High Effectiveness of a 14-Day Concomitant Therapy for Helicobacter pylori Treatment in Primary Care. An Observational Multicenter Study
Abstract
1. Introduction
2. Methods
3. Statistical Analysis
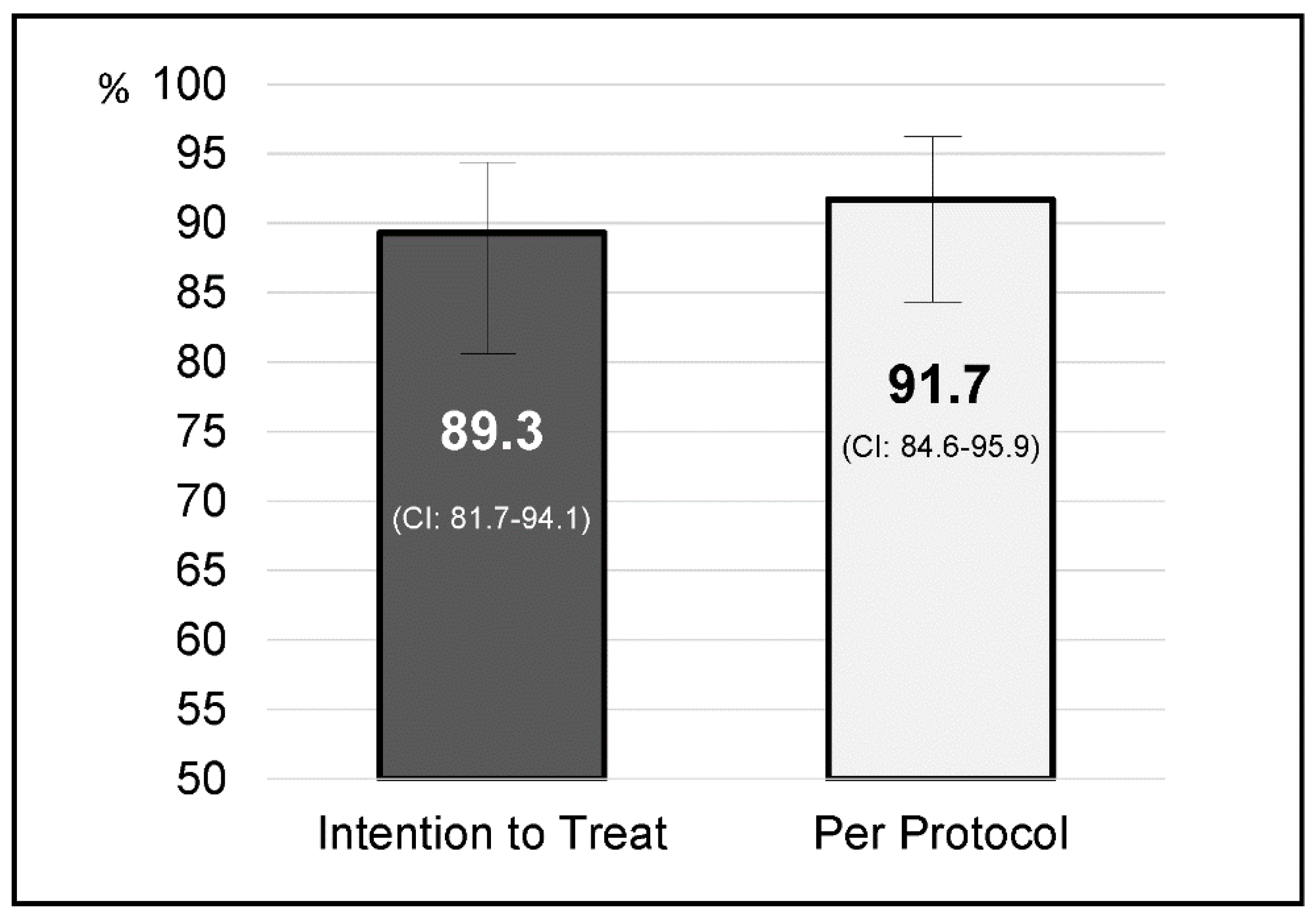
4. Results
Factors Influencing the Efficacy of Therapy
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, B.; Zhao, J.; Cheng, W.-F.; Shi, W.-J.; Liu, W.; Pan, X.-L.; Zhang, G.-X. Efficacy of Helicobacter pylori Eradication Therapy on Functional Dyspepsia: A meta-analysis of randomized controlled studies with 12-month follow-up. J. Clin. Gastroenterol. 2014, 48, 241–247. [Google Scholar] [CrossRef]
- Stasi, R.; Sarpatwari, A.; Segal, J.B.; Osborn, J.; Evangelista, M.L.; Cooper, N.; Provan, D.; Newland, A.; Amadori, S.; Bussel, J.B. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: A systematic review. Blood 2009, 113, 1231–1240. [Google Scholar] [CrossRef]
- Nejati, S.; Karkhah, A.; Darvish, H.; Validi, M.; Ebrahimpour, S.; Nouri, H.R. Influence of Helicobacter pylori virulence factors CagA and VacA on pathogenesis of gastrointestinal disorders. Microb. Pathog. 2018, 117, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kusters, J.G.; Van Vliet, A.H.; Kuipers, E.J. Pathogenesis of Helicobacter pylori Infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Calvet, X. Dealing with uncertainty in the treatment of Helicobacter pylori. Ther. Adv. Chronic Dis. 2018, 9, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.J. Treatment strategies for Helicobacter pylori infection. Gastroenterol. Clin. N. Am. 1993, 22, 183–193. [Google Scholar]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Bazzoli, F.; El-Omar, E.; Graham, D.; Hunt, R.; Rokkas, T.; Vakil, N.; Kuipers, E.J. Management of Helicobacter pylori infection—The Maastricht III Consensus Report. Gut 2007, 56, 772–781. [Google Scholar] [CrossRef]
- Greenberg, E.R.; Anderson, G.L.; Morgan, D.R.; Torres, J.; Chey, W.D.; Bravo, L.E.; Dominguez, R.L.; Ferreccio, C.; Herrero, R.; Lazcano-Ponce, E.C.; et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: A randomised trial. Lancet 2011, 378, 507–514. [Google Scholar] [CrossRef]
- Graham, D.Y.; Fischbach, L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010, 59, 1143–1153. [Google Scholar] [CrossRef]
- Liou, J.; Fang, Y.-J.; Chen, C.-C.; Bair, M.-J.; Chang, C.-Y.; Lee, Y.; Chen, M.-J.; Chen, C.-C.; Tseng, C.; Hsu, Y.-C.; et al. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: A multicentre, open-label, randomised trial. Lancet 2016, 388, 2355–2365. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Lucendo, A.J.; Angueira, T.; Rodriguez-Tellez, M.; Pérez-Aisa, A.; Balboa, A.; Barrio, J.; Martín-Noguerol, E.; Gómez-Rodríguez, B.J.; Botargues-Bote, J.M.; et al. Optimised empiric triple and concomitant therapy for Helicobacter pylori eradication in clinical practice: The OPTRICON study. Aliment. Pharmacol. Ther. 2015, 41, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Romano, M.; Fernandez-Bermejo, M.; Federico, A.; Gravina, A.G.; Pozzati, L.; Garcia–Abadia, E.; Vinagre–Rodriguez, G.; Martinez-Alcala, C.; Hernandez-Alonso, M.; et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology 2013, 145, 121–128. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Molina-Infante, J.; Amador, J.; Bermejo, F.; Bujanda, L.; Calvet, X.; Castro-Fernandez, M.; Cuadrado-Lavín, A.; Elizalde, J.I.; Gene, E.; et al. IV Conferencia Española de Consenso sobre el tratamiento de la infección por Helicobacter pylori. Gastroenterol. Hepatol. 2016, 39, 697–721. [Google Scholar] [CrossRef] [PubMed]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology 2016, 151, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef]
- Sheu, B.-S.; Wu, M.-S.; Chiu, C.-T.; Lo, J.-C.; Wu, D.-C.; Liou, J.-M.; Wu, C.-Y.; Cheng, H.-C.; Lee, Y.; Hsu, P.-I.; et al. Consensus on the clinical management, screening-to-treat, and surveillance of Helicobacter pylori infection to improve gastric cancer control on a nationwide scale. Helicobacter 2017, 22, e12368. [Google Scholar] [CrossRef]
- Smith, S.M.; Boyle, B.; Brennan, D.; Buckley, M.; Crotty, P.; Doyle, M.; Farrell, R.; Hussey, M.; Kevans, D.; Malfertheiner, P.; et al. The Irish Helicobacter pylori Working Group consensus for the diagnosis and treatment of H. pylori infection in adult patients in Ireland. Eur. J. Gastroenterol. Hepatol. 2017, 29, 552–559. [Google Scholar] [CrossRef]
- Cosme, A.; Lizasoan, J.; Montes, M.; Tamayo, E.; Alonso, H.; Mendarte, U.; Martos, M.; Fernández-Reyes, M.; Saraqueta, C.; Bujanda, L. Antimicrobial susceptibility-guided therapy versus empirical concomitant therapy for eradication of Helicobacter pylori in a region with high rate of clarithromycin resistance. Helicobacter 2015, 21, 29–34. [Google Scholar] [CrossRef]
- McNicholl, A.G.; Marín, A.C.; Molina-Infante, J.; Castro, M.; Barrio, J.; Ducons, J.; Calvet, X.; De La Coba, C.; Montoro, M.; Bory, F.; et al. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut 2013, 63, 244–249. [Google Scholar] [CrossRef]
- Apostolopoulos, P.; Koumoutsos, I.; Ekmektzoglou, K.; Dogantzis, P.; Vlachou, E.; Kalantzis, C.; Tsibouris, P.; Alexandrakis, G. Concomitant versus sequential therapy for the treatment of Helicobacter pylori infection: A Greek randomized prospective study. Scand. J. Gastroenterol. 2015, 51, 145–151. [Google Scholar] [CrossRef]
- Zullo, A.; Scaccianoce, G.; De Francesco, V.; Vannella, L.; Ruggiero, V.; Dambrosio, P.; Castorani, L.; Bonfrate, L.; Hassan, C.; Portincasa, P. Sa1909 Concomitant, Sequential, and Hybrid Therapy for H. pylori Eradication: A Pilot Study. Gastroenterology 2013, 144, 647–650. [Google Scholar] [CrossRef]
- McNicholl, A.G.; Amador, J.; Ricote, M.; Cañones-Garzón, P.J.; Gene, E.; Calvet, X.; Gisbert, J.P.; Spanish Primary Care Societies SEMFyC, SEMERGEN and SEMG, the Spanish Association of Gastroenterology; OPTICARE Long-Term Educational Project. Spanish primary care survey on the management of Helicobacter pylori infection and dyspepsia: Information, attitudes, and decisions. Helicobacter 2019, 24, e12593. [Google Scholar] [CrossRef] [PubMed]
- McNicholl, A.G.; Molina-Infante, J.; Bermejo, F.; Harb, Y.; Modolell, I.; Anton, R. Non bismuth quadruple “concomitant” therapies in the eradication of Helicobacter pylori. Standard vs optimized (14 days, high-dose PPI) regimes in clinical practice. Helicobacter 2014, 19 (Suppl. 1), 11. [Google Scholar]

| Variables | Total | p | |
| Age—Years—(Mean ± SD) | 46.7 ± 16.1 | 0.921 | |
| Sex | Men | 50 (44.6%) | 0.404 |
| Women | 62 (55.4%) | ||
| Tobacco | Smoker | 16 (14.3%) | 0.404 |
| Non-smoker | 96 (85.7%) | ||
| Primary care centers (PCC) | PCC Bases de Manresa/PCC Barri Antic. Althaia | 21 (18.8%) | 0.369 |
| PCC Granollers | 54 (48.2%) | ||
| PCC Badia Valés | 8 (7.1%) | ||
| PCC Arbúcies/PCC St Hilari. Girona | 29 (25.9%) | ||
| Main indications | Non-investigated dyspepsia | 93 (83%) | 0.127 |
| Functional dyspepsia | 14 (12.5%) | ||
| Peptic ulcer | 4 (3.6%) | ||
| Others | 1 (0.9%) | ||
| Diagnostic test previous to treatment | Histology | 5 (4.5%) | 0.624 |
| Urea breath test | 23 (20.5%) | ||
| Helicobacter pylori stool antigen | 75 (67%) | ||
| Rapid Urease Test | 9 (8%) | ||
| Treatment adherence | Complete | 104 (92.9%) | 0.004 |
| Partial | 8 (7.1%) | ||
| Mild side effects | Yes | 47 (42%) | 0.004 |
| No | 65 (58%) | ||
| Diagnostic test post treatment | Helicobacter pylori stool antigen | 96 (85.7%) | 0.633 |
| Urea breath test | 16 (14.3%) | ||
| Variables | Total | p | |
| Age—Years—(Mean ± SD) | 46.7 ± 16.1 | 0.921 | |
| Sex | Men | 50 (44.6%) | 0.404 |
| Women | 62 (55.4%) | ||
| Tobacco | Smoker | 16 (14.3%) | 0.404 |
| Non-smoker | 96 (85.7%) | ||
| Primary care centers (PCC) | PCC Bases de Manresa/PCC Barri Antic. Althaia | 21 (18.8%) | 0.369 |
| PCC Granollers | 54 (48.2%) | ||
| PCC Badia Valés | 8 (7.1%) | ||
| PCC Arbúcies/PCC St Hilari. Girona | 29 (25.9%) | ||
| Main indications | Non-investigated dyspepsia | 93 (83%) | 0.127 |
| Functional dyspepsia | 14 (12.5%) | ||
| Peptic ulcer | 4 (3.6%) | ||
| Others | 1 (0.9%) | ||
| Diagnostic test previous to treatment | Histology | 5 (4.5%) | 0.624 |
| Urea breath test | 23 (20.5%) | ||
| Helicobacter pylori stool antigen | 75 (67%) | ||
| Rapid Urease Test | 9 (8%) | ||
| Treatment adherence | Complete | 104 (92.9%) | 0.004 |
| Partial | 8 (7.1%) | ||
| Mild side effects | Yes | 47 (42%) | 0.004 |
| No | 65 (58%) | ||
| Diagnostic test post treatment | Helicobacter pylori stool antigen | 96 (85.7%) | 0.633 |
| Urea breath test | 16 (14.3%) | ||
| Variables | Intention to Treat (95% CI) | Per Protocol (95% CI) | |
|---|---|---|---|
| Sex | Men | 92% (80–97.4) | 93.9% (82.2–98.4) |
| Women | 87.1% (75.6–93,4) | 90% (78.8–95.9) | |
| Tobacco | Smoker | 93.8% (67.8–99.7) | 93.8% (67.8–99.7) |
| Non-smoker | 88.5% (80–93.8) | 91.4% (83.3–96) | |
| Primary care centers (PCC) | PCC Bases de Manresa/PCC Barri Antic. Althaia | 85.7% (62.6–96.2) | 85.7% (62.6–96.2) |
| PCC Granollers | 92.6% (81.3–97.6) | 92.6% (81.3–97.6) | |
| PCC Badia Valés | 100% (59.8–98.8) | 100% (59.8–100) | |
| PCC Arbúcies/PCC St Hilari. Girona | 82.8% (63.6–93.5) | 92.3% (73.4–98.7) | |
| Main indications | Non-investigated dyspepsia | 91.4% (83.3–95.9) | 94.4% (83.3–95.9) |
| Functional dyspepsia | 71,4% (42–90.4) | 71,4% (42–90.4) | |
| Peptic ulcer | 100% (39.6–97.6) | 100% (39.6–100) | |
| Others | 100% (5.4–89.2) | 100% (5.4–100) | |
| Diagnostic test previous to treatment | Histology | 80% (29.9–98.9) | 80% (29.9–98.9) |
| Urea breath test | 91.3% (70.5–98.5) | 95.5% (75.2–99.8) | |
| Helicobacter pylori stool antigen | 88% (80–94) | 90.4% (80.1–95.6) | |
| Rapid Urease Test | 100% (62.9–99) | 100% (62.9–100) | |
| Diagnostic test post treatment | Helicobacter pylori stool antigen | 89.7% (81.4–94.7) | 92.6% (84.8–96.7) |
| Urea breath test | 86.7% (58.4–97.7) | 86.7% (58.4–97.7) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedo, L.; Azagra, R.; Aguyé, A.; Pascual, M.; Calvet, X.; Gené, E. High Effectiveness of a 14-Day Concomitant Therapy for Helicobacter pylori Treatment in Primary Care. An Observational Multicenter Study. J. Clin. Med. 2020, 9, 2410. https://doi.org/10.3390/jcm9082410
Olmedo L, Azagra R, Aguyé A, Pascual M, Calvet X, Gené E. High Effectiveness of a 14-Day Concomitant Therapy for Helicobacter pylori Treatment in Primary Care. An Observational Multicenter Study. Journal of Clinical Medicine. 2020; 9(8):2410. https://doi.org/10.3390/jcm9082410
Chicago/Turabian StyleOlmedo, Llum, Rafael Azagra, Amada Aguyé, Marta Pascual, Xavier Calvet, and Emili Gené. 2020. "High Effectiveness of a 14-Day Concomitant Therapy for Helicobacter pylori Treatment in Primary Care. An Observational Multicenter Study" Journal of Clinical Medicine 9, no. 8: 2410. https://doi.org/10.3390/jcm9082410
APA StyleOlmedo, L., Azagra, R., Aguyé, A., Pascual, M., Calvet, X., & Gené, E. (2020). High Effectiveness of a 14-Day Concomitant Therapy for Helicobacter pylori Treatment in Primary Care. An Observational Multicenter Study. Journal of Clinical Medicine, 9(8), 2410. https://doi.org/10.3390/jcm9082410





