Physiotherapy for Prevention and Treatment of Fecal Incontinence in Women—Systematic Review of Methods
Abstract
1. Introduction
1.1. Factors Determining Fecal Continence
1.2. Physiotherapeutic Diagnosis in Fecal Incontinence
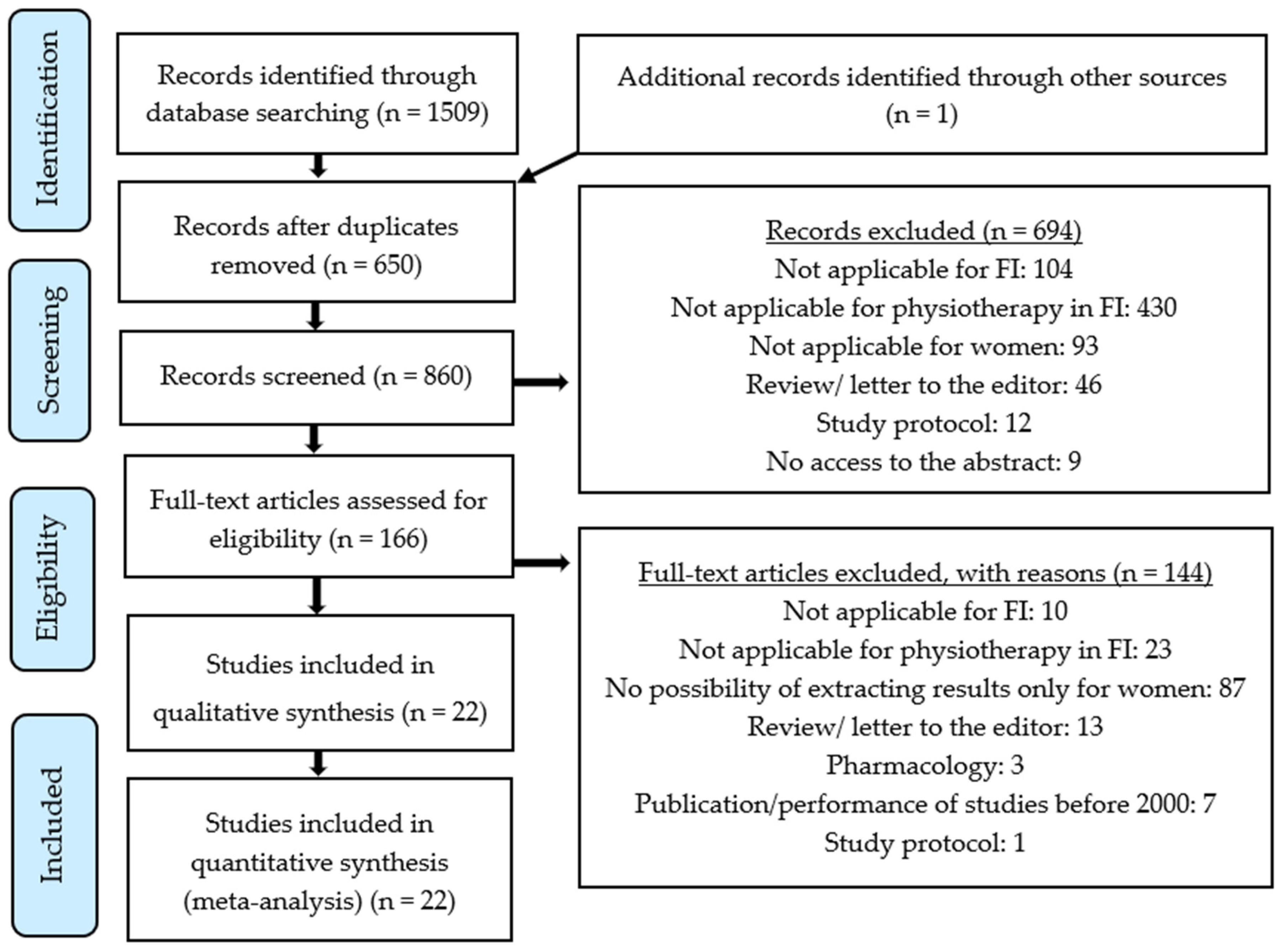
2. Materials and Methods
3. Results
4. Prevention of Fecal Incontinence
5. Physiotherapeutic Techniques for the Treatment of Fecal Incontinence
5.1. Pelvic Floor and Anal Muscle Training for the Treatment of Fecal Incontinence
5.2. PFM Training with Biofeedback for the Treatment of Fecal Incontinence
5.3. PFM Electrostimulation for the Treatment of Fecal Incontinence
5.4. PFM Magnetic Stimulation for the Treatment of Fecal Incontinence
5.5. Reccomendations for Conservative FI Treatment
6. Limitation of the Study
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- D’Ancona, C.; Haylen, B.; Oelke, M.; Abranches-Monteiro, L.; Arnold, E.; Goldman, H.; Hamid, R.; Homma, Y.; Marcelissen, T.; Rademakers, K.; et al. The International Continence Society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol. Urodyn. 2019, 38, 433–477. [Google Scholar] [CrossRef]
- Arbuckle, J.L.; Parden, A.M.; Hoover, K.; Griffin, R.L.; Richter, H.E. Prevalence and Awareness of Pelvic Floor Disorders in Female Adolescents Seeking Gynecologic Care. J. Pediatr Adolesc. Gynecol. 2019, 32, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Thubert, T.; Cardaillac, C.; Fritel, X.; Winer, N.; Dochez, V. Definition, epidemiology and risk factors of obstetric anal sphincter injuries: CNGOF Perineal Prevention and Protection in Obstetrics Guidelines. Gynecol. Obstet. Fertil. Senol. 2018, 46, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.K.; Saga, S.; Biekken, L.E.; Harris, R.; Goodman, C.; Norton, C. The Prevalence, Incidence, and Correlates of Fecal IncontinenceAmong Older People Residing in Care Homes: A Systematic Review. J. Am. Med. Dir. Assoc. 2019, 20, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Wald, A. Update on the management of fecal incontinence for the gastroenterologist. Gastroenterol. Hepatol. 2016, 12, 155–164. [Google Scholar]
- Chabkraborty, S.; Bharucha, A.E. Fecal Incontinence. In Gastrointestinal Motility Disorders; Springer: Cham, Switzerland, 2018; pp. 397–404. ISBN 978-3-319-59352-4. [Google Scholar] [CrossRef]
- Hunt, M.G.; Wong, C.; Aajmain, S.; Dawodu, I. Fecal incontinence in people with self-reported irritable bowel syndrome: Prevalence and quality of life. J. Psychosom. Res. 2018, 113, 45–51. [Google Scholar] [CrossRef]
- Alimohammadian, M.; Ahmadi, B.; Janani, L.; Mahjubi, B. Suffering in silence: A community-based study of fecal incontinence in women. Int. J. Colorectal Dis. 2014, 29, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Petros, P.; Swash, M. The Musculo-Elastic Theory of anorectal function and dysfunction. Pelviperineology 2008, 27, 89–93. [Google Scholar]
- Brochard, C.; Vénara, A.; Bodère, A.; Ropert, A.; Bouguen, G.; Siproudhis, L. Pathophysiology of fecal incontinence in obese patients: A prospective case-matched study of 201 patients. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Freeman, A.; Menees, S. Fecal Incontinence and Pelvic Floor Dysfunction in Women. Gastroenterol. Clin. N. Am. 2016, 45, 217–237. [Google Scholar] [CrossRef]
- Townsend, D.C.; Carrington, E.V.; Grossi, U.; Burgell, R.E.; Wong, J.Y.; Knowles, C.H.; Scott, S.M. Pathophysiology of fecal incontinence differs between men and women: A case-matched study in 200 patients. Neurogastroenterol. Motil. 2016, 28, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- De la Quintana, M.M.; das Graças de Souza Lima, T.; de Gouveia Santos, V.L.C.; de Moraes Lopes, M.H.B. Normal Defecation and Mechanisms for Continence. In Management of Fecal Incontinence for the Advanced Practice Nurse; Bliss, Z.D., Ed.; Springer: Cham, Switzerland, 2018; pp. 63–76. [Google Scholar] [CrossRef]
- Mundet, L.; Cabib, C.; Ortega, O.; Rofes, L.; Tomsen, N.; Marin, S.; Chacón, C.; Clavé, P. Defective Conduction of Anorectal AfferentsIs a Very Prevalent Pathophysiological Factor Associated to Fecal Incontinence in Women. J. Neurogastroenterol. Motil. 2019, 25, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, A.E.; Blandon, R.E.; Lunniss, P.J.; Scott, S.M. Anatomy and Physiology of Continence. In Fecal Incontinence; Ratto, C., Doglietto, G., Lowry, A., Påhlman, L., Romano, G., Eds.; Springer: Milano, Italy, 2007; pp. 3–16. [Google Scholar] [CrossRef]
- Rao, S.S. Diagnosis and Management of Fecal Incontinence. Am. J. Gastroenterol. 2004, 99, 1585–1604. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, A.E.; Dunivan, G.; Goode, P.S.; Lukacz, E.S.; Markland, A.D.; Matthews, C.A.; Mott, L.; Rogers, R.G.; Zinsmeister, A.R.; Whitehead, W.E.; et al. Epidemiology, pathophysiology, and classification of fecal incontinence: State of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am. J. Gastroenterol. 2015, 110, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Cortez, K.C.D.; Mendonça, S.D.S.; Figueiroa, M.D.S. Fecal incontinence as consequence of anorectal surgeries and the physiotherapeutic approach. J. Coloproctol. (Rio Janeiro) 2011, 31, 248–256. [Google Scholar] [CrossRef]
- Rao, S.S. Pathophysiology of adult fecal incontinence. Gastroenterology 2004, 126, 14–22. [Google Scholar] [CrossRef]
- Rao, S.S.; Siddiqui, J. Diagnosis of Fecal Incontinence. In Fecal Incontinence; Ratto, C., Doglietto, G., Lowry, A., Påhlman, L., Romano, G., Eds.; Springer: Milano, Italy, 2007; pp. 95–105. [Google Scholar] [CrossRef]
- Cera, S.M.; Wexner, S.D. Anal Sphincter Repair. Pelvic Floor Dysfunct. 2008, 143–149. [Google Scholar] [CrossRef]
- Weledji, E.P. Electrophysiological Basis of Fecal Incontinence and Its Implications for Treatment. Ann. Coloproctol. 2017, 33, 161–168. [Google Scholar] [CrossRef]
- Hayden, D.M.; Weiss, E.G. Fecal incontinence: Etiology, evaluation, and treatment. Clin. Colon Rectal Surg. 2011, 24, 64–70. [Google Scholar] [CrossRef]
- Makol, A.; Grover, M.; Whitehead, W.E. Fecal incontinence in women: Causes and treatment. Womens Health 2008, 4, 517–528. [Google Scholar] [CrossRef]
- Patcharatrakul, T.; Rao, S.S.C. Update on the Pathophysiology and Management of Anorectal Disorders. Gut Liver 2018, 12, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Laycock, J.; Jerwood, D. Pelvic Floor Muscle Assessment: The PERFECT Scheme. Physiotherapy 2001, 87, 631–642. [Google Scholar] [CrossRef]
- Zhoolideh, P.; Ghaderi, F.; Salahzadeh, Z. Are There Any Relations between Posture and Pelvic Floor Disorders? A Literature Review. Crescent J. Med. Biol. Sci. 2017, 4, 153–159. [Google Scholar]
- Talasz, H.; Kofler, M.; Kalchschmid, E.; Pretterklieber, M.; Lechleitner, M. Breathing with the Pelvic. Floor? Correlation of Pelvic Floor Muscle Function and Expiratory Flows in Healthy Young Nulliparous Women. Int. Urogynecol. J. 2009, 21, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Zachovajeviene, B.; Siupsinskas, L.; Zachovajevas, P.; Venclovas, Z.; Milonas, D. Effect of Diaphragm and Abdominal Muscle Training on Pelvic Floor Strength and Endurance: Results of a Prospective Randomized Trial. Sci. Rep. 2019, 9, 19192. [Google Scholar] [CrossRef]
- Slieker-ten Hove, M.; Pool-Goudzwaard, A.; Eijkemans, M.; Steegers-Theunissen, R.; Burger, C.; Vierhout, M. Face Validity and Reliability of the First Digital Assessment Scheme of Pelvic Floor Muscle Function Conform the New Standardized Terminology of the International Continence Society. Neurourol. Urodyn. 2009, 28, 295–300. [Google Scholar] [CrossRef]
- Tosun, G.; Peker, N.; Tosun, Ö.; Yeniel, Ö.; Ergenoğlu, A.; Elvan, A.; Yıldırım, M. Pelvic Floor Muscle Function and Symptoms of Dysfunctions in Midwifes and Nurses of Reproductive Age with and without Pelvic Floor Dysfunction. Taiwan. J. Obstet. Gynecol. 2019, 58, 505–513. [Google Scholar] [CrossRef]
- Fitz, F.; Stüpp, L.; Costa, T.; Sartori, M.; Girão, M.; Castro, R. Correlation between Maximum Voluntary Contraction and Endurance Measured by Digital Palpation and Manometry: An Observational Study. Rev. Assoc. Med. Bras. 2016, 62, 635–640. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, L.; Li, W.; Sun, X.; Huang, Q.; Tong, B.; Xie, Z. Comparisons of Electromyography and Digital Palpation Measurement of Pelvic Floor Muscle Strength in Postpartum Women with Stress Urinary Incontinence and Asymptomatic Parturients: A Cross-Sectional Study. Gynecol. Obstet. Invest. 2019, 84, 599–605. [Google Scholar] [CrossRef]
- Laycock, J.; Whelan, M.; Dumoulin, C. Therapeutic Management of Incontinence and Pelvic Pain: Pelvic Organ Disorders, 2nd ed.; Haslam, J., Laycock, J., Eds.; Springer: London, UK, 2007; pp. 57–67. ISBN 978-1-84628-661-2. [Google Scholar]
- De Oliveira Camargo, F.; Rodrigues, A.; Arruda, R.; Ferreira Sartori, M.; Girão, M.; Castro, R. Pelvic Floor Muscle Training in Female Stress Urinary Incontinence: Comparison between Group Training and Individual Treatment Using PERFECT Assessment Scheme. Int. Urogynecol. J. 2009, 20, 1455–1462. [Google Scholar] [CrossRef]
- Nyhus, M.; Mathew, S.; Salvesen, Ø.; Salvesen, K.; Stafne, S.; Volløyhaug, I. The Effect of Preoperative Pelvic Floor Muscle Training on Pelvic. Floor Contraction, Symptomatic and Anatomical Pelvic Organ Prolapse after Surgery: A Randomized Controlled Trial. Ultrasound Obstet. Gynecol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Harris-Hayes, M.; Spitznagle, T.; Probst, D.; Foster, S.; Prather, H. A Narrative Review of Musculoskeletal Impairments Associated with Nonspecific Chronic Pelvic Pain. PM&R 2019, 11, S73–S82. [Google Scholar] [CrossRef]
- Bernards, A.; Berghmans, B.; Slieker-ten Hove, M.; Staal, J.; de Bie, R.; Hendriks, E. Dutch Guidelines for Physiotherapy in Patients with Stress Urinary Incontinence: An Update. Int. Urogynecol. J. 2013, 25, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Jorge, M.; Wexner, S. Etiology and Management of Fecal Incontinence. Dis. Colon Rectum 1993, 36, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, T.; Church, J.; Fleshman, J.; Kane, R.; Mavrantonis, C.; Thorson, A.; Wexner, S.; Bliss, D.; Lowry, A. Fecal Incontinence Quality of Life Scale. Dis. Colon Rectum 2000, 43, 9–16. [Google Scholar] [CrossRef]
- Rockwood, T. Incontinence Severity And QOL Scales For Fecal Incontinence. Gastroenterology 2004, 126, S106–S113. [Google Scholar] [CrossRef]
- Bharucha, A.; Locke, G.; Seide, B.; Zinsmeister, A. A New Questionnaire for Constipation and Faecal Incontinence. Alimet. Pharmacol. Ther. 2004, 20, 355–364. [Google Scholar] [CrossRef]
- Kamper, S.J.; Moseley, A.M.; Herbert, R.D.; Maher, C.G.; Elkins, M.R.; Sherrington, C. 15 years of tracking physiotherapy evidence on PEDro, where we are now? Br. J. Sports Med. 2015, 49, 907–909. [Google Scholar] [CrossRef]
- Bø, K.; Haakstad, L.A. Is pelvic floor muscle training effective when taught in a general fitness class in pregnancy? A randomised controlled trial. Physiotherapy 2011, 97, 190–195. [Google Scholar] [CrossRef]
- Stafne, S.N.; Salvesen, K.A.; Romundstad, P.R.; Torjusen, I.H.; Mørkved, S. Does regular exercise including pelvic floor muscle training prevent urinary and anal incontinence during pregnancy? A randomised controlled trial. BJOG 2012, 119, 1270–1280. [Google Scholar] [CrossRef]
- Jahannessen, H.H.; Wibe, A.; Stordahl, A.; Sandvik, L.; Mørkved, S. Do pelvic floor muscle exercises reduce postpartum anal incontinence? A randomised controlled trial. BJOG 2017, 124, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Ilnyckyj, A.; Fachnie, E.; Tougas, G. A randomized-controlled trial comparing an educational intervention alone vs education and biofeedback in the management of faecal incontinence in women. Neurogastroenterol Motil. 2005, 17, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Sjödahl, J.; Walter, S.A.; Johansson, E.; Ingemansson, A.; Ryn, A.K.; Hallböök, O. Combination therapy with biofeedback, loperamide, and stool-bulking agents is effective for the treatment of fecal incontinence in women–a randomized controlled trial. Scand. J. Gastroenterol. 2015, 50, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Peirce, C.; Murphy, C.; Fitzpatrick, M.; Cassidy, M.; Daly, L.; O’Connell, P.; O’Herlihy, C. Randomised controlled trial comparing early home biofeedback physiotherapy with pelvic floor exercises for the treatment of third-degree tears (EBAPT Trial). BJOG 2013, 120, 1240–1247. [Google Scholar] [CrossRef]
- Davis, K.J.; Kumar, D.; Poloniecki, J. Adjuvant biofeedback following anal sphincter repair: A randomized study. Aliment. Pharmacol. Ther. 2004, 20, 539–549. [Google Scholar] [CrossRef]
- Ghahramani, L.; Mohammadipour, M.; Roshanravan, R.; Hajihosseini, F.; Bananzadeh, A.; Izadpanah, A.; Hosseini, S.V. Efficacy of Biofeedback Therapy before and after Sphincteroplasty for Fecal Incontinence because of Obstetric Injury: A Randomized Controlled Trial. Iran. J. Med. Sci. 2016, 41, 126–131. [Google Scholar]
- Sigurdardottir, T.; Steingrimsdottir, T.; Geirsson, R.T.; Halldorsson, T.; Aspelund, T.; Bø, K. Can postpartum pelvic floor muscle training reduce urinary and anal incontinence? An assessor-blinded randomized controlled trial. Am. J. Obstet. Gynecol. 2020, 222, 247. [Google Scholar] [CrossRef]
- Naimy, N.; Lindam, A.; Bakka, A.; Færden, A.; Wiik, P.; Carlsen, E.; Nesheim, B. Biofeedback Vs. Electrostimulation in the Treatment of Postdelivery Anal Incontinence: A Randomized, Clinical Trial. Dis. Colon Rectum 2007, 50, 2040–2046. [Google Scholar] [CrossRef]
- Mahony, R.; Malone, P.; Nalty, J.; Behan, M.; O’Connell, P.; O’Herlihy, C. Randomized Clinical Trial of Intra-Anal Electromyographic Biofeedback Physiotherapy with Intra-Anal Electromyographic Biofeedback Augmented with Electrical Stimulation of the Anal Sphincter in the Early Treatment of Postpartum Fecal Incontinence. Am. J. Obstet. Gynecol. 2004, 191, 885–890. [Google Scholar] [CrossRef]
- Healy, C.; Brannigan, A.; Connolly, E.; Eng, M.; O’Sullivan, M.; McNamara, D.; Cusack, C.; Deasy, J. The Effects of Low-Frequency Endo-Anal Electrical Stimulation on Faecal Incontinence: A Prospective Study. Int. J. Colorectal Dis. 2006, 21, 802–806. [Google Scholar] [CrossRef]
- Cohen-Zubary, N.; Gingold-Belfer, R.; Lambort, I.; Wasserberg, N.; Krissi, H.; Levy, S.; Niv, Y.; Dickman, R. Home electrical stimulation for women with fecal incontinence: A preliminary randomized controlled trial. Int. J. Colorectal Dis. 2015, 30, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, E.O.; Iferikigwe, E.S.; Obi, S.N.; Eleje, G.U.; Ozumba, B.C. Effectiveness of antenatal perineal massage in reducing perineal trauma and post-partum morbidities: A randomized controlled trial. J. Obstet. Gynaecol. Res. 2018, 44, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Coffey, S.W.; Wilder, E.; Majsak, M.J.; Stolove, R.; Quinn, L. The effects of a progressive exercise program with surface electromyographic biofeedback on an adult with fecal incontinence. Phys. Ther. 2002, 82, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Damin, D.; Hommerding, F.; Schirmer, D.; Sanches, P.; Silva Junior, D.; Müller, A.; Thome, P. Patient-Controlled Biofeedback Device for the Treatment of Fecal Incontinence: A Pilot Study. Appl. Psychophysiol. Biofeedback 2017, 42, 133–137. [Google Scholar] [CrossRef]
- Collins, J.; Mazor, Y.; Jones, M.; Kellow, J.; Malcolm, A. Efficacy of Anorectal Biofeedback in Scleroderma Patients with Fecal Incontinence: A Case–Control Study. Scand. J. Gastroenterol. 2016, 51, 1433–1438. [Google Scholar] [CrossRef]
- Lacima, G.; Pera, M.; González-Argenté, X.; Torrents, A.; Valls-Solé, J.; Espuña-Pons, M. Is Electromyography a Predictive Test of Patient Response to Biofeedback in the Treatment of Fecal Incontinence? Neurourol. Urodyn. 2016, 35, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Mathé, M.; Valancogne, G.; Atallah, A.; Sciard, C.; Doret, M.; Gaucherand, P.; Beaufils, E. Early pelvic floor muscle training after obstetrical anal sphincter injuries for the reduction of anal incontinence. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 201–206. [Google Scholar] [CrossRef]
- Worsøe, J.; Fynne, L.; Laurberg, S.; Krogh, K.; Rijkhoff, N.J. Electrical stimulation of the dorsal clitoral nerve reduce incontinence episodes in idiopathic faecal incontinent patients: A pilot study. Colorectal Dis. 2012, 14, 349–355. [Google Scholar] [CrossRef]
- Schobeiri, S.A.; Chesson, R.R.; West, E.C.; Shott, S.; Hoyte, L. A Pilot Study of Extracorporeal Magnetic Stimulation of the Pelvic Floor for the Treatment of Women with Fecal Incontinence and Underactive Pelvic Floor Muscles. J. Pelvic Med. Surg. 2007, 13, 19–26. [Google Scholar] [CrossRef]
- Eogan, M.; Daly, L.; O’Herlihy, C. The effect of regular antenatal perineal massage on postnatal pain and anal sphincter injury: A prospective observational study. J. Matern. Neonatal Med. 2006, 19, 225–229. [Google Scholar] [CrossRef]
- Fernandez-Fraga, X.; Azpiroz, F.; Malagelada, J.R. Significance of pelvic floor muscles in anal incontinence. Gastroenterology 2002, 123, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Schüssler-Fiorenza, C.M.; Wald, A. Current Perspectives: Fecal Incontinence in Older Adults. Aging Health 2007, 3, 751–765. [Google Scholar] [CrossRef]
- Schreiner, L.; Crivelatti, I.; de Oliveira, J.M.; Nygaard, C.C.; Dos Santos, T.G. Systematic review of pelvic floor interventions during pregnancy. Int. J. Gynaecol. Obstet. 2018, 143, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ellington, J.E.; Rizk, B.; Criso, S. Antenatal Perineal Massage Improves Women’s Experience of Childbirth and Postpartum Recovery: A Review to Facilitate Provider and Patient Education on the Technique. J. Womens Health 2017, 6, 2. [Google Scholar] [CrossRef]
- Eason, E.; Labrecque, M.; Marcoux, S.; Mondor, M. Anal incontinence after childbirth. CMAJ. 2002, 166, 326–330. [Google Scholar] [PubMed]
- Seehusen, D.A.; Raleigh, M. Antenatal perineal massage to prevent birth trauma. Am. Fam. Physician 2014, 89, 335–336. [Google Scholar]
- Mei-dan, E.; Walfisch, A.; Raz, I.; Levy, A.; Hallak, M. Perineal massage during pregnancy: A prospective controlled trial. Isr. Med. Assoc. J. 2008, 10, 499–502. [Google Scholar]
- Dieb, A.S.; Shoab, A.Y.; Nabil, H.; Gabr, A.; Abdallah, A.A.; Shaban, M.M.; Attia, A.H. Perineal massage and training reduce perineal trauma in pregnant women older than 35 years: A randomized controlled trial. Int. Urogynecol. J. 2020, 31, 613–619. [Google Scholar] [CrossRef]
- Modi, R.M.; Hinton, A.; Pinkhas, D.; Groce, R.; Meyer, M.M.; Balasubramanian, G.; Levine, E.; Stanich, P.P. Implementation of a Defecation Posture Modification Device: Impact on Bowel Movement Patterns in Healthy Subjects. J. Clin. Gastroenterol. 2019, 53, 216–219. [Google Scholar] [CrossRef]
- Colavita, K.; Andy, U.U. Role of diet in fecal incontinence: A systematic review of the literature. Int. Urogynecol. J. 2016, 27, 1805–1810. [Google Scholar] [CrossRef]
- Bols, E.M.J.; Berghmans, B.C.M.; Hendriks, E.J.M.; de Bie, R.A.; Melenhorst, J.; van Gemert, W.G.; Baeten, C.G.M.I. A randomized physiotherapy trial in patients with fecal incontinence: Design of the PhysioFIT-study. BMC Public Health 2007, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.M. Pelvic floor rehabilitation in the treatment of fecal incontinence. Clin. Colon Rectal Surg. 2014, 27, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Padda, B.S.; Jung, S.A.; Pretorius, D.; Nager, C.W.; Den-Boer, D.; Mittal, R.K. Effects of pelvic floor muscle contraction on anal canal pressure. Am. J. Physiol. Gastrointest Liver Physiol. 2007, 292, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.S.; Lima, T.G.S.; Schmidt, F.M.Q.; Santos, V.L.C.G. Pelvic Floor Rehabilitation in Anal Incontinence. In Fecal Incontinence—Causes, Management and Outcome; Catto-Smith, A.G., Ed.; IntechOpen: Rijeka, Croatia, 2014; pp. 107–122. [Google Scholar] [CrossRef]
- Norton, C.; Cody, J.D. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults (Review). Cochrane Database Syst. Rev. 2012, 11, 3–4. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.; Kołomańska-Bogucka, D.; Nowakowski, C.; Tim, S. Urinary Incontinence in Women: Modern Methods of Physiotherapy as a Support for Surgical Treatment or Independent Therapy. J. Clin. Med. 2020, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- ICS. ICS Standards 2019—Volume 1; Blurb: San Francisco, CA, USA, 2019; pp. 395–397. ISBN 9780464170907. [Google Scholar]
- Abrams, P.; Cardozo, L.; Wagg, A.; Wein, A. Incontinence, 6th ed.; ICI-ICS. International Continence Society: Bristol, UK, 2017; pp. 1993–2087. ISBN 978-0956960733. [Google Scholar]
- Duelund-Jakobsen, J.; Worsoe, J.; Lundby, L.; Christensen, P.; Krogh, K. Management of patients with faecal incontinence. Therap. Adv. Gastroenterol. 2016, 9, 86–97. [Google Scholar] [CrossRef]
- Puthanmadhom Narayanan, S.; Sharma, M.; Fletcher, J.; Karwoski, R.; Holmes, D., III; Bharucha, A. Comparison of Changes in Rectal Area and Volume During MR Evacuation Proctography in Healthy and Constipated Adults. Neurogastroenterol. Motil. 2019, e13608. [Google Scholar] [CrossRef]
- Parker, C.H.; Henry, S.; Liu, L.W.C. Efficacy of Biofeedback Therapy in Clinical Practice for the Management of Chronic Constipation and Fecal Incontinence. J. Can. Assoc. Gastroenterol. 2018, 2, 126–131. [Google Scholar] [CrossRef][Green Version]
- Pucciani, F. Rehabilitation and Biofeedback. In Fecal Incontinence; Ratto, C., Doglietto, G., Lowry, A., Påhlman, L., Romano, G., Eds.; Springer: Milano, Italy, 2007; pp. 167–170. [Google Scholar] [CrossRef]
- Ussing, A.; Dahn, I.; Due, U.; Sørensen, M.; Petersen, J.; Bandholm, T. Efficacy of Supervised Pelvic Floor Muscle Training and Biofeedback vs Attention-Control Treatment in Adults With Fecal Incontinence. Clin. Gastroenterol. Hepatol. 2019, 17, 2253–2261. [Google Scholar] [CrossRef]
- Rodas, M.; Garcia-Perdomo, H. From Kegel exercises to Pelvic. floor rehabilitation: A physiotherapeutic perspective. Rev. Mex. Urol. 2018, 78, 402–411. [Google Scholar] [CrossRef]
- Rogers, R.; Abed, H.; Fenner, D. Current Diagnosis and Treatment Algorithms for Anal Incontinence. BJU Int. 2006, 98, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, S.A. Fecal incontinence responses to anal electricalstimulation. Biosci. Res. 2019, 16, 1167–1173. [Google Scholar]
- Bø, K.; Berghmans, B.; Morkved, S.; van Kampen, M. Evidence-Based Physical Therapy for the Pelvic Floor, 2nd ed.; Churchill Livingstone, C., Ed.; Elsevier: London, UK, 2015; pp. 321–322. ISBN 9780702044434. [Google Scholar]
- Bakar, Y.; Cinar Özdemir, O.; Ozengin, N.; Duran, B. The use of extracorporeal magnetic innervation for the treatment of stress urinary incontinence in older women: A pilot study. Arch. Gynecol. Obstet. 2011, 284, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.R.; Moore, K.H. Extracorporeal Magnetic Stimulation. In Pelvic Floor Re-Education; Springer: London, UK, 2008; pp. 196–200. [Google Scholar] [CrossRef]
- Brusciano, L.; Gambardella, C.; Gualtieri, G.; Terracciano, G.; Tolone, S.; Schiano di Visconte, M.; Grossi, U.; Genio, G.; Docimo, L. Effects of Extracorporeal Magnetic Stimulation in Fecal Incontinence. Open Med. 2020, 15, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Quek, P. A critical review on magnetic stimulation: What is its role in the management of pelvic floor disorders? Curr. Opin. Urol. 2005, 15, 231–235. [Google Scholar] [CrossRef] [PubMed]
| P | Performance | Strength of maximum voluntary contraction (MVC) evaluated in the Oxford Modified Scale | |
| 0 | no contraction | ||
| 1 | flickering or pulsation | ||
| 2 | poor tension without lifting the vaginal walls | ||
| 3 | moderate tension with vaginal walls lifting without resistance | ||
| 4 | contraction with lifting of vaginal walls leading to join fingers in the vagina without therapist resistance | ||
| 5 | strong contraction leading to join fingers in the vagina against resistance | ||
| E | Endurance | Muscle strength assessed in seconds (0–10) as the ability to maintain an MVC until it falls to 50% of MVC | |
| R | Repetition | Number of MVC repetitions (0–10) of the length diagnosed in the endurance section | |
| F | Fast | Performance of fast twitch fibers evaluated as the number of repetitions (0–10) of one-second MVC | |
| E | Elevation | Cefalo-ventral elevation of PFM | |
| C | Co-contraction | Reflex co-contraction of the transverse abdominal muscle during the contraction of the PFM | |
| T | Timing | Involuntary PFM contraction during coughing provocation | |
| Study | EC * | RA | CA | BC | BS | BT | BA | AF | ITA | BGC | PEaV | TS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bø, 2011 [44] | + | + | + | + | - | - | - | - | - | + | + | 5 |
| Stafne, 2012 [45] | + | + | + | + | - | - | - | + | + | + | + | 7 |
| Johannessen, 2017 [46] | + | + | - | + | - | - | - | - | + | + | + | 5 |
| Ilnyckyj, 2005 [47] | + | + | - | - | - | - | - | - | - | + | - | 2 |
| Sjödahl, 2015 [48] | + | + | + | + | - | - | - | + | - | + | + | 6 |
| Peirce, 2013 [49] | + | + | + | - | - | - | - | + | - | + | - | 4 |
| Davis, 2004 [50] | + | + | - | + | - | - | - | - | - | + | + | 4 |
| Ghahramani, 2016 [51] | + | + | + | + | + | + | + | + | + | + | + | 10 |
| Sigurdardottir, 2020 [52] | + | + | - | + | - | - | + | + | - | + | + | 6 |
| Naimy, 2007 [53] | + | + | - | - | - | - | - | - | - | + | + | 3 |
| Mahony, 2004 [54] | + | + | + | + | - | - | + | + | - | + | + | 7 |
| Healy, 2006 [55] | - | + | - | - | - | - | - | - | - | + | + | 3 |
| Cohen-Zubary, 2015 [56] | + | + | + | + | - | - | - | - | + | + | + | 6 |
| Ugwu, 2018 [57] | + | + | + | + | - | - | - | + | - | + | + | 6 |
| Study | Qol | Depression | UI | FI | CI | SPF | PFMF | Other |
|---|---|---|---|---|---|---|---|---|
| Bø, 2011 [44] | + | + | GI | |||||
| Stafne, 2012 [45] | + | + | + | TD | ||||
| Johannessen, 2017 [46] | + | + | + | EaU | ||||
| Ilnyckyj, 2005 [47] | + | + | BD | |||||
| Sjödahl, 2015 [48] | + | + | BD, EaU | |||||
| Peirce, 2013 [49] | + | + | + | + | + | + | EaU | |
| Coffey, 2002 [58] | + | + | + | |||||
| Damin, 2017 [59] | + | + | + | |||||
| Collins, 2016 [60] | + | + | + | |||||
| Davis, 2004 [50] | + | + | + | + | EaU | |||
| Ghahramani, 2016 [51] | + | + | EaU | |||||
| Lacima, 2016 [61] | + | + | + | EaU, PNTML, BD | ||||
| Sigurdardottir, 2020 [52] | + | + | + | + | ||||
| Mathé, 2016 [62] | + | + | + | + | GI | |||
| Naimy, 2007 [53] | + | + | + | |||||
| Worsøe, 2011 [63] | + | + | + | + | BD | |||
| Mahony, 2004 [54] | + | + | + | EaU | ||||
| Healy, 2006 [55] | + | + | EaU, PNTML | |||||
| Cohen-Zubary, 2015 [56] | + | + | + | + | GI | |||
| Shobeiri, 2007 [64] | + | + | + | EvU | ||||
| Ugwu, 2018 [57] | + | + | + | GI | ||||
| Eogan, 2006 [65] | + | + | EaU, PP |
| Reference | Main Objective | Participants | Intervention | Outcome |
|---|---|---|---|---|
| (A) Pelvic floor muscle training (PFMT) in fecal incontinence prevention | ||||
| Bø et al. (2011) Norway [44] A randomized controlled trial study | The effectiveness of PFM exercises conducted during general fitness classes in pregnant women for mitigating postpartum incontinence. | 84 pregnant women Exp.: 42 (aged 31.2 ± 3.7 years) Con.: 42 (aged 30.3 ± 4.4 years) | Exp.: 1 h general fitness classes with PMFT (3 × 8–12 PFM contractions, hold 6–8 s); 2–3 times a week per 12 weeks + 30 min additional home exercises Con.: w/o interventionAssessment: SI, ICIQ–UI SH, | No efficacy of PFMT conducted during general fitness classes in reduction of FI symptoms assessed 6–8 weeks after childbirth. |
| Stafne (2012) Norway [45] A randomized controlled trial study | Comparison of the effectiveness of general exercise course including PFMT with standard care in the prevention of UI and FI in late pregnancy | 761 pregnant women Exp.: 396 (aged 30.5 ± 4.4 years) Con.: 365 (aged 30.4 ± 4.3 years) | Exp.: general exercise with PFMT (1 h, once a week under physiotherapist supervision + 45 min at home, twice a week per 12 weeks) Con.: standard care w/o exercise Assessment: SI, St. M.S, questionnaire about PFMT | Pregnant women who regularly participated in the training program reported UI and FI less frequently in late pregnancy. |
| (B) Pelvic floor muscle training (PFMT) in fecal incontinence treatment | ||||
| Johannessen (2017) Norway [46] A randomized controlled trial study | Assessment of the effectiveness of individualized PFM training in the treatment of FI in the postpartum period | 109 women with FI 1 year postpartum Exp.: 54 (aged 29.7 ± 4.3 years) Con.: 55 (aged 30.6 ± 3.8 years) | Exp.: individually adapted home PFMT program: 3 sets of 8–10 maximum PFM contractions per day, 3 s long with progression to 10 or 12 s with 3 fast contraction at the end, for 6 months Con.: written information about PFMT, training was not obligatory. Assessment: St. M.S, EaU, manometry, VPFMC | Both interventions reduced the symptoms of FI, howeverregular, individualized PFMT in the postpartum period reduced them significantly more, which has been described as a clinically significant effect. |
| Mathé et al. (2016) France [62] A retrospective observational study | Comparison of the effectiveness of early PFMT and/or standard rehabilitation for FI symptoms after vaginal deliveries complicated by ≥3rd degree of perineal tears | 167 women with ≥3rd degree of perineal tears after vaginal delivery Con.: 83 (age: 29.5 ± 4.7 years) Exp.: 84 (age: 30.6 ± 4.1 years) | Con.: standard rehabilitation, PFMT from 6–8 weeks of puerperium + BF as supports + education Exp.: early rehabilitation, PFMT after 30 days postpartum (6–10 series of PFMT twice a day) + standard rehabilitation (as in the group Con.) Assessment: Modified version of the Jorge and Wexner questionnaire | The implementation of early rehabilitation significantly reduces FI, GI, and UI in women after childbirth complicated by massive perineal damage, this result was significantly better than that obtained after standard rehabilitation. |
| Reference | Main Objective | Participants | Intervention | Outcome |
|---|---|---|---|---|
| Ugwu et al. (2018) Nigeria [57] A randomized controlled trial study | Evaluation of the effectiveness of APM in the prevention of perineal injuries and FI development. | 108 primiparous at 34–36 weeks of pregnancy MG: 53 (Average Age: 28.02 Years) Con.: 55 (Average Age: 28.77 Years) | MG: perineal massage, 10 min a day until delivery Con.: no intervention Follow up after 12 weeks Assessment: Diary of APM | APM reduces the frequency of incisions and other perineal injuries. Moreover, it lowers the risk of FI after childbirth. |
| Eogan et al. (2006) [65] A prospective observational study | Assessment of the impact of APM on the prevention of stool disorders | MG: 100 primiparous at 34 weeks of pregnancy (Average Age: 30.00 Years) Con.: 79 (Average Age: 25.9 Years) | MG: perineal massage, 5 min a day until delivery Con.: no intervention Follow up after 3 days and 3 months of postpartum Assessment: PS, Continence Score, Anal manometry, EaU | APM reduces postpartum perineal pain. There were no significant differences in the manometry results between the two groups. |
| Reference | Main Objective | Participants | Intervention | Outcome |
|---|---|---|---|---|
| Ilnyckyj et al. (2005) Canada, [47] A randomized controlled trial study | Assessing whether BF has any specific effect beyond the standard educational intervention | 18 women with FI history ≥ 6 months (aged 26–75 years) Con.: 11 Exp.: 7 | Con.: education on FI and PFM and verbal instruction of PFM exercises (6 times a day, 5 contractions with maximum possible force and holding time separated by a 20 s pause). Exp.: education and PFM exercise as in the Con.: group + BF training Assessment: manometry, diary | Education with exercise instruction and BF effectively minimize the symptoms of FI in women. In both groups, there was a significant improvement in squeeze duration; however, resting and squeeze pressures improved only in the group with BF. |
| Sjödahl et al. (2015), Sweden [48] A randomized controlled trial study | Evaluation of the therapeutic effect of BF alone or in combination with pharmacotherapy in the treatment of FI. | 57 women with FI history ≥ 1 episode of FI within 2 weeks BF + Pharmaco: 29 (median age 62 years) Pharmaco + BF: 28 (median age 57 years) | A combination of BF therapy and subsequent pharmacotherapy or vice versa BF: education, behavioral instructions and surface electro-BF training with anal plug (1–6 session during 4–6 months with individual home exercise program) Pharmaco: loperamide and stool-bulking agents for 2 months Assessment: 14 day symptoms diary, 3D-EAUS, anal function | Both type of combined interventions significantly reduced the FI symptoms and was more effectiveness than BF or pharmacotherapy alone (significant reduction in urgency, number of loose stools, leakages without forewarning, and passive leakages were observed). |
| Peirce et al. (2013), Ireland [49] A randomized controlled trial study | Comparison of early home BF physiotherapy with PFM exercises in the initial management of women sustaining a primary third-degree perineal tear. | 120 Primiparous women with a primary third-degree perineal tear Exp.: 30 (aged nd) Con.: 90 (aged nd) | Exp.: home EMG BF therapy with the intra-anal probe, twice a day for 3 months (10 contractions, 5 s duration, 10 s rest) Con.: home PFMT (5 min standard Kegel exercise) twice a day for 3 months. Assessment: anorectal manometry, EAU, CCCS, RFI QoL | No additional benefits of BF therapy compared to PFMT have been demonstrated. There was no difference in the manometric test, FI symptoms and quality of life between the groups. |
| Coffey et al. (2002), USA [58] Case Report | Evaluation of the effectiveness of multifactorial therapy designed to reduce the PFM dysfunction and FI symptoms. | 30 year old woman with FI symptoms from 8 years which started after the first delivery (vacuum extraction, perineal incision, child’s weight 4.16 kg) | Education, EMG BF, strengthening exercises, PFM relaxation training, soft tissue techniques, Follow up: 4 months Assessment: authors questionnaire, digital palpation, EMG BF | As a result of the therapy improvements have been achieved in such aspects as the quality of life, PFM strength, endurance and control. FI symptoms was also improved. |
| Damin et al. (2017), Brazil [59] A pilot study | Verification of the usefulness of a novel portable biofeedback device in the FI treatment | 10 women with FI, without any previous treatment (aged 50–73 years) | BF training: daily for 28 days, 20 min in three phases (1) 5 series of 10 2-s contractions with a 2-s rest between contractions, (2) 2-min break, (3) 5 series of 10 contractions during 5 s with a 5 -second rest. PFM contractions of maximum strength Assessment: Wexner scale, FIQL | A reduction in FI symptoms and an increase in QoL were achieved. The BF device allows for effective training for FI treatment at home. |
| Collins et al. (2016), Australia [60] A case-control study | Assessment of the effectiveness of anorectal BF in the treatment of FI and QoL improvement among patients with scleroderma compared to patients with functional FI. | 39 women with FI G.I.: 13 women with FI and scleroderma (median age 59, IQR 47–65 years) G.II.: 26 women with functional FI (age- and parity-matched) | Both groups: supervised BF training (30–60 min), once a week for 6 weeks, education, BF and PFMT learning, anal sensory training. Follow up: 6 weeks and 6 months after the end of therapy Assessment: FISI, manometry | BF training improved quality of life and stool control in both groups. Patients with scleroderma benefit from BF comparable as patients with functional FI. |
| Davis et al. (2004), United Kingdom [50] A randomized controlled trial study | To evaluate the effect of BF as adjuvant therapy in women after anal sphincter surgery. | 31 women with FI referred to a secondary/tertiary colorectal unit Con.: 17 (aged 60.29 ± 13.59 years) Exp.: 14 (aged: 60.71 ±10.04 years) | Con.: anal sphincter surgery Exp.: BF 3 months after sphincter surgery; session duration 1 h, once a week for 6 weeks, education, BF, and PFMT twice a day at home. Assessment: CGSS, manometry, EaU | Anal sphincter surgery significantly reduces the FI symptoms. Postoperative BF therapy improves the long-term QoL of patients. |
| Ghahramani et al. (2016), Iran [51] A randomized controlled trial study | Assessment of the effect of BF applied before and/or after surgery on FI symptoms in women with sphincter damage. | 27 women with the anal sphincters damage during childbirth Exp1: 9 (aged: 41.1 ± 13.12 years), Exp2: 9 (aged: 36.8 ± 16.45 years), Con.: 9 (aged: 44.85 ± 15.32 years), | Exp1: BF 3 months before and 6 months after surgery Exp2: BF 6 months after surgery Con.: operation only BF: supervised training—10 sphincter muscle contractions maintained for 5 s, education; at home—100 contractions of the anal sphincter muscles twice a day Assessment: The Wexner scale, manometers, EaU | Surgery alone and in combination with BF reduce the FI symptoms in women. The use of BF before and/or after surgery provides better FI improvement than surgery alone (Wexner scale), but not in manometry. |
| Lacima et al. (2016), Spain [61] Observational study | Prospective identification of clinical factors that can predict the efficacy of BF for FI treatment and evaluate the utility of tests in predicting outcomes of treatment. | 135 women with fecal incontinence of varying etiology (aged: 60.6 ± 11.5 years) | A minimum of 4 BF sessions + anal sphincter exercises at home; 10 min, twice a day Assessment: manometry, rectal sensory testing, EaU, PNTML, questionnaire about symptoms | BF therapy was effective in the treatment of FI. Clinical factors and tests to predict treatment outcomes could not be described |
| Sigurdardottir et al. (2020), Iceland [52] A randomized controlled trial study | Assessment of the effects of PFMT with BF facilitation in the early postpartum period on UI and FI symptoms and related problems, as well as the strength and endurance of PFM. | 84 women with UI and FI symptoms Exp.: 41 (mean age: 28 ± 4.3 years) Con.: 43 (mean age: 29 ± 5.3 years) | Exp.: supervised PFMT with vaginal EMG-BF to facilitate—once a week for 12 weeks, session duration 45–60 min; home PFMT 3 x 10 PFM contractions. Training started in the 9th week post-partum Con.: no intervention Assessment: APFQ, manometry | PFMT with BF facilitation increased the strength and endurance of both PFM and anal sphincter, but the frequency of UI and FI after 6 and 12 months remained unchanged. |
| Reference | Main Objective | Participants | Intervention | Outcome |
|---|---|---|---|---|
| Naimy et al. (2007), Norway [53] A randomized controlled trial study | Comparison of the effect of BF vs. ES in the treatment of postdelivery FI. | 49 women with FI (≥3rd degree of perineal tears) (aged 22–44 years) BF: 24 ES: 25 | 2 sessions with a therapist, and then twice a day for 8 weeks at home. BF: 30 min, 5 sets of 3 s, 10 s and as long as it can be kept with a minimum 50 percent of amplitude of the three-second contraction ES: with anal probe; frequency 30–40 Hz, pulse width: 200 ms, up to 80 mAmp, time: 20 min. Assessment: Wexner score, FIQL, RQL | No improvement was observed after BF or ES therapy in FI symptoms (Wexner score) and quality of life (FIQL) in women with postdelivery FI problem. Both therapies improved the subjective perception of fecal incontinence control by patients. |
| Worsøe (2011), Denmark [63] A prospective descriptive study | Assessment of the effects of DGN stimulation on FI symptoms. | Nine women with idiopathic FI (median age, 60 years; 34–68 years) | ES: twice a day for 3 weeks; 15 min, pulse width 200 μs; frequency 20 Hz. Assessment: VAS, FIQL, bowel habit diary, EA USG, Wexner score, St. Mark’s scale, manometry | After electrostimulation of DGN, the symptoms of FI were reduced (Wexner score, St. Mark’s scale), and the effect was maintained also 3 weeks after the end of treatment. |
| Mahony et al. (2004), Ireland [54] A randomized controlled trial study | Comparison of the effectiveness of intra-anal EMG-BF with intra-anal EMG-BF combined with anal sphincter ES in the treatment of postpartum FI, as well as QoL of treated patients. | 54 women with FI after obstetric injury BF + ES: 28 (median age 35 years, range 23–39), BF: 26 (median age 32 years, range 22–42) | In both groups: daily PFMT for 12 weeks BF + ES: PFEs with EMG-BF or intra-anal BF and intra-anal electrical stimulation for 20 min, once per week. BF: PFEs with EMG-BF or intra-anal BF once per week. Assessment: FIQL, questionnaire to determine continence score, manometry, EA USG | In both groups, there was significant improvement in FI symptoms and quality of life. ES did not bring any additional benefits. |
| Healy et al. (2006), Ireland [55] A prospective study | Comparison of the effectiveness of home and hospital therapy using low-frequency endoanal electrostimulation in alleviating FI symptoms. | 38 women with FI (mean age: 55 years; range 40–78) G.I: 21 G.II: 17 | G.I: low-frequency endo-anal ES at home with sequence of 3, 10, 20, 30, 40, 10 Hz frequencies (4 s on/4 s off), 1 h daily for 3 months G.II: low frequency endo-anal ES (15 min 10 Hz + 15 min 40 Hz) with biofeedback; 2 series 15 min; once a week under the supervision of a physiotherapist for 3 months Assessment: manometry, Wexner score, QoL | Low-frequency ES significantly reduced the symptoms of FI and improved quality of life. Daily use of home ES significantly improved rectal pressure (resting and squeeze pressure). |
| Cohen-Zubary et al. (2015) Israel [56] A randomized controlled trial study | Comparison of the effectiveness and costs of ES at home with BF training in women with FI. | 36 women with chronic FI (mean age: 67.45 ± 7.2 years) ES: 18 (mean age: 66.6 ± 6.6) BF + PFMT: 18 (mean age: 68.3 ± 6.9) | ES: stimulation twice daily (25 min) for 6 weeks BF + PFMT: once a week supervised PFMT with BF (30–45 min) for 6 weeks and PFMT at home twice a day, 3 series, 10 contractions for 10 s. Assessment: VAS, VIS, HADS, intra-anal surface EMG | In both groups there was an increase in muscle strength as well as a decrease in FI symptoms. There were no adverse side effects. |
| Reference | Main Objective | Participants | Intervention | Outcome |
|---|---|---|---|---|
| Shobeiri et al. (2007), USA [64] A prospective cohort pilot study | Assessment of the usefulness of EXMI in alleviating FI symptoms in women with underactive pelvic floor. | 16 women with FI and underactive PFM (mean age 57 years) | EXMI in a sitting position on a chair inducing an alternating magnetic field, frequency from 5 to 50 Hz, pulse 8 s, rest 4 s, for 20 min, twice a week for 8 weeks. Follow-up after 12 weeks Assessment: CCFIS, MR, endovaginal ultrasound, PFM examination with a Kegel Perineometer | EXMI alleviates the FI symptoms among women with an underactive pelvic floor (CCFIS). Pelvic floor rest and squeeze pressures have improved significantly. |
| Method | Level of Evidence | Grade of Recommendation | Line of Treatment (ICI) |
|---|---|---|---|
| PFMT | 2 | B [82] | Primary |
| BF | 1 | A [82] | Secondary |
| ES | 2 | B [83] | Secondary |
| MS | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur-Bialy, A.I.; Kołomańska-Bogucka, D.; Opławski, M.; Tim, S. Physiotherapy for Prevention and Treatment of Fecal Incontinence in Women—Systematic Review of Methods. J. Clin. Med. 2020, 9, 3255. https://doi.org/10.3390/jcm9103255
Mazur-Bialy AI, Kołomańska-Bogucka D, Opławski M, Tim S. Physiotherapy for Prevention and Treatment of Fecal Incontinence in Women—Systematic Review of Methods. Journal of Clinical Medicine. 2020; 9(10):3255. https://doi.org/10.3390/jcm9103255
Chicago/Turabian StyleMazur-Bialy, Agnieszka Irena, Daria Kołomańska-Bogucka, Marcin Opławski, and Sabina Tim. 2020. "Physiotherapy for Prevention and Treatment of Fecal Incontinence in Women—Systematic Review of Methods" Journal of Clinical Medicine 9, no. 10: 3255. https://doi.org/10.3390/jcm9103255
APA StyleMazur-Bialy, A. I., Kołomańska-Bogucka, D., Opławski, M., & Tim, S. (2020). Physiotherapy for Prevention and Treatment of Fecal Incontinence in Women—Systematic Review of Methods. Journal of Clinical Medicine, 9(10), 3255. https://doi.org/10.3390/jcm9103255





