A Prospective Clinical Cohort Investigation on Zirconia Implants: 5-Year Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Null Hypothesis
2.2. Design of the Investigation
2.3. Oral Implant Devices, Surgical and Prosthetic Procedures, Follow-Ups
2.4. Clinical Peri-Implant Soft Tissue Evaluation
2.5. Patient-Reported Outcome Measures (PROMs; Patient’s Assessment)
2.6. Implant Success Rating and Bone Loss Criteria
2.7. Statistical Analysis
3. Results
3.1. Status of Follow-Up
3.2. Marginal Bone Loss
3.3. Peri-Implant Soft Tissue Evaluation (Table 5)
3.4. Patient Assessment: Patient-Reported Outcome Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanz, M.; Noguerol, B.; Sanz-Sanchez, I.; Hämmerle, C.H.F.; Schliephake, H.; Renouard, F.; Sicilia, A.; Cordaro, L.; Jung, R.; Klinge, B.; et al. European association for osseointegration delphi study on the trends in implant dentistry in europe for the year 2030. Clin. Oral Implants Res. 2019, 30, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Roehling, S.; Schlegel, K.A.; Woelfler, H.; Gahlert, M. Performance and outcome of zirconia dental implants in clinical studies: A meta-analysis. Clin. Oral Implants Res. 2018, 29, 135–153. [Google Scholar] [CrossRef]
- Pieralli, S.; Kohal, R.J.; Jung, R.E.; Vach, K.; Spies, B.C. Clinical outcomes of zirconia dental implants: A systematic review. J. Dent. Res. 2017, 96, 38–46. [Google Scholar] [CrossRef]
- Rabel, K.; Spies, B.C.; Pieralli, S.; Vach, K.; Kohal, R.J. The clinical performance of all-ceramic implant-supported single crowns: A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, 196–223. [Google Scholar] [CrossRef]
- Pieralli, S.; Kohal, R.J.; Rabel, K.; von Stein-Lausnitz, M.; Vach, K.; Spies, B.C. Clinical outcomes of partial and full-arch all-ceramic implant-supported fixed dental prostheses. A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Balmer, M.; Spies, B.C.; Kohal, R.J.; Hämmerle, C.H.; Vach, K.; Jung, R.E. Zirconia implants restored with single crowns or fixed dental prostheses: 5-year results of a prospective cohort investigation. Clin. Oral Implants Res. 2020, 31, 452–462. [Google Scholar] [CrossRef]
- Koller, M.; Steyer, E.; Theisen, K.; Stagnell, S.; Jakse, N.; Payer, M. Two-piece zirconia versus titanium implants after 80 months: Clinical outcomes from a prospective randomized pilot trial. Clin. Oral Implants Res. 2020, 31, 388–396. [Google Scholar] [CrossRef]
- Lorenz, J.; Giulini, N.; Holscher, W.; Schwiertz, A.; Schwarz, F.; Sader, R. Prospective controlled clinical study investigating long-term clinical parameters, patient satisfaction, and microbial contamination of zirconia implants. Clin. Implant Dent. Relat. Res. 2019, 21, 263–271. [Google Scholar] [CrossRef]
- Jung, R.E.; Sailer, I.; Hämmerle, C.H.; Attin, T.; Schmidlin, P. In vitro color changes of soft tissues caused by restorative materials. Int. J. Periodontics Restor. Dent. 2007, 27, 251–257. [Google Scholar]
- Kniha, K.; Kniha, H.; Grunert, I.; Edelhoff, D.; Holzle, F.; Modabber, A. Esthetic evaluation of maxillary single-tooth zirconia implants in the esthetic zone. Int. J. Periodontics Restor. Dent. 2019, 39, e195–e201. [Google Scholar] [CrossRef]
- Mombelli, A.; Hashim, D.; Cionca, N. What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clin. Oral Implants Res. 2018, 29, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Chrcanovic, B.; Mölne, J.; Wennerberg, A. Foreign body reactions, marginal bone loss and allergies in relation to titanium implants. Int. J. Oral Implantol. 2018, 11, s37–s46. [Google Scholar]
- Jacobi-Gresser, E.; Huesker, K.; Schutt, S. Genetic and immunological markers predict titanium implant failure: A retrospective study. Int. J. Oral Maxillofac. Surg. 2013, 42, 537–543. [Google Scholar] [CrossRef]
- Rimondini, L.; Cerroni, L.; Carrassi, A.; Torricelli, P. Bacterial colonization of zirconia ceramic surfaces: An in vitro and in vivo study. Int. J. Oral Maxillofac. Implants 2002, 17, 793–798. [Google Scholar]
- Degidi, M.; Artese, L.; Scarano, A.; Perrotti, V.; Gehrke, P.; Piattelli, A. Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. J. Periodontol. 2006, 77, 73–80. [Google Scholar] [CrossRef]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef]
- de Avila, E.D.; Avila-Campos, M.J.; Vergani, C.E.; Spolidorio, D.M.; de Assis Mollo, F.J. Structural and quantitative analysis of a mature anaerobic biofilm on different implant abutment surfaces. J. Prosthet. Dent. 2016, 115, 428–436. [Google Scholar] [CrossRef]
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic steel? Nature 1975, 258, 703–704. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Bethke, A.; Pieralli, S.; Kohal, R.J.; Burkhardt, F.; von Stein-Lausnitz, M.; Vach, K.; Spies, B.C. Fracture resistance of zirconia oral implants in vitro: A systematic review and meta-analysis. Materials 2020, 13, 562. [Google Scholar] [CrossRef]
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef]
- Kohal, R.J.; Wolkewitz, M.; Mueller, C. Alumina-reinforced zirconia implants: Survival rate and fracture strength in a masticatory simulation trial. Clin. Oral Implants Res. 2010, 21, 1345–1352. [Google Scholar] [CrossRef]
- Spies, B.C.; Sauter, C.; Wolkewitz, M.; Kohal, R.J. Alumina reinforced zirconia implants: Effects of cyclic loading and abutment modification on fracture resistance. Dent. Mater. 2015, 31, 262–272. [Google Scholar] [CrossRef]
- Kohal, R.J.; Bächle, M.; Renz, A.; Butz, F. Evaluation of alumina toughened zirconia implants with a sintered, moderately rough surface: An experiment in the rat. Dent. Mater. 2016, 32, 65–72. [Google Scholar] [CrossRef]
- Spies, B.C.; Sperlich, M.; Fleiner, J.; Stampf, S.; Kohal, R.J. Alumina reinforced zirconia implants: 1-year results from a prospective cohort investigation. Clin. Oral Implants Res. 2016, 27, 481–490. [Google Scholar] [CrossRef]
- Spies, B.C.; Balmer, M.; Patzelt, S.B.; Vach, K.; Kohal, R.J. Clinical and patient-reported outcomes of a zirconia oral implant: Three-year results of a prospective cohort investigation. J. Dent. Res. 2015, 94, 1385–1391. [Google Scholar] [CrossRef]
- STROBE Statement. Strengthening the Reporting of Observational Studies in Epidemiology. Available online: https://www.strobe-statement.org/index.php?id=strobe-home (accessed on 4 February 2020).
- EQUATOR Network. Enhancing the Quality and Transparency of Health Research. Available online: http://www.equator-network.org (accessed on 4 February 2020).
- Mombelli, A.; van Oosten, M.A.C.; Schürch, J.E.; Lang, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef]
- Östman, P.O.; Hellman, M.; Albrektsson, T.; Sennerby, L. Direct loading of nobel direct and nobel perfect one-piece implants: A 1-year prospective clinical and radiographic study. Clin. Implant Dent. Relat. Res. 2007, 18, 409–418. [Google Scholar] [CrossRef]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- de Oliveira Limirio, J.P.J.; Lemos, C.A.A.; de Luna Gomes, J.M.; Minatel, L.; Alves Rezende, M.C.R.; Pellizzer, E.P. A clinical comparison of 1-piece versus 2-piece implants: A systematic review and meta-analysis. J. Prosthet. Dent. 2019. [Google Scholar] [CrossRef]
- Jung, R.E.; Zembic, A.; Pjetursson, B.J.; Zwahlen, M.; Thoma, D.S. Systematic review of the survival rate and the incidence of biological, technical and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implants Res. 2012, 23, 2–21. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (fdps) after a mean observation period of at least 5 years. Clin. Oral Implants Res. 2012, 23, 22–38. [Google Scholar] [CrossRef]
- Balmer, M.; Spies, B.C.; Vach, K.; Kohal, R.J.; Hämmerle, C.H.F.; Jung, R.E. Three-year analysis of zirconia implants used for single-tooth replacement and three-unit fixed dental prostheses: A prospective multicenter study. Clin. Oral Implants Res. 2018, 29, 290–299. [Google Scholar] [CrossRef]
- Bormann, K.H.; Gellrich, N.C.; Kniha, H.; Schild, S.; Weingart, D.; Gahlert, M. A prospective clinical study to evaluate the performance of zirconium dioxide dental implants in single-tooth edentulous area: 3-year follow-up. BMC Oral Health 2018, 18, 181–189. [Google Scholar] [CrossRef]
- Cheng, Q.; Su, Y.Y.; Wang, X.; Chen, S. Clinical outcomes following immediate loading of single-tooth implants in the esthetic zone: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implants 2020, 35, 167–177. [Google Scholar] [CrossRef]
- Schou, S.; Holmstrup, P.; Stoltze, K.; Hjorting-Hansen, E.; Fiehn, N.E.; Skovgaard, L.T. Probing around implants and teeth with healthy or inflamed peri-implant mucosa/gingiva. A histologic comparison in cynomolgus monkeys (Macaca fascicularis). Clin. Oral Implants Res. 2002, 13, 113–126. [Google Scholar] [CrossRef]
- Ericsson, I.; Lindhe, J. Probing depth at implants and teeth. An experimental study in the dog. J. Clin. Periodontol. 1993, 20, 623–627. [Google Scholar] [CrossRef]
- Gerber, J.A.; Tan, W.C.; Balmer, T.E.; Salvi, G.E.; Lang, N.P. Bleeding on probing and pocket probing depth in relation to probing pressure and mucosal health around oral implants. Clin. Oral Implants Res. 2009, 20, 75–78. [Google Scholar] [CrossRef]
- Cutrim, E.S.; Peruzzo, D.C.; Benatti, B. Evaluation of soft tissues around single tooth implants in the anterior maxilla restored with cemented and screw-retained crowns. J. Oral Implantol. 2012, 38, 700–705. [Google Scholar] [CrossRef]
- Wolleb, K.; Sailer, I.; Thoma, A.; Menghini, G.; Hämmerle, C.H. Clinical and radiographic evaluation of patients receiving both tooth- and implant-supported prosthodontic treatment after 5 years of function. Int. J. Prosthodont. 2012, 25, 252–259. [Google Scholar]
- Listgarten, M.A.; Lang, N.P.; Schroeder, H.E.; Schroeder, A. Periodontal tissues and their counterparts around endosseous implants. Clin. Oral Implants Res. 1991, 2, 1–19. [Google Scholar] [CrossRef]
- Norton, M.R.; Gamble, C. Bone classification: An objective scale of bone density using the computerized tomography scan. Clin. Oral Implants Res. 2001, 12, 79–84. [Google Scholar] [CrossRef]
- Molly, L. Bone density and primary stability in implant therapy. Clin. Oral Implants Res. 2006, 17 (Suppl. 2), 124–135. [Google Scholar] [CrossRef] [PubMed]
- Glauser, R.; Ree, A.; Lundgren, A.; Gottlow, J.; Hämmerle, C.H.; Schärer, P. Immediate occlusal loading of Brånemark implants applied in various jawbone regions: A prospective, 1-year clinical study. Clin. Implant Dent. Relat. Res. 2001, 3, 204–213. [Google Scholar] [CrossRef]
- Jaffin, R.A.; Berman, C.L. The excessive loss of Brånemark fixtures in type IV bone: A 5-year analysis. J. Periodontol. 1991, 62, 2–4. [Google Scholar] [CrossRef]
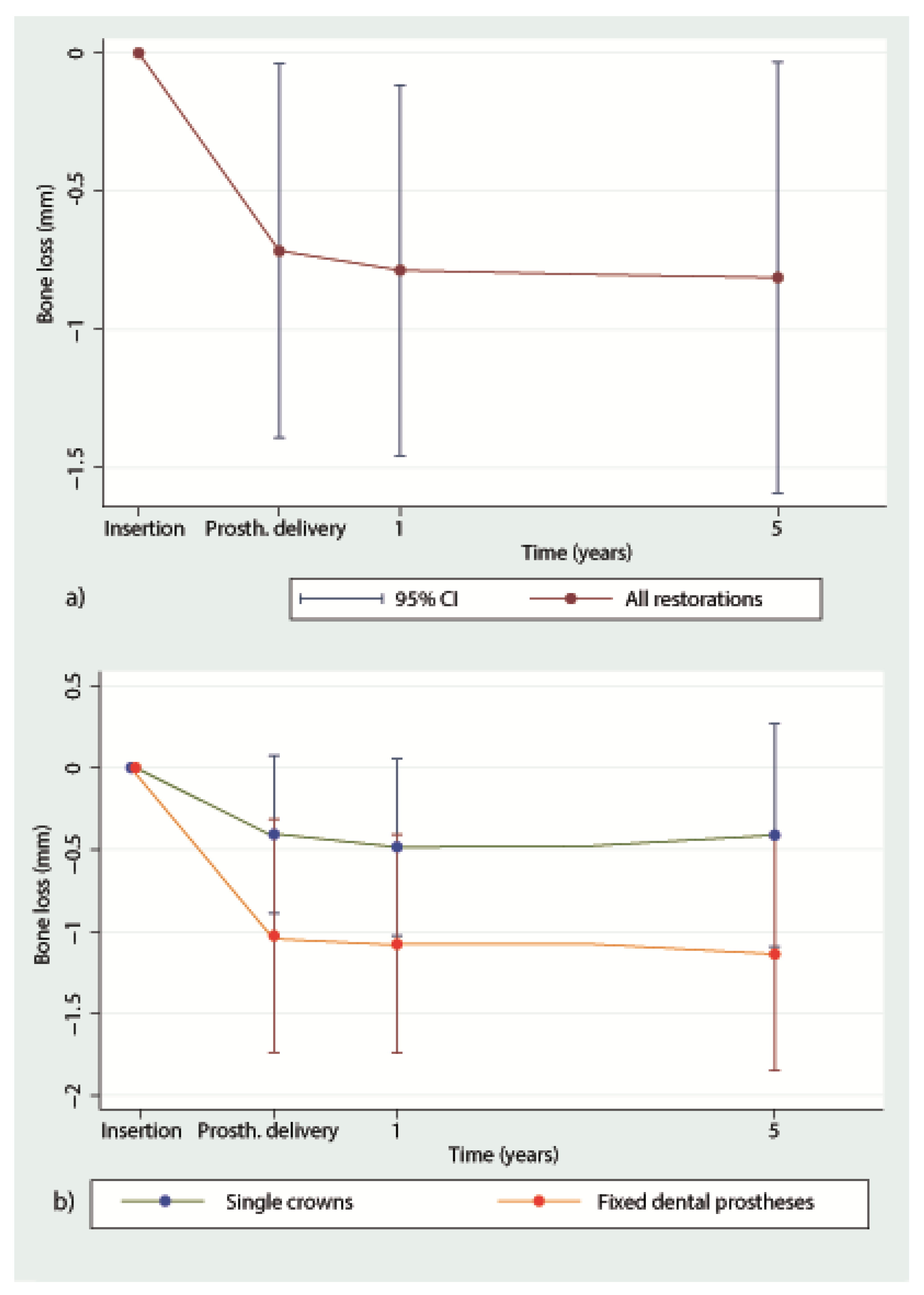

| Time | N of Implants | Losses | Survival (%) | Survival Worst-Case Scenario (%) |
|---|---|---|---|---|
| Baseline to delivery | 53 | 3 | 94.3 | 94.3 |
| at delivery | 50 | 0 | 94.3 | 94.3 |
| Delivery to 1-year follow-up | 50 | 0 | 94.3 | 94.3 |
| 1-year to 5-year follow-up | 48 | 0 (2 *) | 94.3 | 90 * |
| Prosthetic Delivery | 1-y Follow-Up | 5-y Follow-Up | Significance (p) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | Mean | SD | I → D | D → 1y | D → 5y | 1y → 5y | |
| Position | |||||||||||||||
| Mesial tooth | 30 | 0.06 | 0.92 | 35 | 0.11 | 0.63 | 32 | 0.39 | 1.50 | 0.39 | 1.50 | 0.593 | 0.523 | 0.294 | 0.292 |
| Implant | 45 | 0.71 | 0.67 | 48 | 0.78 | 0.67 | 48 | 0.81 | 0.78 | 0.81 | 0.77 | <0.001 * | 0.548 * | 0.611 * | 0.892 * |
| Distal tooth | 24 | 0.12 | 0.64 | 25 | 0.35 | 0.58 | 23 | 0.41 | 0.90 | 0.41 | 0.89 | 0.170 | 0.029 | 0.151 | 0.734 |
| Insertion to Prosthetic Delivery | Insertion to 5-Year Follow-Up | |||
|---|---|---|---|---|
| Bone loss in mm | n | % | n | % |
| <0 | 7 | 15.6 | 5 | 10.4 |
| 0 | 1 | 2.2 | 0 | 0 |
| >0 –0.5 | 9 | 20 | 12 | 25 |
| >0.5–1.0 | 14 | 31.1 | 11 | 22.9 |
| >1.0–1.5 | 10 | 22.2 | 11 | 22.9 |
| >1.5–2.0 | 2 | 4.4 | 5 | 10.4 |
| >2.0–2.5 | 2 | 4.4 | 3 | 6.25 |
| >2.5–3.0 | 0 | 0 | 1 | 2.1 |
| Total | 45 | 100 | 48 | 100 |
| Variable | n | Mean (in mm) | Standard Deviation | p-Value |
|---|---|---|---|---|
| Gender | ||||
| female | 23 | 0.87 | 0.78 | 0.645 |
| male | 25 | 0.76 | 0.78 | |
| Jaw type | ||||
| mandible | 32 | 0.78 | 0.79 | 0.680 |
| maxilla | 15 | 0.89 | 0.78 | |
| Ant-post | ||||
| posterior implants | 44 | 0.81 | 0.79 | 0.855 |
| anterior implants | 3 | 0.87 | 0.63 | |
| Implant diameter in mm | ||||
| 3 | 6 | 1.05 | 0.57 | 0.205 |
| 4 | 29 | 0.89 | 0.83 | |
| 5 | 12 | 0.50 | 0.71 | |
| Implant length in mm | ||||
| 9 | 15 | 1.14 | 0.78 | 0.071 |
| 12 | 28 | 0.72 | 0.71 | |
| 14 | 4 | 0.25 | 0.93 | |
| Bone quality | ||||
| 1 and 2 | 36 | 0.78 | 0.77 | 0.207 |
| 3 | 11 | 1.05 | 0.79 | |
| Bone quantity | ||||
| A | 20 | 0.65 | 0.79 | 0.245 |
| B and C | 27 | 0.93 | 0.76 | |
| Anchorage | ||||
| no cortical | 2 | 0.60 | 0.57 | n.e.o. |
| monocortical | 42 | 0.80 | 0.81 | |
| bicortical | 3 | 1.07 | 0.60 | |
| Grafting | ||||
| no | 22 | 0.82 | 0.66 | 0.987 |
| yes | 25 | 0.75 | 0.81 | |
| Flap design | ||||
| w/o releasing incisions | 39 | 0.86 | 0.80 | 0.477 |
| w releasing incisions | 8 | 0.65 | 0.60 | |
| Torque | ||||
| > 30 ≤ 35 Ncm | 32 | 0.94 | 0.78 | 0.064 |
| > 35 Ncm | 15 | 0.55 | 0.72 | |
| Prosthetic Delivery | 5-y Follow-Up | Significance (p) | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | D → 5y | |
| PD in mm | |||||||
| Mesial tooth | 35 | 2.19a | 0.74 | 35 | 2.56a | 1.41 | 0.005 |
| Implant | 47 | 2.67b | 0.75 | 48 | 3.27b | 0.64 | <0.001 * |
| Distal tooth | 26 | 2.38b | 0.63 | 24 | 2.44a | 0.64 | 0.787 |
| CAL in mm | |||||||
| Mesial tooth | 35 | 2.68ab | 1.00 | 35 | 2.8a | 0.85 | 0.293 |
| Implant | 47 | 2.64a | 0.92 | 48 | 2.68b | 0.80 | 0.986 |
| Distal tooth | 26 | 2.74b | 0.84 | 24 | 2.59a | 0.98 | 0.142 |
| GR in mm | |||||||
| Mesial tooth | 35 | 0.44a | 0.63 | 35 | 0.52a | 0.54 | 0.127 |
| Implant | 47 | 0.34a | 0.43 | 48 | 0.25b | 0.30 | 0.005 |
| Distal tooth | 26 | 0.54a | 0.59 | 24 | 0.46ab | 0.57 | 0.955 |
| mPI | |||||||
| Mesial tooth | 35 | 0.52a | 0.60 | 35 | 0.61a | 0.52 | 0.277 |
| Implant | 47 | 0.29b | 0.41 | 48 | 0.49b | 0.47 | <0.001 * |
| Distal tooth | 26 | 0.76c | 0.61 | 24 | 0.69a | 0.58 | 0.022 |
| mBI | |||||||
| Mesial tooth | 35 | 0.36a | 0.39 | 35 | 0.20a | 0.29 | <0.001 |
| Implant | 47 | 0.51a | 0.41 | 48 | 0.87b | 0.54 | <0.001 * |
| Distal tooth | 26 | 0.43a | 0.42 | 24 | 0.38c | 0.55 | 0.708 |
| Assessment Parameter | Pre | D | 1-Year Follow-Up | 5-Year Follow-Up | Significance (p) | |||
|---|---|---|---|---|---|---|---|---|
| Pre → D | D →1 year | 1 year → 5 years | ||||||
| Function | n | 40 | 34 | 37 | 35 | |||
| Mean (%) | 65.3 | 86 | 94.7 | 95 | <0.0001 | 0.001 | 0.220 | |
| SD | 27.8 | 19.7 | 9.8 | 9.1 | ||||
| Esthetics | n | 40 | 34 | 37 | 35 | |||
| Mean (%) | 61.5 | 84.8 | 87.7 | 91.9 | <0.0001 | 0.313 | 0.012 | |
| SD | 29.5 | 16.5 | 17.1 | 12.2 | ||||
| Sense | n | 40 | 34 | 37 | 35 | |||
| Mean (%) | 33.9 | 81 | 83.8 | 92.6 | <0.0001 | 0.606 | 0.001 | |
| SD | 33.8 | 26.7 | 23.5 | 10.1 | ||||
| Speech | n | 40 | 34 | 37 | 35 | |||
| Mean (%) | 85.1 | 92.7 | 96.9 | 96.8 | 0.026 | 0.020 | 0.689 | |
| SD | 19.5 | 12.3 | 7.5 | 3.5 | ||||
| Self-esteem | n | 40 | 34 | 37 | 35 | |||
| Mean (%) | 85.2 | 93.5 | 96.8 | 95.9 | 0.032 | 0.004 | 0.491 | |
| SD | 23.1 | 9.2 | 7.3 | 8 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohal, R.-J.; Spies, B.C.; Vach, K.; Balmer, M.; Pieralli, S. A Prospective Clinical Cohort Investigation on Zirconia Implants: 5-Year Results. J. Clin. Med. 2020, 9, 2585. https://doi.org/10.3390/jcm9082585
Kohal R-J, Spies BC, Vach K, Balmer M, Pieralli S. A Prospective Clinical Cohort Investigation on Zirconia Implants: 5-Year Results. Journal of Clinical Medicine. 2020; 9(8):2585. https://doi.org/10.3390/jcm9082585
Chicago/Turabian StyleKohal, Ralf-Joachim, Benedikt Christopher Spies, Kirstin Vach, Marc Balmer, and Stefano Pieralli. 2020. "A Prospective Clinical Cohort Investigation on Zirconia Implants: 5-Year Results" Journal of Clinical Medicine 9, no. 8: 2585. https://doi.org/10.3390/jcm9082585
APA StyleKohal, R.-J., Spies, B. C., Vach, K., Balmer, M., & Pieralli, S. (2020). A Prospective Clinical Cohort Investigation on Zirconia Implants: 5-Year Results. Journal of Clinical Medicine, 9(8), 2585. https://doi.org/10.3390/jcm9082585








