet in Croatia is grown in crop rotation where neonicotinoids are already prohibited (maize, oilseed rape, wheat, etc.,), there should be a limited risk of bioaccumulation and transfer to other crops but the risk for succeeding crops needs to be further assessed.
Neonicotinoid seed treatment of sugar beet is still allowed in many other regions of the world (except the EU). Increase in the wide use of insecticides, in particular pyrethroid insecticides, against aphids and flea beetles (depending on the growing area) is expected in areas where neonicotinoids are banned. The status of neonicotinoids for sugar beet seed treatment will possibly be further investigated by various regulatory authorities around the world.