Epidemiological Features of the Bladder Neck Rest Position and Mobility
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pomian, A.; Majkusiak, W.; Kociszewski, J.; Tomasik, P.; Horosz, E.; Zwierzchowska, A.; Lisik, W.; Barcz, E. Demographic features of female urethra length. Neurourol. Urodyn. 2018, 37, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Huisman, A.B. Aspects on the anatomy of the female urethra with special relation to urinary continence. Contrib. Gynecol. Obstet. 1983, 10, 1–31. [Google Scholar] [PubMed]
- Ashton-Miller, J.A.; DeLancey, J.O. Functional anatomy of the female pelvic floor. Ann. N. Y. Acad. Sci. 2007, 1101, 266–296. [Google Scholar] [CrossRef] [PubMed]
- Pirpiris, A.; Shek, K.L.; Dietz, H.P. Urethral mobility and urinary incontinence. Ultrasound Obstet. Gynecol. 2010, 36, 507–511. [Google Scholar] [CrossRef]
- DeLancey, J.O.; Trowbridge, E.R.; Miller, J.M.; Morgan, D.M.; Guire, K.; Fenner, D.E.; Weadock, W.J.; Ashton-Miller, J.A. Stress urinary incontinence: Relative importance of urethral support and urethral closure pressure. J. Urol. 2008, 179, 2286–2290. [Google Scholar] [CrossRef]
- Jundt, K.; Scheer, I.; Schiessl, B.; Karl, K.; Friese, K.; Peschers, U.M. Incontinence, bladder neck mobility, and sphincter ruptures in primiparous women. Eur. J. Med. Res. 2010, 15, 246–252. [Google Scholar] [CrossRef]
- DeLancey, J.O. Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am. J. Obstet. Gynecol. 1994, 170, 1713–1720. [Google Scholar] [CrossRef]
- Petros, P.E.; Ulmsten, U.I. An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet. Gynecol. Scand. Suppl. 1990, 153, 7–31. [Google Scholar] [CrossRef]
- Volløyhaug, I.; van Gruting, I.; van Delft, K.; Sultan, A.H.; Thakar, R. Is bladder Neck and urethral mobility associated with urinary incontinence and mode of delivery 4 years after childbirth? Neurourol. Urodyn. 2017, 36, 1403–1410. [Google Scholar] [CrossRef]
- Karram, M.M.; Bhatia, N.N. The Q-tip test: Standardization of the technique and its interpretation in women with urinary incontinence. Obstet. Gynecol. 1988, 71, 807–811. [Google Scholar]
- McGuire, E.J.; Lytton, B.; Pepe, V.; Kohorn, E.I. Stress Urinary Incontinence. Obstet. Gynecol. 1976, 47, 255–264. [Google Scholar] [PubMed]
- Hanzal, E.; Joura, E.M.; Haeusler, G.; Koelbl, H. Influence of catheterisation on the results of sonographic urethrocystography in patients with genuine stress incontinence. Arch. Gynecol. Obstet. 1994, 255, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Hol, M.; van Bolhuis, C.; Vierhout, M.E. Vaginal ultrasound studies of bladder neck mobility. Br. J. Obstet. Gynaecol. 1995, 102, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Pregazzi, R.; Sartore, A.; Bortoli, P.; Grimaldi, E.; Troiano, L.; Guaschino, S. Perineal ultrasound evaluation of urethral angle and bladder neck mobility in women with stress urinary incontinence. BJOG 2002, 109, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Van Geelen, H.; Ostergard, D.; Sand, P. A review of the impact of pregnancy and childbirth on pelvic floor function as assessed by objective measurement techniques. Int. Urogynecol. J. 2018, 29, 327–338. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, C.; Shek, K.L.; Martin, A.J.; Dietz, H.P. What is normal bladder Neck anatomy? Int. Urogynecol. J. 2016, 27, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Shek, K.L.; Kruger, J.; Dietz, H.P. The effect of pregnancy on hiatal dimensions and urethral mobility: An observational study. Int. Urogynecol. J. 2012, 23, 1561–1567. [Google Scholar] [CrossRef]
- Dietz, H.P.; Eldridge, A.; Grace, M.; Clarke, B. Does pregnancy affect pelvic organ mobility? Aust. N. Z. J. Obstet. Gynaecol. 2004, 44, 517–520. [Google Scholar] [CrossRef]
- Reed, H.; Freeman, R.M.; Waterfield, A.; Adekanmi, O. Prevalence of bladder Neck mobility in asymptomatic non-pregnant nulliparous volunteers. BJOG 2004, 111, 172–175. [Google Scholar] [CrossRef]
- Peschers, U.M.; Fanger, G.; Schaer, G.N.; Vodusek, D.B.; DeLancey, J.O.; Schuessler, B. Bladder neck mobility in continent nulliparous women. BJOG 2001, 108, 320–324. [Google Scholar]
- Tunn, R.; Albrich, S.; Beilecke, K.; Kociszewski, J.; Lindig-Knopke, C.; Reisenauer, C.; Schwertner-Tiepelmann, N.; Kuhn, A.; Viereck, V.; Bjelic Radisic, V.; et al. Interdisciplinary S2k guideline: Sonography in urogynecology: Short Version—AWMF registry number: 015/055. Geburtshilfe Frauenheilkd. 2014, 74, 1093–1098. [Google Scholar]
- Wlazlak, E.; Kociszewski, J.; Suzin, J.; Dresler, M.; Surkont, G. Urethral length measurement in women during sonographic urethrocystography—An analysis of repeatability and reproducibility. J. Ultrason. 2016, 16, 25–31. [Google Scholar] [PubMed]
- Whitcomb, E.L.; Lukacz, E.S.; Lawrence, J.M.; Nager, C.W.; Luber, K.M. Prevalence and degree of bother from pelvic floor disorders in obese women. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2009, 20, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Dell’Atti, L.; Galosi, A.B. Female Urethra Adenocarcinoma. Clin. Genitourin. Cancer 2018, 16, e263–e267. [Google Scholar] [CrossRef] [PubMed]
- Galosi, A.B.; Dell’Atti, L. Ultrasound Study of the Urethra. In Atlas of Ultrasonography in Urology, Andrology, and Nephrolog; Martino, P., Galosi, A.B., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 211–226. [Google Scholar]
- Dietz, H.P.; Eldridge, A.; Grace, M.; Clarke, B. Pelvic organ descent in young nulligravid women. Am. J. Obstet. Gynecol. 2004, 191, 95–99. [Google Scholar] [CrossRef]
- Dietz, H.P.; Hansell, N.K.; Grace, M.E.; Eldridge, A.M.; Clarke, B.; Martin, N.G. Bladder neck mobility is a heritable trait. BJOG 2005, 112, 334–339. [Google Scholar] [CrossRef]
- Matthews, C.A.; Whitehead, W.E.; Townsend, M.K.; Grodstein, F. Risk factors for urinary, fecal, or dual incontinence in the Nurses’ Health Study. Obstet. Gynecol. 2013, 122, 539–545. [Google Scholar] [CrossRef]
- Rortveit, G.; Daltveit, A.K.; Hannestad, Y.S.; Hunskaar, S. Norwegian EPINCONT Study. Urinary incontinence after vaginal delivery or cesarean section. N. Engl. J. Med. 2003, 348, 900–907. [Google Scholar] [CrossRef]
- Toozs-Hobson, P.; Balmforth, J.; Cardozo, L.; Khullar, V.; Athanasiou, S. The effect of mode of delivery on pelvic floor functional anatomy. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2008, 19, 407–416. [Google Scholar] [CrossRef]
- Dietz, H.P.; Bennett, M.J. The effect of childbirth on pelvic organ mobility. Obstet. Gynecol. 2003, 102, 223–228. [Google Scholar] [CrossRef]
- Zhu, L.; Lang, J.; Wang, H.; Han, S.; Huang, J. The prevalence of and potential risk factors for female urinary incontinence in Beijing, China. Menopause 2008, 15, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.K.; Danforth, K.N.; Rosner, B.; Curhan, G.C.; Resnick, N.M.; Grodstein, F. Body mass index, weight gain, and incident urinary incontinence in middle-aged women. Obstet. Gynecol. 2007, 110, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Khullar, V.; Sexton, C.C.; Thompson, C.L.; Milsom, I.; Bitoun, C.E.; Coyne, K.S. The relationship between BMI and urinary incontinence subgroups: Results from EpiLUTS. Neurourol. Urodyn. 2014, 33, 392–399. [Google Scholar] [CrossRef] [PubMed]
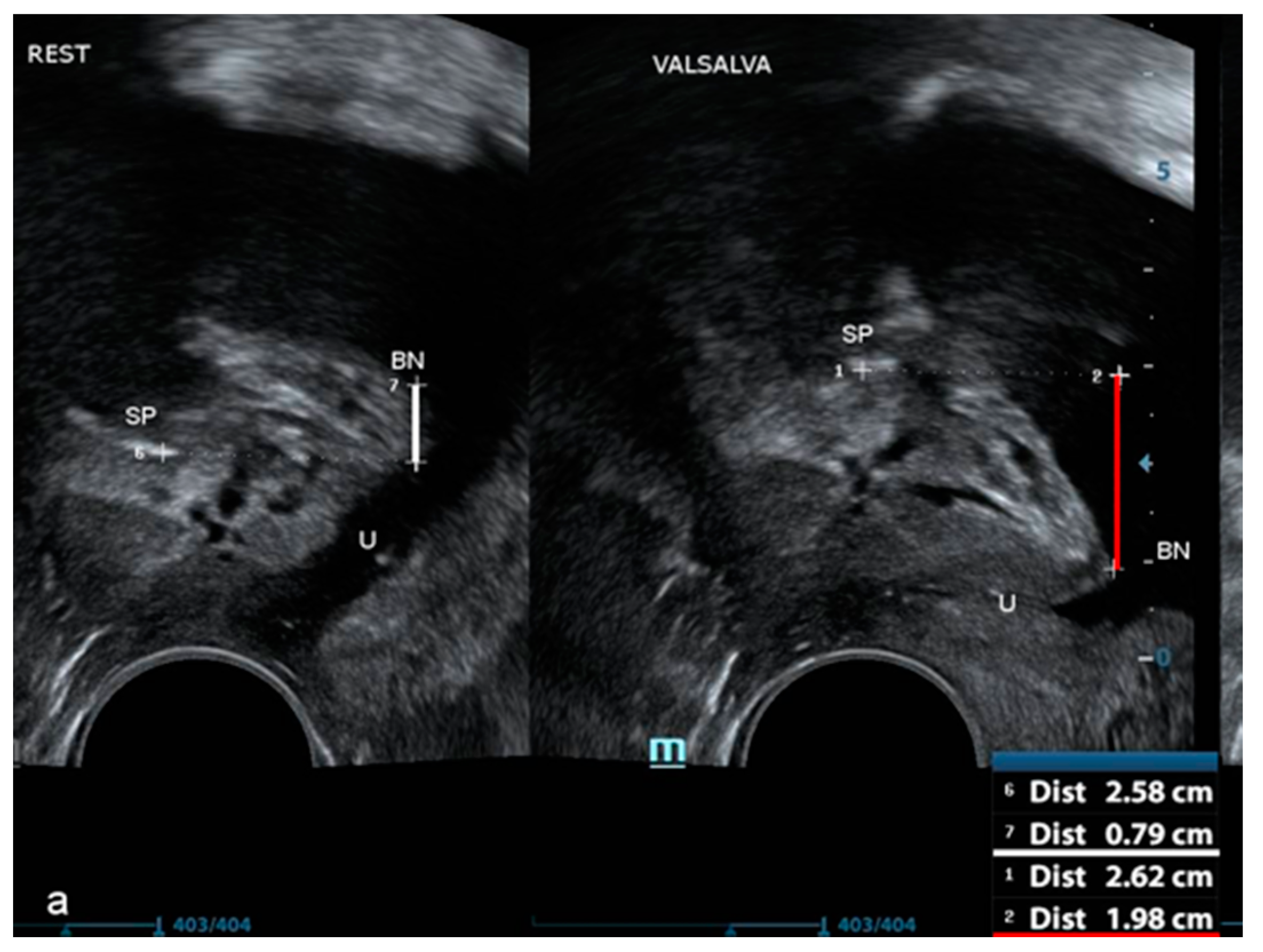




| Variable | Mean | Minimum | Maximum | Standard Deviation |
|---|---|---|---|---|
| Age (years) | 53.4 | 18.0 | 85.0 | 13.3 |
| Height (cm) | 164.2 | 145.0 | 190.0 | 6.1 |
| Weight (kg) | 85.6 | 44.0 | 176.0 | 25.3 |
| BMI | 31.7 | 16.6 | 70.5 | 8.8 |
| No of deliveries | 1.9 | 0.0 | 10.0 | 1.1 |
| No of VDs | 1.7 | 0.0 | 10.0 | 1.2 |
| No of cesarean sections | 0.2 | 0.0 | 3.0 | 0.5 |
| No of vacuum/forceps deliveries | 0.0 | 0.0 | 2.0 | 0.1 |
| Average birth weight (VDs only) (g) | 3432 | 650 | 5050 | 500 |
| Maximum birth weight (VDs only) (g) | 3602 | 650 | 5850 | 555 |
| Cumulative birth weight (VDs only) (g) | 7045 | 650 | 30,800 | 3460 |
| Age at 1st VD (years) | 23.9 | 14.0 | 40.0 | 3.9 |
| Age at 2nd VD (years) | 27.5 | 15.0 | 43.0 | 4.6 |
| Age at last VD (years) | 28.6 | 17.0 | 46.0 | 5.3 |
| Urethral length (mm) | 30.2 | 19.0 | 48.1 | 4.3 |
| BN position at rest (mm) | 10.0 | −31.8 | 29.9 | 7.0 |
| BN position during the VM | −4.8 | −37.1 | 24.8 | 9.3 |
| BN mobility (rest–VM) (mm) | 14.9 | 0.3 | 42.9 | 7.1 |
| BN | At Rest [mm] | During the VM [mm] | BN Mobility (rest-VM) [mm] | |
|---|---|---|---|---|
| Position Patients | ||||
| Whole Group n = 796 | 10.0 ± 7.0 | −4.8 ± 9.3 | 14.9 ± 7.1 | |
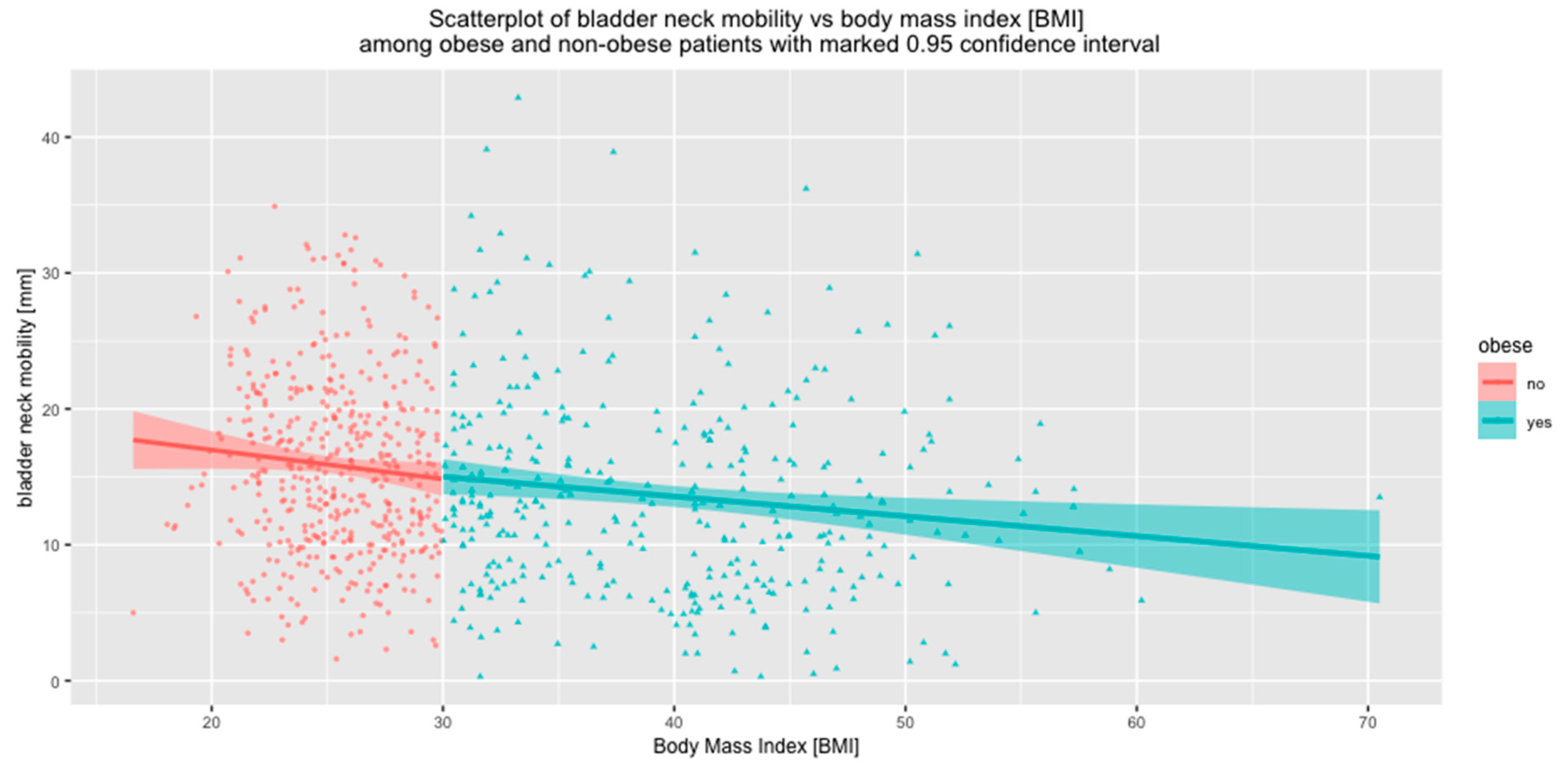
| Obese n = 352 | 11.7 ± 6.8 | −2.0 ± 9.6 | 13.6 ± 7.4 | |
| Non-Obese n = 444 | 8.7 ± 6.9 | −7.1 ± 8.5 | 15.8 ± 6.6 | |
| p | <0.001 | <0.001 | <0.001 | |
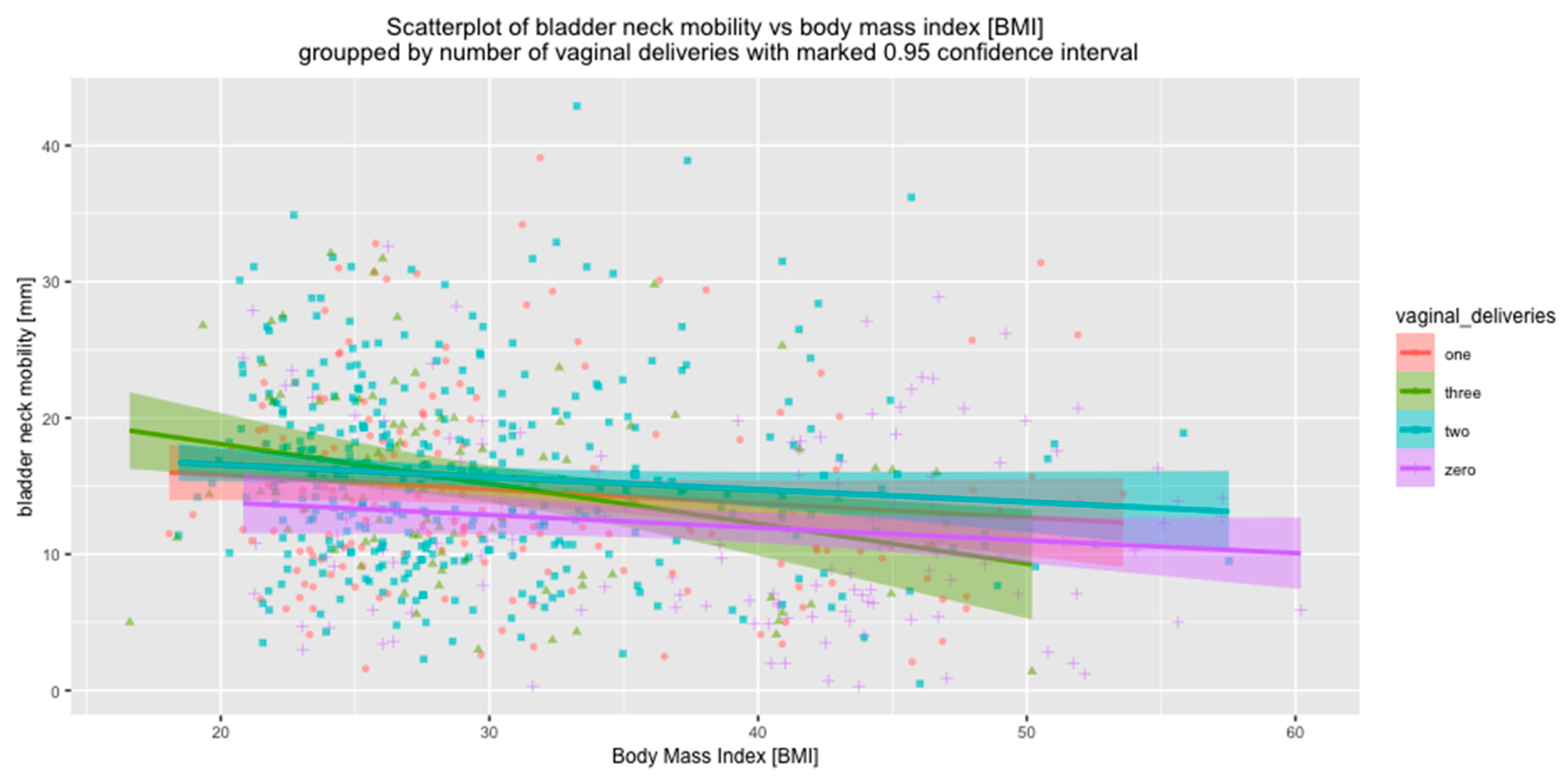
| No of VDs = 0 n = 146 | 13.4 ± 6.0 | 1.3 ± 8.5 | 12.1 ± 6.7 | |
| No of VDs > 0 n = 650 | 9.2 ± 7.0 | −6.2 ± 8.9 | 15.5 ± 7.0 | |
| p | <0.001 | <0.001 | <0.001 | |
| <65 years n = 613 | 11.1 ± 6.4 | −4.1 ± 9.2 | 15.1 ± 7.2 | |
| ≥65 years n = 182 | 6.4 ± 7.7 | −7.4 ± 9.1 | 13.8 ± 6.3 | |
| p | <0.001 | <0.001 | <0.05 | |
| Obese with VD = 0 n = 102 | 14.5 ± 5.9 | 3.3 ± 7.8 | 11.2 ± 6.3 | |
| Obese with VD > 0 n = 250 | 10.5 ± 6.8 | −4.1 ± 9.3 | 14.6 ± 7.6 | |
| p | <0.001 | <0.001 | <0.001 | |
| Non-obese with VD = 0 n = 44 | 10.8 ± 5.4 | −3.4 ± 8.0 | 14.2 ± 7.2 | |
| Non-obese with VD > 0 n = 400 | 8.5 ± 7.0 | −7.6 ± 8.4 | 16 ± 6.5 | |
| p | <0.05 | <0.01 | Ns | |
| Obese < 65 years n = 281 | 12.8 ± 6.3 | −1.1 ± 9.6 | 13.9 ± 7.8 | |
| Obese ≥ 65 years n = 71 | 7.0 ± 6.8 | −5.5 ± 8.1 | 12.5 ± 5.5 | |
| p | <0.001 | <0.001 | Ns | |
| Non-obese < 65 years n = 332 | 9.6 ± 6.2 | −6.6 ± 8.1 | 16.2 ± 6.5 | |
| Non-obese ≥ 65 years n = 111 | 6.1 ± 8.2 | −8.6 ± 9.4 | 14.7 ± 6.6 | |
| p | <0.001 | <0.05 | <0.05 | |
| BN Position | At Rest [mm] | During the VM [mm] | BN Mobility (Rest-VM) [mm] | |
|---|---|---|---|---|
| Patients | ||||
| Not scheduled for anti-incontinence surgery n = 426 | 11.1 ± 7.7 | −2.7 ± 10.3 | 13.8 ± 7.2 | |
| Scheduled for anti-incontinence surgery n = 370 | 8.7 ± 5.8 | −7.4 ± 7.2 | 16.1 ± 6.8 | |
| p | p < 0.001 | p < 0.001 | p < 0.001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horosz, E.; Pomian, A.; Zwierzchowska, A.; Lisik, W.; Majkusiak, W.; Tomasik, P.; Rutkowska, B.; Skalska, J.; Siemion, M.; Banasiuk, D.; et al. Epidemiological Features of the Bladder Neck Rest Position and Mobility. J. Clin. Med. 2020, 9, 2413. https://doi.org/10.3390/jcm9082413
Horosz E, Pomian A, Zwierzchowska A, Lisik W, Majkusiak W, Tomasik P, Rutkowska B, Skalska J, Siemion M, Banasiuk D, et al. Epidemiological Features of the Bladder Neck Rest Position and Mobility. Journal of Clinical Medicine. 2020; 9(8):2413. https://doi.org/10.3390/jcm9082413
Chicago/Turabian StyleHorosz, Edyta, Andrzej Pomian, Aneta Zwierzchowska, Wojciech Lisik, Wojciech Majkusiak, Paweł Tomasik, Beata Rutkowska, Joana Skalska, Małgorzata Siemion, Dominika Banasiuk, and et al. 2020. "Epidemiological Features of the Bladder Neck Rest Position and Mobility" Journal of Clinical Medicine 9, no. 8: 2413. https://doi.org/10.3390/jcm9082413
APA StyleHorosz, E., Pomian, A., Zwierzchowska, A., Lisik, W., Majkusiak, W., Tomasik, P., Rutkowska, B., Skalska, J., Siemion, M., Banasiuk, D., & Barcz, E. (2020). Epidemiological Features of the Bladder Neck Rest Position and Mobility. Journal of Clinical Medicine, 9(8), 2413. https://doi.org/10.3390/jcm9082413






