Impact of Women Obesity and Obesity Severity on Live Birth Rate after In Vitro Fertilization
Abstract
1. Introduction
2. Experimental Section
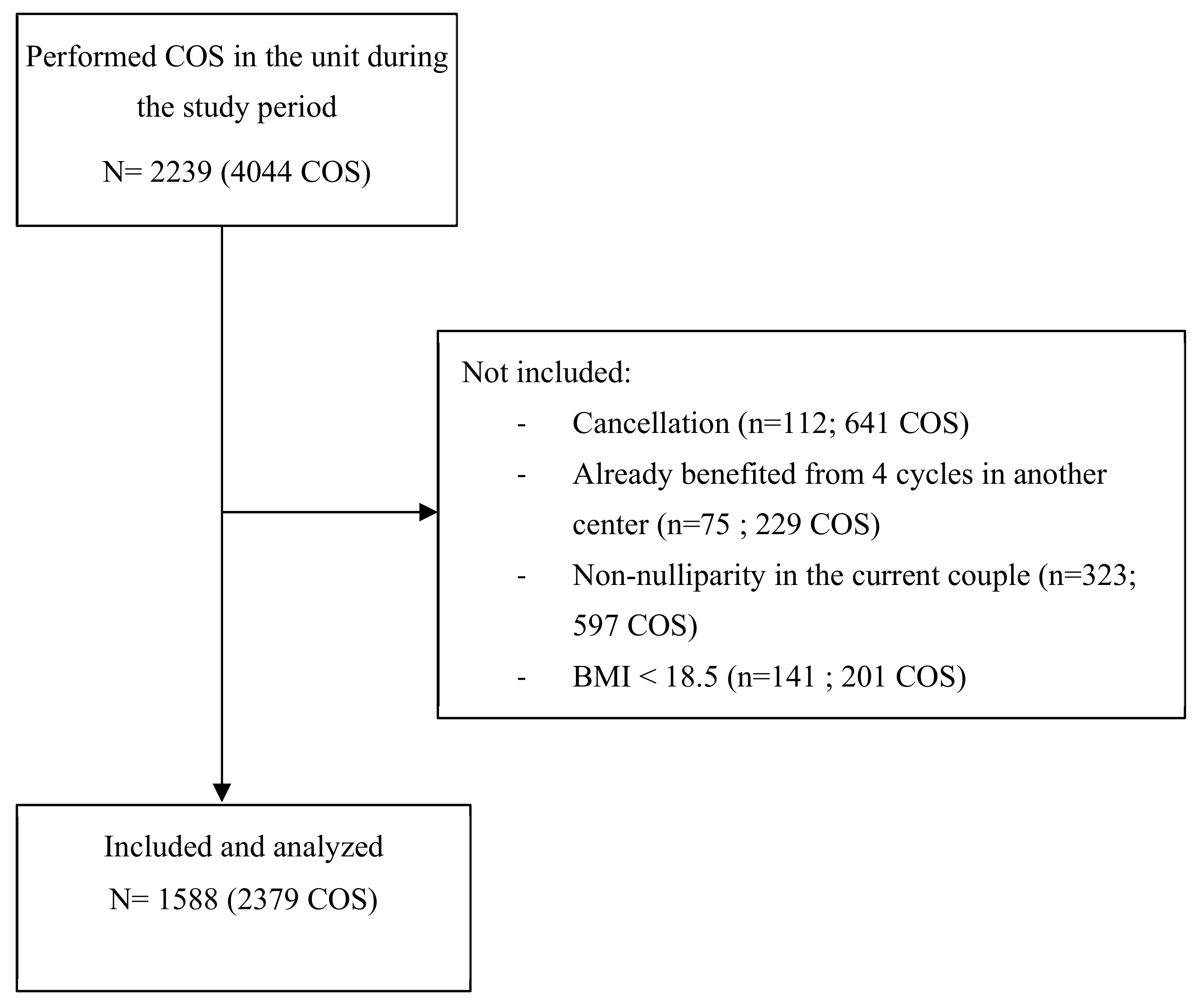
2.1. Population and Design
2.2. IVF Procedure
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. COS Characteristics
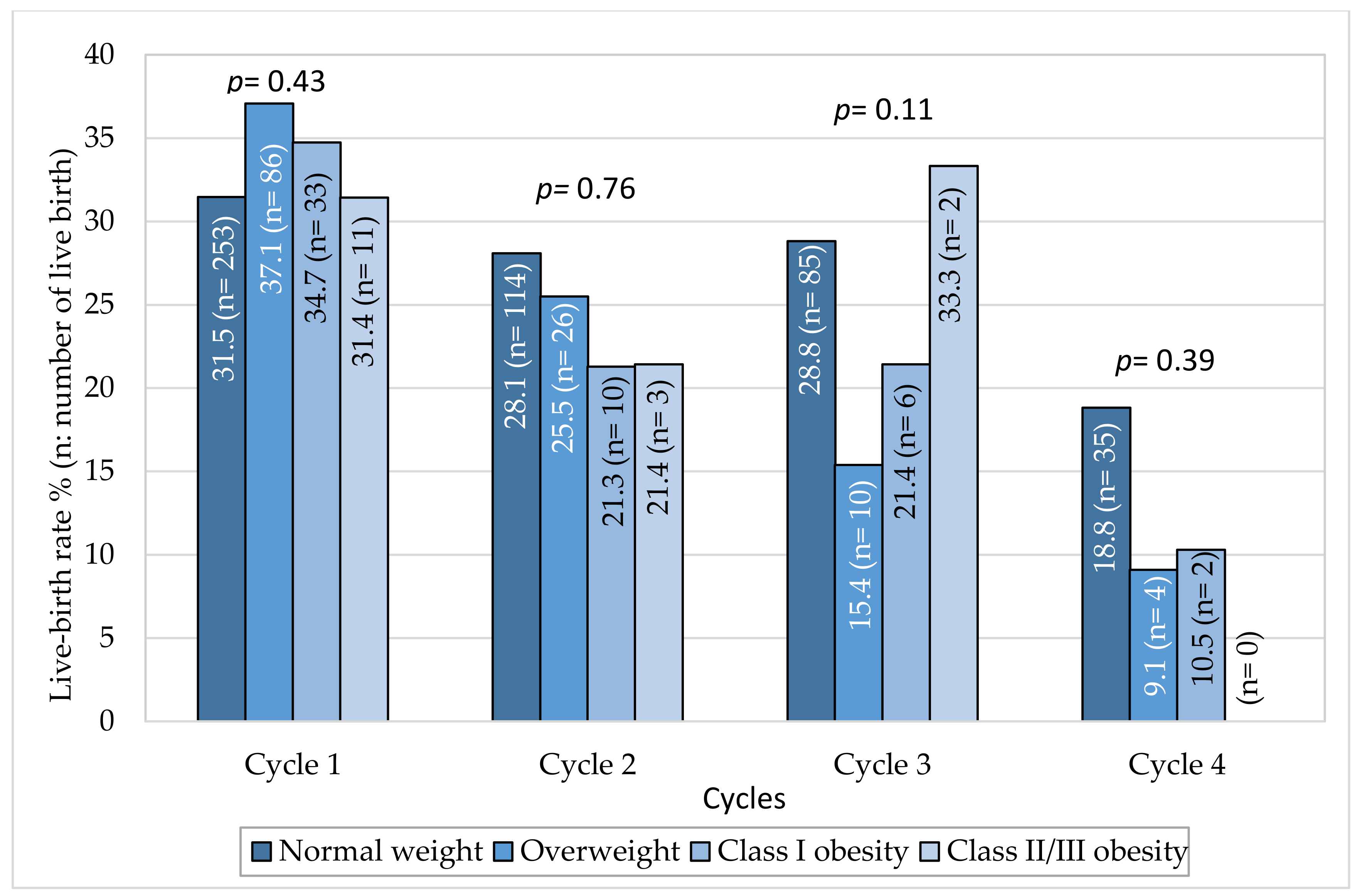
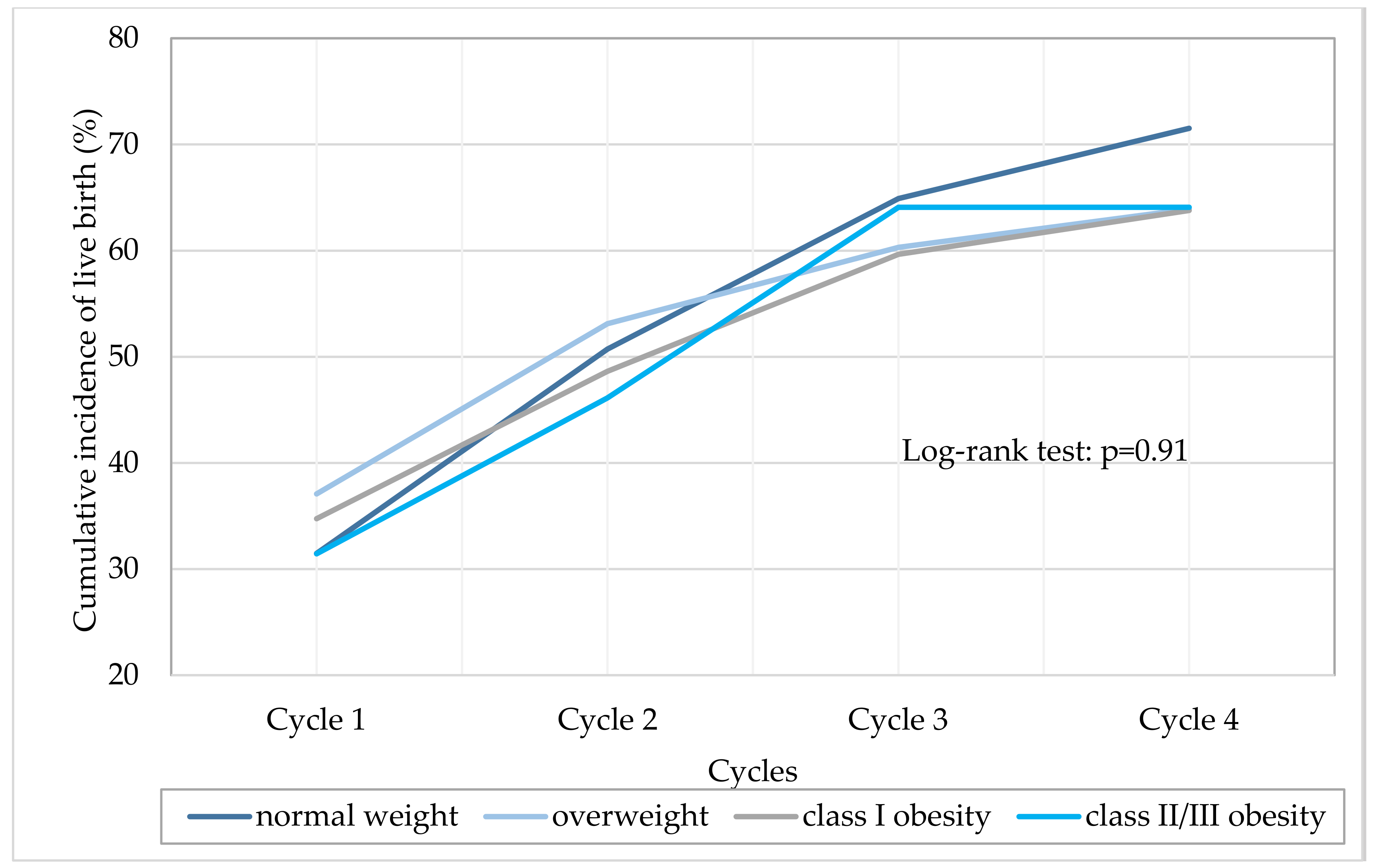
3.3. Impact of Obesity on Live Birth Rate Per Cycle and on Cumulative Live Birth Rate
3.4. Impact of Obesity on Rank of Transfer, Fresh, or Frozen Embryo Transfer and Miscarriage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hassan, M.A.; Killick, S.R. Negative lifestyle is associated with a significant reduction in fecundity. Fertil. Steril. 2004, 81, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Rothman, K.J.; Mikkelsen, E.M.; Sorensen, H.T.; Riis, A.; Hatch, E.E. An internet-based prospective study of body size and time-to-pregnancy. Hum. Reprod. 2010, 25, 253–264. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health Care Excellence. Fertility Problems: Assessment and Treatment; NICE: London, UK, 2013. [Google Scholar]
- Farquhar, C.M.; Gillett, W.R. Prioritising for fertility treatments-should a high BMI exclude treatment? BJOG 2006, 113, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- RANZCOG (The Royal Australian and New Zealand College of Obstetricians and Gynaecologists). Ovarian Stimulation in Assisted Reproduction; RANZCOG: Melbourne, Australia, 2014. [Google Scholar]
- Provost, M.P.; Acharya, K.S.; Acharya, C.R.; Yeh, J.S.; Steward, R.G.; Eaton, J.L.; Goldfarb, J.M.; Muasher, S.J. Pregnancy outcomes decline with increasing body mass index: Analysis of 239,127 fresh autologous in vitro fertilization cycles from the 2008–2010 Society for Assisted Reproductive Technology registry. Fertil. Steril. 2016, 105, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Sarais, V.; Pagliardini, L.; Rebonato, G.; Papaleo, E.; Candiani, M.; Vigano, P. A Comprehensive Analysis of Body Mass Index Effect on in Vitro Fertilization Outcomes. Nutrients 2016, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Friedler, S.; Cohen, O.; Liberty, G.; Saar-Ryss, B.; Meltzer, S.; Lazer, T. Should high BMI be a reason for IVF treatment denial? Gynecol. Endocrinol. 2017, 33, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhang, F.L.; Liu, X.C.; Hu, L.L.; Dai, S.J.; Li, G.; Kong, H.J.; Guo, Y.H. Impact of Female Obesity on Cumulative Live Birth Rates in the First Complete Ovarian Stimulation Cycle. Front. Endocrinol. Lausanne 2019, 10, 516. [Google Scholar] [CrossRef]
- Bellver, J.; Ayllon, Y.; Ferrando, M.; Melo, M.; Goyri, E.; Pellicer, A.; Remohi, J.; Meseguer, M. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil. Steril. 2010, 93, 447–454. [Google Scholar] [CrossRef]
- Zander-Fox, D.L.; Henshaw, R.; Hamilton, H.; Lane, M. Does obesity really matter? The impact of BMI on embryo quality and pregnancy outcomes after IVF in women aged ≤38 years. Aust. N. Z. J. Obs. Gynaecol. 2012, 52, 270–276. [Google Scholar] [CrossRef]
- Kawwass, J.F.; Kulkarni, A.D.; Hipp, H.S.; Crawford, S.; Kissin, D.M.; Jamieson, D.J. Extremities of body mass index and their association with pregnancy outcomes in women undergoing in vitro fertilization in the United States. Fertil. Steril. 2016, 106, 1742–1750. [Google Scholar] [CrossRef]
- Provost, M.P.; Acharya, K.S.; Acharya, C.R.; Yeh, J.S.; Steward, R.G.; Eaton, J.L.; Goldfarb, J.M.; Muasher, S.J. Pregnancy outcomes decline with increasing recipient body mass index: An analysis of 22,317 fresh donor/recipient cycles from the 2008–2010 Society for Assisted Reproductive Technology Clinic Outcome Reporting System registry. Fertil. Steril. 2016, 105, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Bellver, J.; Pellicer, A.; Garcia-Velasco, J.A.; Ballesteros, A.; Remohi, J.; Meseguer, M. Obesity reduces uterine receptivity: Clinical experience from 9587 first cycles of ovum donation with normal weight donors. Fertil. Steril. 2013, 100, 1050–1058. [Google Scholar] [CrossRef]
- Sermondade, N.; Huberlant, S.; Bourhis-Lefebvre, V.; Arbo, E.; Gallot, V.; Colombani, M.; Freour, T. Female obesity is negatively associated with live birth rate following IVF: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Caillon, H.; Freour, T.; Bach-Ngohou, K.; Colombel, A.; Denis, M.G.; Barriere, P.; Masson, D. Effects of female increased body mass index on in vitro fertilization cycles outcome. Obes. Res. Clin. Pr. 2015, 9, 382–388. [Google Scholar] [CrossRef]
- Comstock, I.A.; Kim, S.; Behr, B.; Lathi, R.B. Increased body mass index negatively impacts blastocyst formation rate in normal responders undergoing in vitro fertilization. J. Assist. Reprod. Genet. 2015, 32, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Legge, A.; Bouzayen, R.; Hamilton, L.; Young, D. The impact of maternal body mass index on in vitro fertilization outcomes. J. Obs. Gynaecol. Can. 2014, 36, 613–619. [Google Scholar] [CrossRef]
- Pinborg, A.; Gaarslev, C.; Hougaard, C.O.; Nyboe-Andersen, A.; Andersen, P.K.; Boivin, J.; Schmidt, L. Influence of female bodyweight on IVF outcome: A longitudinal multicentre cohort study of 487 infertile couples. Reprod. Biomed. Online 2011, 23, 490–499. [Google Scholar] [CrossRef]
- Coyne, K.; Whigham, L.D.; O’Leary, K.; Yaklic, J.K.; Maxwell, R.A.; Lindheim, S.R. Gestational carrier BMI and reproductive, fetal and neonatal outcomes: Are the risks the same with increasing obesity? Int. J. Obes. Lond. 2016, 40, 171–175. [Google Scholar] [CrossRef]
- Moragianni, V.A.; Jones, S.M.; Ryley, D.A. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil. Steril. 2012, 98, 102–108. [Google Scholar] [CrossRef]
- Luke, B.; Brown, M.B.; Stern, J.E.; Missmer, S.A.; Fujimoto, V.Y.; Leach, R.; Group, S.W. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum. Reprod. 2011, 26, 245–252. [Google Scholar] [CrossRef]
- Tremellen, K.; Wilkinson, D.; Savulescu, J. Should obese women’s access to assisted fertility treatment be limited? A scientific and ethical analysis. Aust. N. Z. J. Obs. Gynaecol. 2017, 57, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Rittenberg, V.; Seshadri, S.; Sunkara, S.K.; Sobaleva, S.; Oteng-Ntim, E.; El-Toukhy, T. Effect of body mass index on IVF treatment outcome: An updated systematic review and meta-analysis. Reprod. Biomed. Online 2011, 23, 421–439. [Google Scholar] [CrossRef]
- Martinuzzi, K.; Ryan, S.; Luna, M.; Copperman, A.B. Elevated body mass index (BMI) does not adversely affect in vitro fertilization outcome in young women. J. Assist. Reprod. Genet. 2008, 25, 169–175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ozekinci, M.; Seven, A.; Olgan, S.; Sakinci, M.; Keskin, U.; Akar, M.E.; Ceyhan, S.T.; Ergun, A. Does obesity have detrimental effects on IVF treatment outcomes? BMC Womens Health 2015, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, S.; Bergh, C.; Friberg, B.; Pinborg, A.; Klajnbard, A.; Karlstrom, P.O.; Kluge, L.; Larsson, I.; Loft, A.; Mikkelsen-Englund, A.L.; et al. Weight reduction intervention for obese infertile women prior to IVF: A randomized controlled trial. Hum. Reprod. 2017, 32, 1621–1630. [Google Scholar] [CrossRef]
- Kluge, L.; Bergh, C.; Einarsson, S.; Pinborg, A.; Mikkelsen-Englund, A.L.; Thurin-Kjellberg, A. Cumulative live birth rates after weight reduction in obese women scheduled for IVF: Follow-up of a randomized controlled trial. Hum. Reprod. Open 2019, 2019, hoz030. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Lawlor, D.A. Predicting live birth, preterm delivery, and low birth weight in infants born from in vitro fertilisation: A prospective study of 144,018 treatment cycles. PLoS Med. 2011, 8, e1000386. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Cnattingius, S.; Naslund, I.; Roos, N.; Trolle-Lagerros, Y.; Granath, F.; Stephansson, O.; Neovius, M. Outcomes of pregnancy after bariatric surgery. N. Engl. J. Med. 2015, 372, 814–824. [Google Scholar] [CrossRef]
- Pandey, S.; Maheshwari, A.; Bhattacharya, S. Should access to fertility treatment be determined by female body mass index? Hum. Reprod. 2010, 25, 815–820. [Google Scholar] [CrossRef][Green Version]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
| Variables | Normal Weight(n = 1115) | Overweight(n = 309) | Class I Obesity(n = 126) | Class II/III Obesity(n = 38) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 33.3 ± 4.9 | 32.6 ± 5.6 | 33.5 ± 5.7 | 32.7 ± 4.8 | 0.32 |
| Smoking (%) | 26.2 | 21.2 | 22.7 | 20 | 0.28 |
| Infertility etiology | |||||
| Dysovulation (%) | 7.5 | 16.8 | 16.7 | 31.6 | <0.001 |
| Tubal factor (%) | 14.1 | 15.5 | 16.7 | 18.4 | 0.72 |
| Endometriosis (%) | 21.6 | 15.9 | 8.7 | 7.9 | <0.001 |
| PCOS (%) | 6.6 | 13.3 | 15.9 | 21 | <0.001 |
| Male infertility (%) | 55.7 | 57.3 | 63.5 | 65.8 | 0.25 |
| Idiopathic (%) | 13.5 | 9.1 | 10.3 | 0 | 0.015 |
| Other (%) | 1.5 | 1.9 | 1.6 | 0 | 0.91 |
| Gestity and parity | |||||
| Gestity | 0.48 ± 0.98 | 0.59 ± 1.06 | 0.57 ± 1.00 | 0.53 ± 1.2 | 0.26 |
| Parity | 0.10 ± 0.44 | 0.11 ± 0.43 | 0.17 ± 0.57 | 0.05 ± 0.23 | 0.24 |
| Ovarian reserve | |||||
| AMH | 3.6 ± 3.4 | 4.1 ± 4.5 | 3.4 ± 2.4 | 4.7 ± 3.8 | 0.005 |
| FSH | 7.5 ± 4.5 | 7.0 ± 4.3 | 6.6 ± 1.7 | 5.9 ± 1.6 | <0.001 |
| Cycle 1 | Cycle 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal Weight(n = 804) | Over-Weight(n = 232) | Class I Obesity(n = 95) | Class II/III Obesity(n = 35) | p- Value | Normal Weight(n = 406) | Over-Weight(n = 102) | Class I Obesity(n = 47) | Class II/III Obesity(n = 14) | p-Value | |
| Protocol (%) | ||||||||||
| Agonist | 44.7 | 50.4 | 45.3 | 40 | 0.41 | 37.9 | 42.2 | 44.7 | 42.9 | 0.73 |
| Antagonist | 55.3 | 49.6 | 54.7 | 60 | 62.1 | 57.8 | 55.3 | 57.1 | ||
| ART technique (%) | ||||||||||
| IVF | 40.8 | 40.7 | 39.8 | 25 | 0.36 | 24.4 | 23.7 | 15.6 | 23.1 | 0.65 |
| ICSI | 59.2 | 59.3 | 60.2 | 75 | 75.6 | 76.3 | 84.4 | 76.9 | ||
| Gonadotrophin | ||||||||||
| Starting dose (UI) | 206 ± 74 | 199 ± 72 | 231 ± 61 | 236 ± 75 | <0.001 | 226 ± 75 | 223 ± 76 | 247 ± 71 | 261 ± 78 | 0.19 |
| Total dose (UI) | 2138 ± 850 | 2158 ± 884 | 2449 ± 774 | 2624 ± 992 | <0.001 | 2275 ± 871 | 2307 ± 909 | 2689 ± 972 | 3138 ± 1346 | 0.009 |
| Duration (days) | 10.3 ± 1.3 | 10.6 ± 1.6 | 10.4 ± 1.4 | 10.8 ± 1.6 | 0.05 | 10.1 ± 1.4 | 10.2 ± 1.6 | 10.6 ± 1.9 | 11.5 ± 2.5 | 0.009 |
| Number of oocytes collected | 10.6 ± 6.0 | 10.7 ± 5.9 | 10.9 ± 6.2 | 10.8 ± 6.1 | 0.88 | 9.8 ± 5.7 | 10.3 ± 5.4 | 10.7 ± 6.3 | 8.7 ± 5.0 | 0.45 |
| Number of mature oocytes | 5.1 ± 5.8 | 5.1 ± 5.4 | 5.3 ± 5.9 | 5.9 ± 5.8 | 0.62 | 5.7 ± 5.1 | 6.1 ± 5.5 | 6.5 ± 4.9 | 5.6 ± 5.1 | 0.66 |
| Number of embryos obtained at day 2 | 5.8 ± 4.4 | 5.6 ± 3.9 | 5.0 ± 4.1 | 5.2 ± 4.7 | 0.31 | 5.2 ± 3.9 | 5.4 ± 4.2 | 4.0 ± 3.3 | 5.6 ± 4.7 | 0.19 |
| Number of fresh transferred embryos | 1.2 ± 0.8 | 1.1 ± 0.8 | 1.3 ± 0.8 | 0.9 ± 0.9 | 0.006 | 1.4 ± 0.9 | 1.4 ± 0.9 | 1.3 ± 0.9 | 1.2 ± 0.8 | 0.45 |
| Total number of transferred embryos | 1.9 ± 1.2 | 1.8 ± 1.1 | 1.8 ± 1.2 | 1.5 ± 1.1 | 0.04 | 2.0 ± 1.3 | 2.2 ± 1.3 | 1.6 ± 1.3 | 1.4 ± 0.8 | 0.006 |
| Adjusted OR [95% CI] ‡ | ||||||||
|---|---|---|---|---|---|---|---|---|
| BMI Classes | N | Cycle 1 | N | Cycle 2 | N | Cycle 3 | N | Cycle 4 |
| Normal weight | 804 | 1 | 406 | 1 | 295 | 1 | 186 | 1 |
| Overweight | 232 | 1.11 (0.78–1.58)p = 0.55 | 102 | 0.83 (0.47–1.46)p = 0.51 | 65 | 0.45 (0.20–1.03)p = 0.06 | 44 | 0.44 (0.12–1.56)p = 0.20 |
| Class I obesity | 95 | 1.17 (0.70–1.95)p = 0.55 | 47 | 0.68 (0.29–1.55)p = 0.36 | 28 | 0.97 (0.36–2.64)p = 0.95 | 19 | 0.59 (0.11–3.11)p = 0.54 |
| Class II/III obesity | 35 | 1.05 (0.48–2.31)p = 0.90 | 14 | 0.69 (0.14–3.37)p = 0.65 | 6 | 0.87 (0.08–9.39)p = 0.91 | 1 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunet, C.; Aouinti, S.; Huguet, F.; Macioce, V.; Ranisavljevic, N.; Gala, A.; Avignon, A.; Mura, T.; Sultan, A. Impact of Women Obesity and Obesity Severity on Live Birth Rate after In Vitro Fertilization. J. Clin. Med. 2020, 9, 2414. https://doi.org/10.3390/jcm9082414
Brunet C, Aouinti S, Huguet F, Macioce V, Ranisavljevic N, Gala A, Avignon A, Mura T, Sultan A. Impact of Women Obesity and Obesity Severity on Live Birth Rate after In Vitro Fertilization. Journal of Clinical Medicine. 2020; 9(8):2414. https://doi.org/10.3390/jcm9082414
Chicago/Turabian StyleBrunet, Cécile, Safa Aouinti, Fanchon Huguet, Valérie Macioce, Noémie Ranisavljevic, Anna Gala, Antoine Avignon, Thibault Mura, and Ariane Sultan. 2020. "Impact of Women Obesity and Obesity Severity on Live Birth Rate after In Vitro Fertilization" Journal of Clinical Medicine 9, no. 8: 2414. https://doi.org/10.3390/jcm9082414
APA StyleBrunet, C., Aouinti, S., Huguet, F., Macioce, V., Ranisavljevic, N., Gala, A., Avignon, A., Mura, T., & Sultan, A. (2020). Impact of Women Obesity and Obesity Severity on Live Birth Rate after In Vitro Fertilization. Journal of Clinical Medicine, 9(8), 2414. https://doi.org/10.3390/jcm9082414






