Pyrenophora teres and Rhynchosporium secalis Establishment in a Mediterranean Malt Barley Field: Assessing Spatial, Temporal and Management Effects
Abstract
1. Introduction
2. Materials and Methods
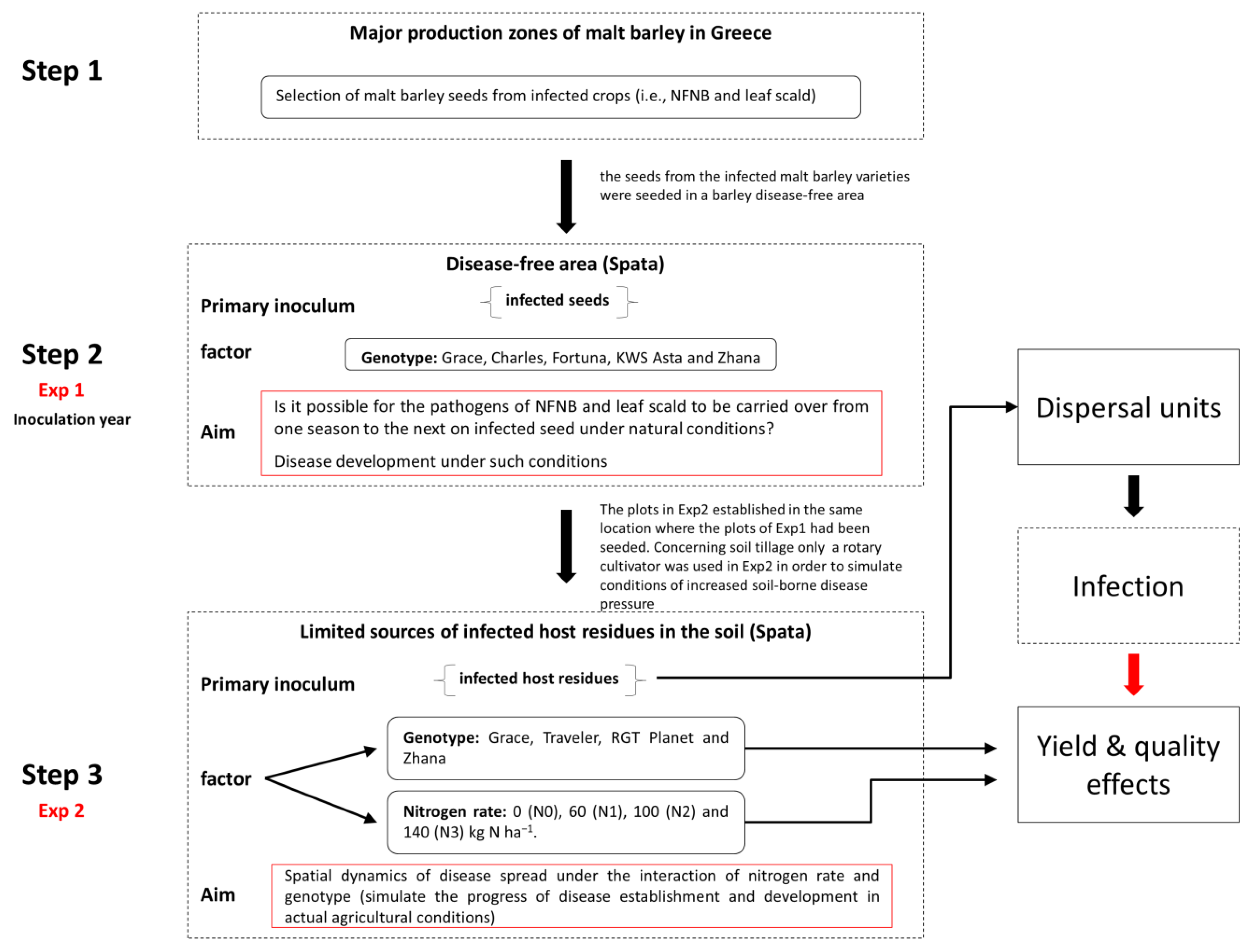
2.1. Study Site and Experimental Design
2.2. Disease Assessment
2.3. Yield and Malt Character Measurements
2.4. Spatial Statistical Analysis
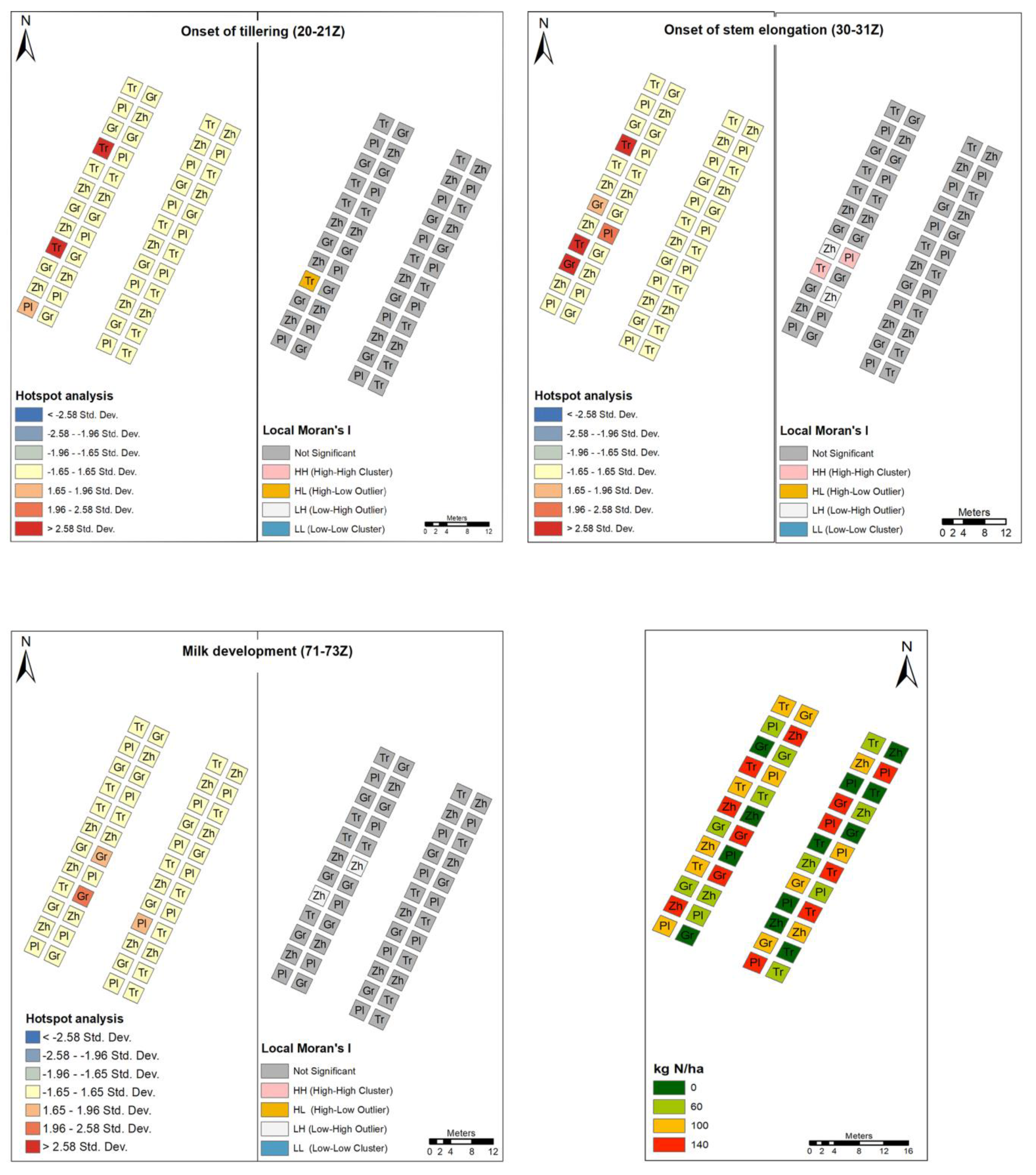
2.4.1. Hotspot Analysis
2.4.2. Cluster and Outlier Analysis
2.5. Statistical Analysis
3. Results
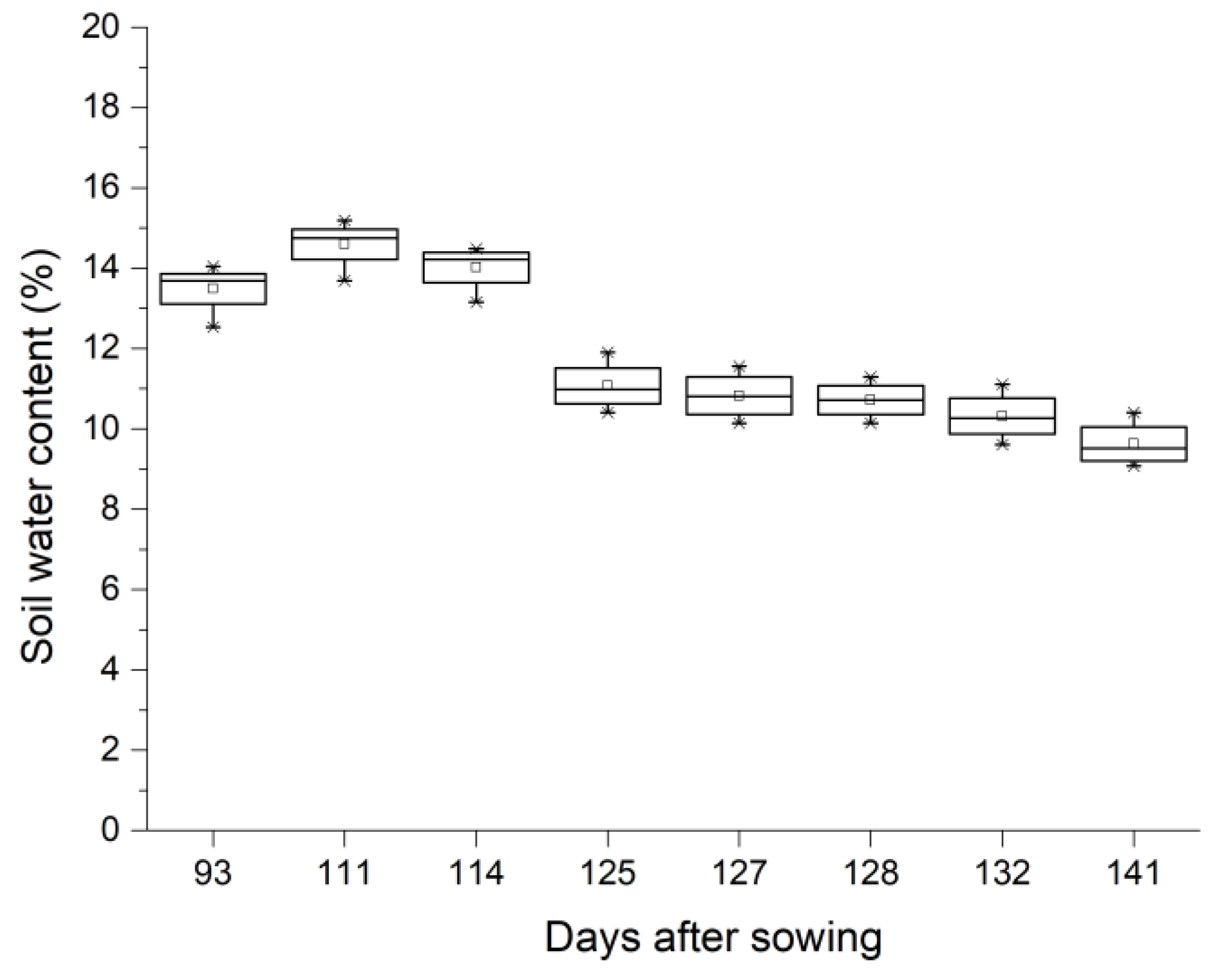
3.1. Weather Conditions
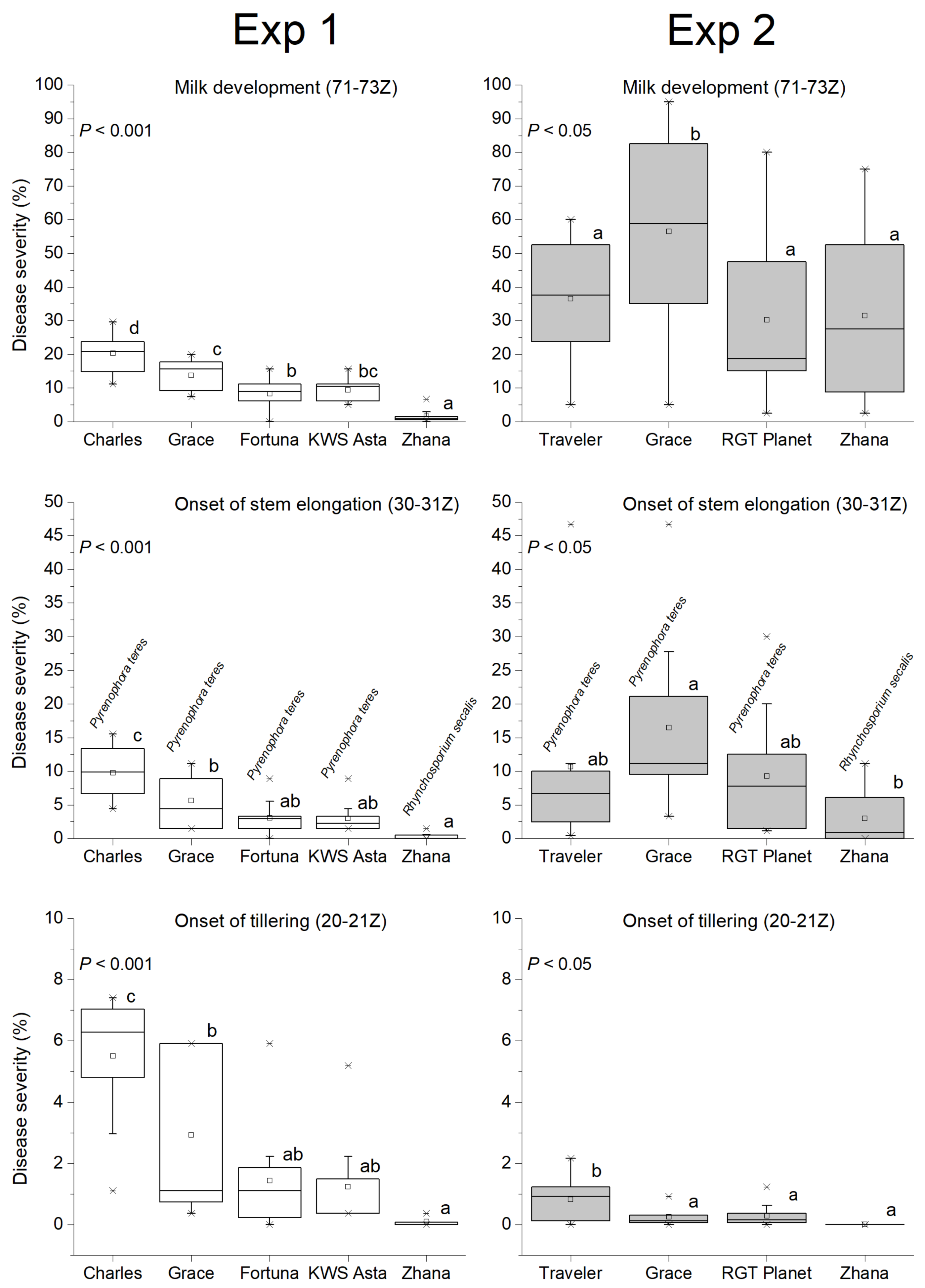
3.2. Temporal and Genotypic Effects
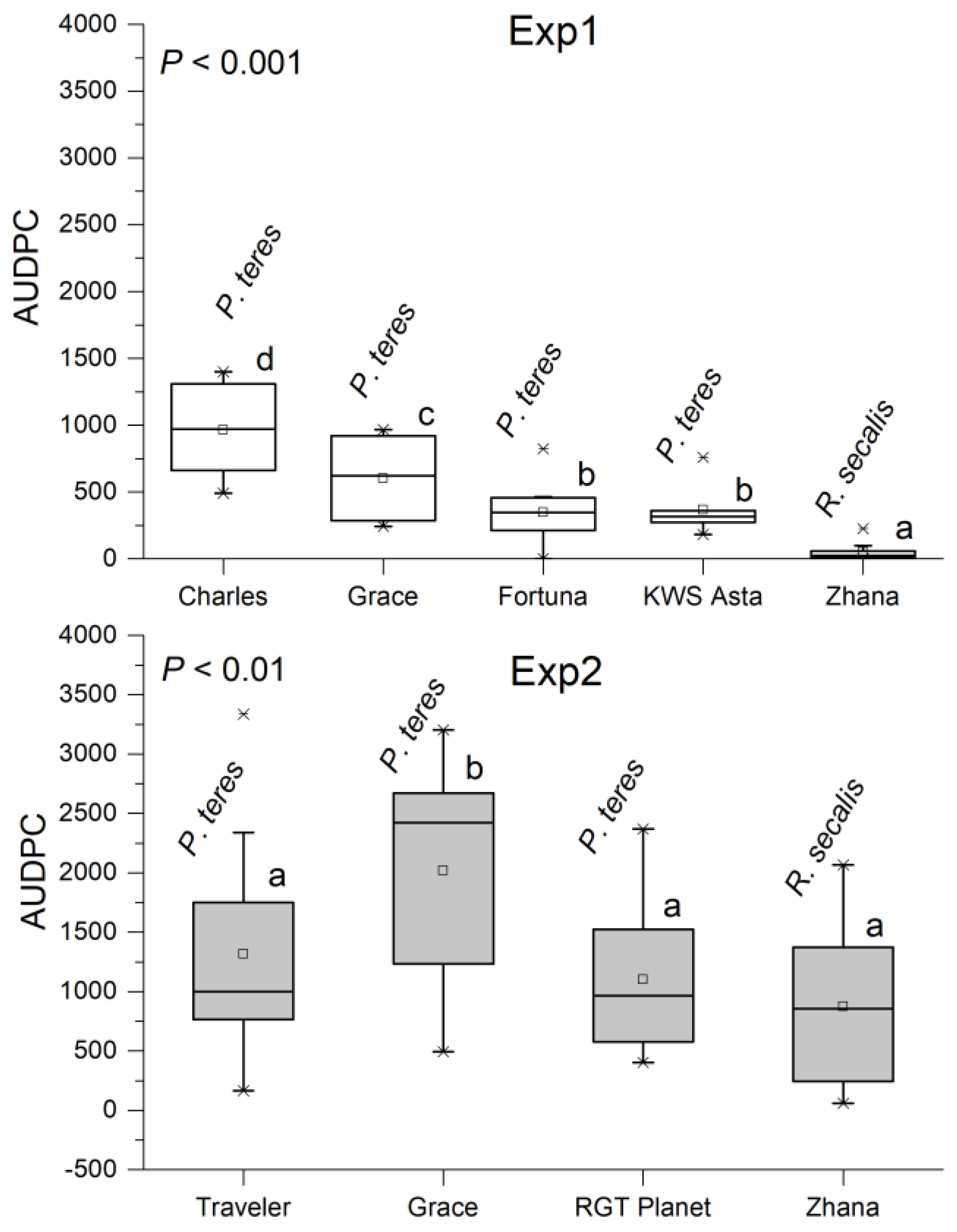
3.3. The Area under Disease Progress Curve (AUDPC)
3.4. Epidemiology Assessment When Nitrogen Rate and Genotype Are the Main Sources of Variation
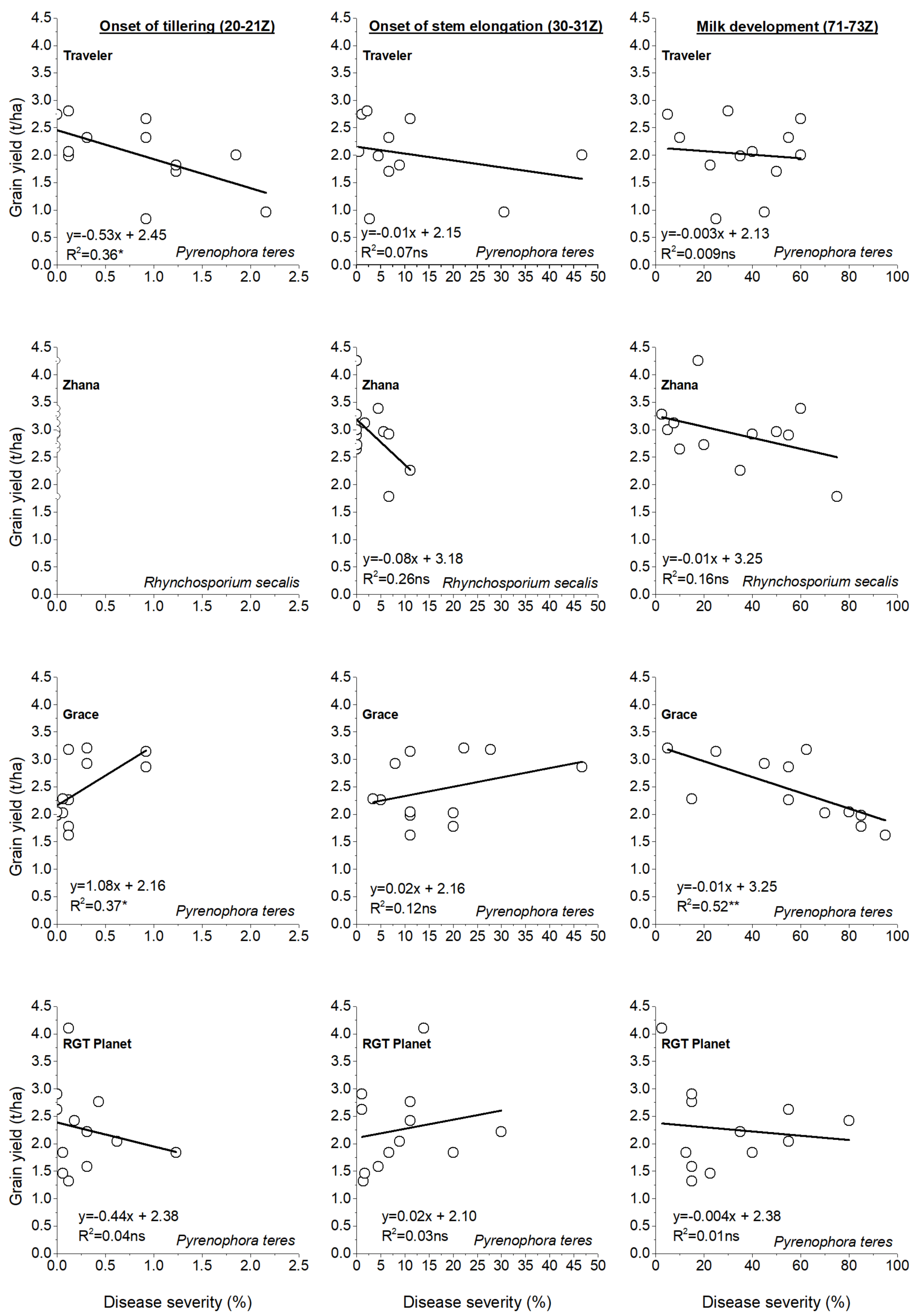
3.5. Quantifying the Effects of the Rate of Nitrogen Application and the Distance from the Nearest Hotspot on Crop Disease Severity
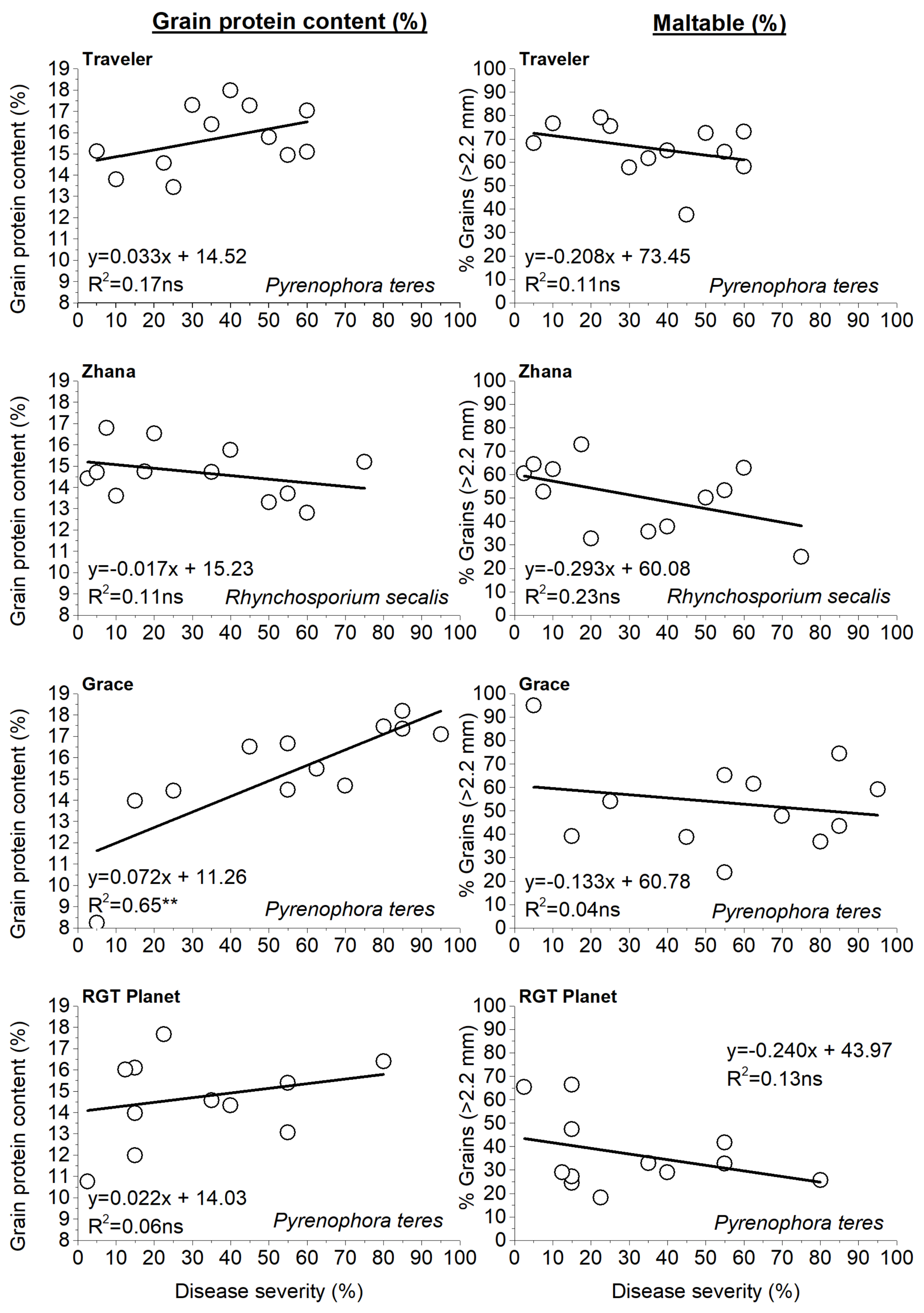
3.6. Effect of N and Genotype on Grain Yield and Quality Characters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Friedt, W. Barley breeding history, progress, objectives, and technology. In Barley Production, Improvement, and Uses, 1st ed.; Ullrich, S.E., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 160–220. [Google Scholar]
- Meussdoerffer, F.; Zarnkow, M. Starchy Raw Materials. In Handbook of Brewing: Processes, Technology, Markets, 1st ed; Eßlinger, H.M., Ed.; Wiley: Weinheim, Germany, 2009; pp. 43–83. [Google Scholar]
- Zhan, J.; Fitt, B.D.L.; Pinnschmidt, H.O.; Oxley, S.J.P.; Newton, A.C. Resistance, epidemiology and sustainable management of Rhynchosporium secalis populations on barley. Plant Pathol. 2008, 57, 1–14. [Google Scholar] [CrossRef]
- Liu, Z.; Ellwood, S.R.; Oliver, R.P.; Friesen, T.L. Pyrenophora teres: Profile of an increasingly damaging barley pathogen. Mol. Plant Pathol. 2011, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Paulitz, T.C.; Steffenson, B.J. Biotic stress in barley: Disease problems and solutions. In Barley Production, Improvement, and Uses, 1st ed.; Ullrich, S.E., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 307–354. [Google Scholar]
- Shipton, W.A. Effect of net blotch infection of barley on grain yield and quality. Aust. J. Exp. Agric. Anim. Husb. 1966, 6, 437–440. [Google Scholar] [CrossRef]
- Shipton, W.A.; Boyd, W.J.R.; Ali, S.M. Scald of barley. Rev. Plant. Pathol. 1974, 53, 839–861. [Google Scholar]
- Martin, R.A. Disease progression and yield loss in barley associated with net blotch, as influenced by fungicide seed treatment. Can. J. Plant Pathol. 1985, 7, 83–90. [Google Scholar] [CrossRef]
- Khan, T.N. Relationship between net blotch (Drechslera teres) and losses in grain yield of barley in Western Australia. Aust. J. Agric. Res. 1987, 38, 671–679. [Google Scholar] [CrossRef]
- El Yousfi, B.; Ezzahiri, B. Net blotch in semi-arid regions of Marocco II: Yield and yield-loss modeling. Field Crops Res. 2002, 73, 81–93. [Google Scholar] [CrossRef]
- Murray, G.M.; Brennan, J.P. Estimating disease losses to the Australian barley industry. Australas. Plant Pathol. 2010, 39, 85–96. [Google Scholar] [CrossRef]
- McCartney, H.A.; Fitt, B.D.L. Dispersal of foliar fungal plant pathogens: Mechanisms, gradients and spatial patterns. In The Epidemiology of Plant Diseases, 1st ed.; Jones, D.G., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 138–160. [Google Scholar]
- Gibson, G.J. Investigating mechanisms of spatiotemporal epidemic spread using stochastic models. Phytopathology 1997, 87, 139–146. [Google Scholar] [CrossRef]
- Gibson, G.J. Markov chain Monte Carlo methods for fitting spatiotemporal epidemic stochastic models in plant epidemiology. J. R. Stat. Soc. Ser. C 1997, 46, 215–233. [Google Scholar] [CrossRef]
- Madden, L.V. Botanical epidemiology: Some key advances and its continuing role in disease management. Eur. J. Plant Pathol. 2006, 115, 3–23. [Google Scholar] [CrossRef]
- Scherm, H.; Ngugi, H.K.; Ojiambo, P.S. Trends in theoretical plant epidemiology. Eur. J. Plant Pathol. 2006, 115, 61–73. [Google Scholar] [CrossRef]
- Luo, W.; Pietravalle, S.; Parnell, S.; van den Bosch, F.; Gottwald, T.R.; Irey, M.S.; Parke, S.R. An improved regulatory sampling method for mapping and representing plant disease from a limited number of samples. Epidemics 2012, 4, 68–77. [Google Scholar] [CrossRef]
- El Yousfi, B.; Ezzahiri, B. Net blotch in semi arid regions of Morocco. I Epidemiology. Field Crops Res. 2001, 73, 35–46. [Google Scholar] [CrossRef]
- Baethgen, W.E.; Christianson, C.B.; Lamothe, A.G. Nitrogen fertilizer effects on growth, grain yield, and yield components of malting barley. Field Crops Res. 1995, 43, 87–99. [Google Scholar] [CrossRef]
- Abeledo, L.G.; Calderini, D.F.; Slafer, G.A. Nitrogen economy in old and modern malting barleys. Field Crops Res. 2008, 106, 171–178. [Google Scholar] [CrossRef]
- Albrizio, R.; Todorovic, M.; Matic, T.; Stellacci, A.M. Comparing the interactive effects of water and nitrogen on durum wheat and barley grown in a Mediterranean environment. Field Crops Res. 2010, 115, 179–190. [Google Scholar] [CrossRef]
- Dordas, C. Variation in dry matter and nitrogen accumulation and remobilization in barley as affected by fertilization, cultivar, and source–sink relations. Eur. J. Agron. 2012, 37, 31–42. [Google Scholar] [CrossRef]
- Grant, C.A.; Gauer, L.E.; Gehl, D.T.; Bailey, L.D. Protein production and nitrogen utilization by barley cultivars in response to nitrogen fertilizer under varying moisture conditions. Can. J. Plant Sci. 1991, 7l, 997–1009. [Google Scholar] [CrossRef]
- Grashoff, C.; D’Antuono, L.F. Effect of shading and nitrogen application on yield, grain size distribution and concentrations of nitrogen and water soluble carbohydrates in malting spring barley (Hordeum vulgare L.). Eur. J. Agron. 1997, 6, 275–293. [Google Scholar] [CrossRef]
- Boonchoo, S.; Fukai, S.; Hetherington, S.E. Barley yield and grain protein concentration as affected by assimilate and nitrogen availability. Aust. J. Agric. Res. 1998, 49, 695–706. [Google Scholar] [CrossRef]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. The effects of biochar, compost and their mixture and nitrogen fertilizer on yield and nitrogen use efficiency of barley grown on a Nitisol in the highlands of Ethiopia. Sci. Total Environ. 2016, 569–570, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Jenkyn, J.F.; Griffiths, E. Relationships between the severity of leaf blotch (Rhynchosporium secalis) and the water-soluble carbohydrate and nitrogen contents of barley plants. Ann. Appl. Biol. 1978, 90, 35–44. [Google Scholar] [CrossRef]
- Krupinsky, J.M.; Halvorson, A.D.; Tanaka, D.L.; Merrill, S.D. Nitrogen and tillage effects on wheat leaf spot diseases in the northern great plains. Agron. J. 2007, 99, 562–569. [Google Scholar] [CrossRef]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 33–46. [Google Scholar] [CrossRef]
- Kangor, T.; Sooväli, P.; Tamm, Y.; Tamm, I.; Koppel, M. Malting barley diseases, yield and quality—Responses to using various agro-technology regimes. Proc. Latv. Acad. Sci. Sect. B 2017, 71, 57–62. [Google Scholar] [CrossRef]
- Turkington, T.K.; O’Donovan, J.T.; Edney, M.J.; Juskiw, P.E.; McKenzie, R.H.; Harker, K.N.; Clayton, G.W.; Xi, K.; Lafond, G.P.; Irvine, R.B.; et al. Effect of crop residue, nitrogen rate and fungicide application on malting barley productivity, quality, and foliar disease severity. Can. J. Plant Sci. 2012, 92, 577–588. [Google Scholar] [CrossRef]
- Bingham, I.J.; Blake, J.; Foulkes, M.J.; Spink, J. Is barley yield in the UK sink limited? II. Factors affecting potential grain size. Field Crops Res. 2007, 101, 212–220. [Google Scholar] [CrossRef]
- Ugarte, C.; Calderini, D.F.; Slafer, G.A. Grain weight and grain number responsiveness to pre-anthesis temperature in wheat barley and triticale. Field Crops Res. 2007, 100, 240–248. [Google Scholar] [CrossRef]
- Bertholdsson, N.O. Characterization of malting barley cultivars with more or less stable grain protein content under varying environmental conditions. Eur. J. Agron. 1999, 10, 1–8. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Eyal, Z.; Scharen, A.L.; Prescott, J.M.; van Ginkel, M. The Septoria Disease of Wheat: Concepts and Methods of Disease Management; CIMMYT: Mexico City, Mexico, 1987; Available online: https://repository.cimmyt.org/xmlui/bitstream/handle/10883/1113/13508.pdf?sequence=1&isAllowed=y (accessed on 17 November 2020).
- Sharma, P.; Duveiller, E.; Sharma, R.C. Effect of mineral nutrient on spot blotch severity in wheat and associated increases in grain yield. Field Crops Res. 2006, 95, 426–430. [Google Scholar] [CrossRef]
- Saxesena, R.R.; Mishra, V.K.; Chand, R.; Chowdhury, A.K.; Bhattacharya, P.M.; Joshi, A.K. Pooling together spot blotch resistance, high yield with earliness in wheat for eastern Gangetic plains of south Asia. Field Crop. Res. 2017, 214, 291–300. [Google Scholar] [CrossRef]
- Saari, E.E.; Prescott, J.M. Scale for appraising the foliar intensity of wheat diseases. Plant Dis. Rep. 1975, 59, 377–380. [Google Scholar]
- Shaner, G.; Finney, R. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in knox wheat. Phytopathology 1977, 67, 1051–1056. [Google Scholar] [CrossRef]
- Vahamidis, P.; Stefopoulou, A.; Kotoulas, V.; Lyra, D.; Dercas, N.; Economou, G. Yield, grain size, protein content and water use efficiency of null-LOX malt barley in a semiarid Mediterranean agroecosystem. Field Crops Res. 2017, 206, 115–127. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Zhang, H.; Tripathi, N.K. Geospatial hotspot analysis of lung cancer patients correlated to fine particulate matter (PM2.5) and industrial wind in Eastern Thailand. J. Clean. Prod. 2018, 170, 407–424. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, L.; Xu, W.; Ledwith, V. Use of local Moran’s I and GIS to identify pollution hotspots of Pb in urban soils of Galway, Ireland. Sci. Total Environ. 2008, 398, 212–221. [Google Scholar] [CrossRef]
- Lalor, G.C.; Zhang, C. Multivariate outlier detection and remediation in geochemical databases. Sci. Total Environ. 2001, 281, 99–109. [Google Scholar] [CrossRef]
- Nimon, K.; Lewis, M.; Kane, R.; Haynes, R.M. An R package to compute commonality coefficients in the multiple regression case: An introduction to the package and a practical example. Behav. Res. Methods 2008, 40, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Ray-Mukherjee, J.; Nimon, K.; Mukherjee, S.; Morris, D.W.; Slotow, R.; Hamer, M. Using commonality analysis in multiple regressions: A tool to decompose regression effects in the face of multicollinearity. Methods Ecol. Evol. 2014, 5, 320–328. [Google Scholar] [CrossRef]
- Jordan, V.W.L. Aetiology of barley net blotch caused by Pyrenophora teres and some effects on yield. Plant. Pathol. 1981, 30, 77–87. [Google Scholar] [CrossRef]
- McLean, M.S.; Howlett, B.J.; Hollaway, G.J. Epidemiology and control of spot form of net blotch (Pyrenophora teres f. maculata) of barley: A review. Crop. Pasture Sci. 2009, 60, 303–315. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Creighton, N.F.; Lacey, M.E.; McCartney, H.A. Effects of rainfall intensity and duration on dispersal of Rhynchosporium secalis conidia from infected barley leaves. Trans. Br. Mycol. Soc. 1986, 86, 611–618. [Google Scholar] [CrossRef]
- Shjerve, R.A.; Faris, J.D.; Brueggeman, R.S.; Yan, C.; Zhu, Y.; Koladia, V.; Friesen, T.L. Evaluation of a Pyrenophora teres f. teres mapping population reveals multiple independent interactions with the barley 6H chromosome region. Fungal Genet. Biol. 2014, 70, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Linsell, K.J.; Keiper, F.J.; Forgan, A.; Oldach, K.H. New insights into the infection process of Rhynchosporium secalis in barley using GFP. Fungal Genet. Biol. 2011, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Jalli, M. Grain yield, net blotch and scald of barley in Finnish official variety trials. Agric. Food Sci. 1997, 6, 399–408. [Google Scholar] [CrossRef]
- Jalakas, P.; Tulva, I.; Kangor, T.; Sooväli, P.; Rasulov, B.; Tamm, Ü.; Koppel, M.; Kollist, H.; Merilo, E. Gas exchange-yield relationships of malting barley genotypes treated with fungicides and biostimulants. Eur. J. Agron. 2018, 99, 129–137. [Google Scholar] [CrossRef]
- Cossani, C.M.; Savin, R.; Slafer, G.A. Contrasting performance of barley and wheat in a wide range of conditions in Mediterranean Catalonia (Spain). Ann. Appl. Biol. 2007, 151, 167–173. [Google Scholar] [CrossRef]
- Cossani, C.M.; Slafer, G.A.; Savin, R. Yield and biomass in wheat and barley under a range of conditions in a Mediterranean site. Field Crops Res. 2009, 112, 205–213. [Google Scholar] [CrossRef]
- Bingham, I.J.; Hoad, S.P.; Thomas, W.T.B.; Newton, A.C. Yield response to fungicide of spring barley genotypes differing in disease susceptibility and canopy structure. Field Crops Res. 2012, 139, 9–19. [Google Scholar] [CrossRef]
- Agostinetto, L.; Casa, R.T.; Bogo, A.; Sachs, C.; Souza, C.A.; Reis, E.M.; da Cunha, I.C. Barley spot blotch intensity, damage, and control response to foliar fungicide application in southern Brazil. Crop. Prot. 2015, 67, 7–12. [Google Scholar] [CrossRef]



| Source of Variation | GY | GPC | Maltable | AUDPC a | DSSE | DSGF |
|---|---|---|---|---|---|---|
| Cultivar | ** | ns | *** | ** | * | * |
| Nitrogen | ns | *** | ns | ns | ns | ns |
| Cultivar x Nitrogen | * | ns | ns | ns | ns | ns |
| Cultivar | Unique and Common Effects | Onset of Stem Elongation | Onset of Grain Filling (Milk Development) | ||
|---|---|---|---|---|---|
| Coefficient | % Total | Coefficient | % Total | ||
| Traveler | Unique to Distance a | 0.4547 | 72.51 | 0.0008 | 0.22 |
| Unique to Nitrogen b | 0.0004 | 0.07 | 0.3493 | 93.17 | |
| Common to Distance and Nitrogen | 0.1720 | 27.42 | 0.0248 | 6.61 | |
| Total | 0.6271 | 100.00 | 0.3748 | 100.00 | |
| Zhana | Unique to Distance | 0.1678 | 67.81 | 0.0819 | 79.91 |
| Unique to Nitrogen | 0.0089 | 3.59 | 0.0241 | 23.51 | |
| Common to Distance and Nitrogen | 0.0708 | 28.61 | −0.0035 | −3.42 | |
| Total | 0.2475 | 100.00 | 0.1025 | 100.00 | |
| Grace | Unique to Distance | 0.3837 | 97.65 | 0.1641 | 22.26 |
| Unique to Nitrogen | 0.0105 | 2.66 | 0.2850 | 38.66 | |
| Common to Distance and Nitrogen | −0.0012 | −0.31 | 0.2881 | 39.08 | |
| Total | 0.3930 | 100.00 | 0.7373 | 100.00 | |
| RGT Planet | Unique to Distance | 0.1912 | 38.76 | 0.3672 | 83.26 |
| Unique to Nitrogen | 0.0925 | 18.75 | 0.0020 | 0.46 | |
| Common to Distance and Nitrogen | 0.2096 | 42.49 | 0.0718 | 16.29 | |
| Total | 0.4933 | 100.00 | 0.4411 | 100.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vahamidis, P.; Stefopoulou, A.; Lagogianni, C.S.; Economou, G.; Dercas, N.; Kotoulas, V.; Kalivas, D.; Tsitsigiannis, D.I. Pyrenophora teres and Rhynchosporium secalis Establishment in a Mediterranean Malt Barley Field: Assessing Spatial, Temporal and Management Effects. Agriculture 2020, 10, 553. https://doi.org/10.3390/agriculture10110553
Vahamidis P, Stefopoulou A, Lagogianni CS, Economou G, Dercas N, Kotoulas V, Kalivas D, Tsitsigiannis DI. Pyrenophora teres and Rhynchosporium secalis Establishment in a Mediterranean Malt Barley Field: Assessing Spatial, Temporal and Management Effects. Agriculture. 2020; 10(11):553. https://doi.org/10.3390/agriculture10110553
Chicago/Turabian StyleVahamidis, Petros, Angeliki Stefopoulou, Christina S. Lagogianni, Garyfalia Economou, Nicholas Dercas, Vassilis Kotoulas, Dionissios Kalivas, and Dimitrios I. Tsitsigiannis. 2020. "Pyrenophora teres and Rhynchosporium secalis Establishment in a Mediterranean Malt Barley Field: Assessing Spatial, Temporal and Management Effects" Agriculture 10, no. 11: 553. https://doi.org/10.3390/agriculture10110553
APA StyleVahamidis, P., Stefopoulou, A., Lagogianni, C. S., Economou, G., Dercas, N., Kotoulas, V., Kalivas, D., & Tsitsigiannis, D. I. (2020). Pyrenophora teres and Rhynchosporium secalis Establishment in a Mediterranean Malt Barley Field: Assessing Spatial, Temporal and Management Effects. Agriculture, 10(11), 553. https://doi.org/10.3390/agriculture10110553






