Effects of Aerobic Exercise on Cortisol Stress Reactivity in Response to the Trier Social Stress Test in Inpatients with Major Depressive Disorders: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
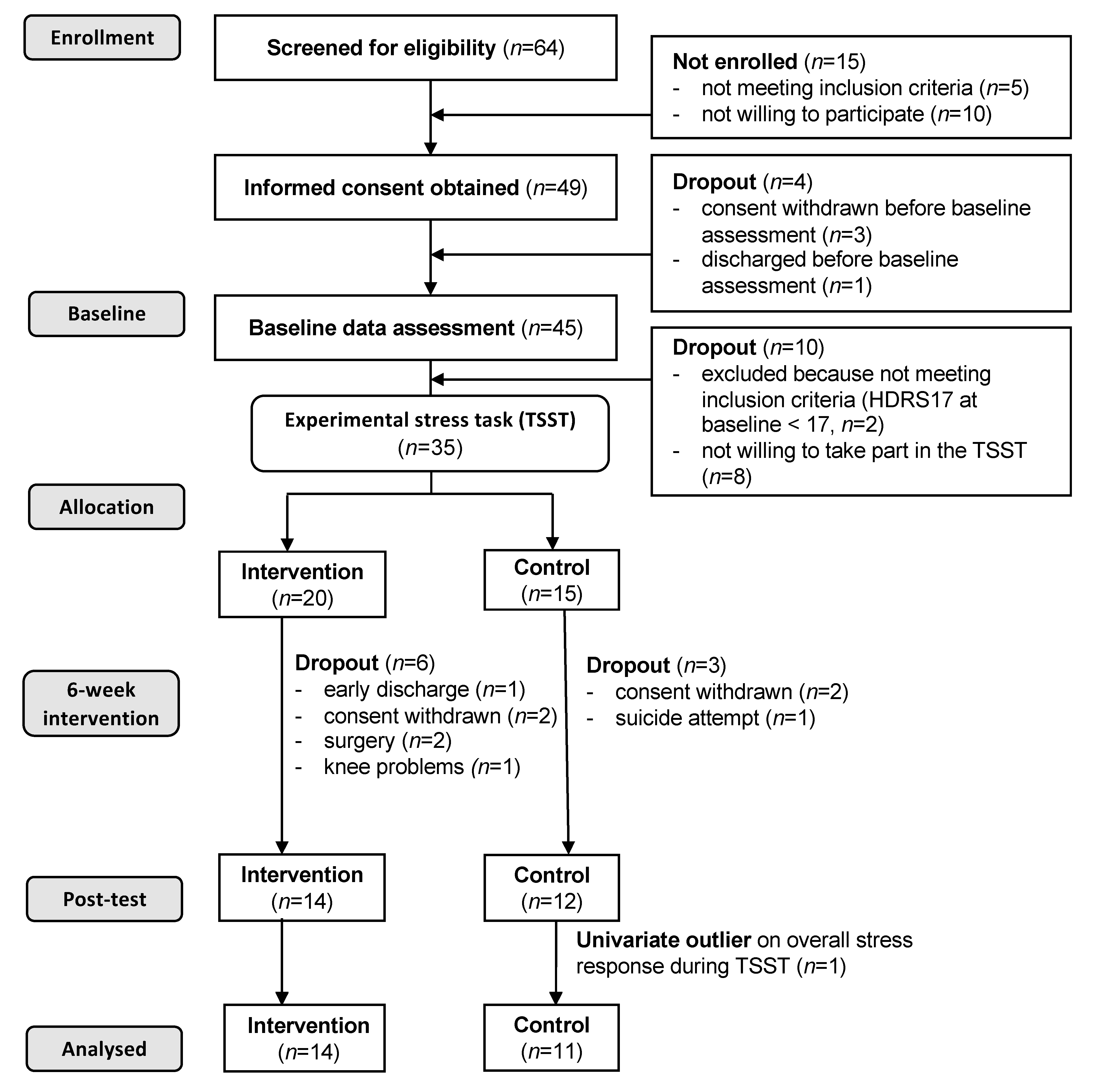
2.1. Participants and Procedures
2.2. Intervention vs. Control Condition
2.3. Trier Social Stress Test
2.4. Assessment of the Adrenocortical Stress Response
2.5. Assessment of Covariates
2.5.1. Depressive Symptom Severity
2.5.2. Physical Activity
2.5.3. Further Potential Confounders
2.6. Statistical Analyses
3. Results
3.1. Sample Characteristics and Descriptive Statistics
3.2. Reactivity in Response to the TSST at the Baseline Data Assessment
3.3. Associations Between Potential Confounders and Stress Reactivity
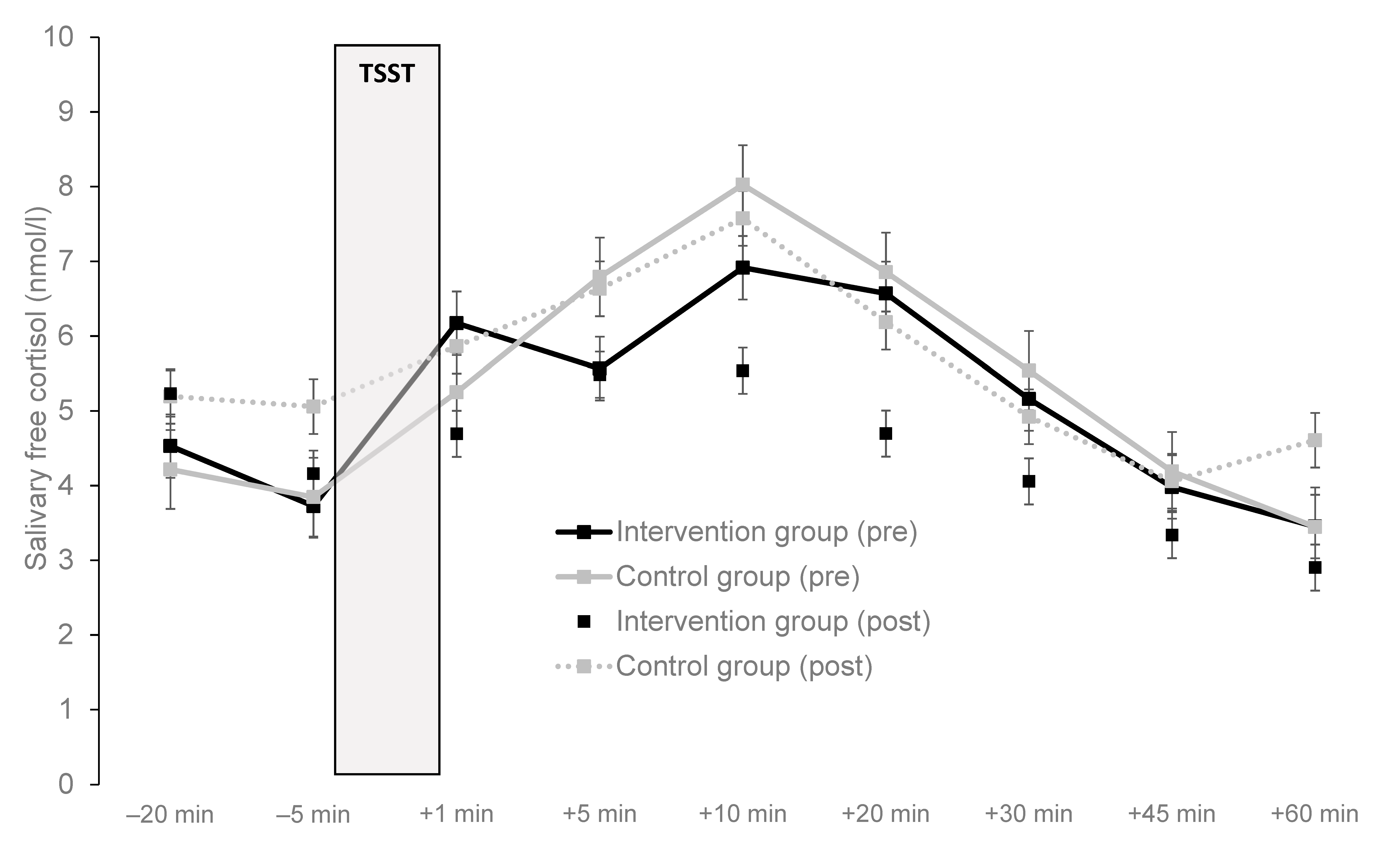
3.4. Impact on Aerobic Exercise Training on Stress Reactivity
3.5. Correlations of Change Between Depressive Symptoms and Cortisol Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baer, N.; Schuler, D.; Füglister-Dousse, S.; Moreau-Gruet, F. Depressionen in der Schweizer Bevölkerung. Daten zur Epidemiologie, Behandlung und Sozialberuflichen Integration (No. 56); Schweizerisches Gesundheitsobservatorium: Neuchâtel, Switzerland, 2013. [Google Scholar]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jein, R.; Koretz, D.; Merikangas, K.R.; Rush, A.J.; Walters, E.E.; Wang, P.S. The epidemiology of Major Depressive Disorder. Results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003, 18, 3095–3105. [Google Scholar] [CrossRef]
- WHO. The World Health Report 2001-mental Health: New Understanding, New Hope; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Lépine, J.-P.; Briley, M. The increasing burden of depression. Neuropsychiatr. Dis. Treat. 2011, 7, 3–7. [Google Scholar] [PubMed] [Green Version]
- Qin, D.-D.; Rizak, J.; Feng, X.-L.; Yang, X.-C.; Lü, L.-B.; Pan, L.; Yin, Y.; Hu, Y.-T. Prolonged secretion of cortisol as a possible mechanism underlying stress and depressive behaviour. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Hammen, C. Stress and depression. Annu. Rev. Clin. Psychol. 2005, 1, 293–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, A.C.; Carroll, D.; Der, G. Negative life events and symptoms of depression and anxiety: Stress causation and/or stress generation. Anxiety Stress Coping 2015, 28, 357–371. [Google Scholar] [CrossRef] [Green Version]
- Siegrist, J. Chronic psychosocial stress at work and risk of depression: Evidence from prospective studies. Eur. Arch. Psychiatry Clin. Neurosci. 2008, 258, 115–119. [Google Scholar] [CrossRef]
- Monroe, K.L.; Harkness, S.M. Life stress, the “kindling” hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychol. Rev. 2005, 112, 417–445. [Google Scholar] [CrossRef] [Green Version]
- Ising, M.; Horstmann, S.; Kloiber, S.; Lucae, S.; Binder, E.B.; Kern, N.; Künzel, H.E.; Pfenning, A.; Uhr, M.; Holsboer, F. Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression-a potential biomarker? Biol. Psychiatry 2007, 62, 47–54. [Google Scholar] [CrossRef]
- Gotlib, I.H.; Joormann, J.; Minor, K.L.; Hallmayer, J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol. Psychiatry 2008, 63, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Schüle, C. Neuroendocrinological mechanisms of actions of antidepressantdrugs. J. Neuroendocrinol. 2007, 19, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Scheel-Krüger, J.; Belzung, C. The neurobiology of depression andantidepressant action. Neurosci. Biobehav. Rev. 2013, 37, 2331–2371. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.E.; Chen, E.; Zhou, E.S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 2007, 133, 25–45. [Google Scholar] [CrossRef] [Green Version]
- Chida, Y.; Steptoe, A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biol. Psychol. 2009, 80, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Chida, Y.; Hamer, M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: A quantitative review of 30 years of investigations. Psychol. Bull. 2008, 134, 829–885. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.L.; Smith, T.L.; Hauger, R.L.; Nicassio, P.M.; Patterson, T.L.; McClintick, J.; Costlow, C.; Irwin, M.R. Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosom. Med. 1997, 59, 447–457. [Google Scholar] [CrossRef]
- Holsboer, F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000, 23, 477–501. [Google Scholar] [CrossRef] [Green Version]
- Modell, S.; Yassouridis, A.; Huber, J.; Holsboer, F. Cortico-steroid receptor function is decreased in depressed patients. Neuroendocrinology 1997, 65, 216–222. [Google Scholar] [CrossRef]
- Stetler, C.; Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef]
- Belvederi Murri, M.; Pariante, C.; Mondelli, V.; Masotti, M.; Atti, A.R.; Mellacqua, Z.; Antonioli, M.; Ghio, L.; Menchetti, M.; Zanetidiou, S.; et al. HPA axis and aging in depression: Systematic review and meta-analysis. Psychoneuroendocrinology 2014, 41, 46–62. [Google Scholar] [CrossRef]
- Knorr, U.; Vinberg, M.; Kessing, L.V.; Wetterslev, J. Salivary cortisol in depressed patients versus control persons: A systematic review and meta-analysis. Psychoneuroendocrinology 2010, 35, 1275–1286. [Google Scholar] [CrossRef]
- Bremmer, M.A.; Deeg, D.J.; Beekman, A.T.; Penninx, B.W.; Lips, P.; Hoogendijk, W.J. Major depression in late life is associated with both hypo- and hypercortisolemia. Biol. Psychiatry 2007, 62, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Oldehinkel, A.J.; van den Berg, M.D.; Flentge, F.; Bouhuys, A.L.; ter Horst, G.J.; Ormel, J. Urinary free cortisol excretion in elderly persons with minor and major depression. Psychiatry Res. 2001, 104, 39–47. [Google Scholar] [CrossRef]
- Dickerson, S.S.; Kemeny, M.E. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004, 130, 355–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handwerger, K. Differential patterns of HPA activity and reactivity in adult post-traumatic stress disorder and major depressive disordr. Harv. Rev. Psychiatry 2009, 17, 184–205. [Google Scholar] [CrossRef] [PubMed]
- Rao, U.; Hammen, C.; Ortiz, L.R.; Chen, L.A.; Poland, R.E. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol. Psychiatry 2008, 64, 521–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, J.G.; Mazurka, R.; Bond, L.; Wynne-Edwards, K.E.; Harkness, K.L. Rumination and impaired contisol recovery following a social stressor in adolescent depression. J. Abnorm. Child Psychol. 2013, 41, 1015–1026. [Google Scholar] [CrossRef]
- Harkness, K.L.; Stewart, J.G.; Wynne-Edwards, K.E. Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrinology 2011, 36, 173–181. [Google Scholar] [CrossRef]
- Luby, J.L.; Heffelfinger, A.; Mrakotsky, C.; Brown, K.W.; Hessler, M.; Spitznagel, E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric no-disorder comparison groups. Arch. Gen. Psychiatry 2003, 60, 1248–1255. [Google Scholar] [CrossRef]
- Burke, H.M.; Davis, S.C.; Otte, C.; Mohr, D.C. Depression and cortisol response to psychological stress: A meta-analysis. Psychoneuroendocrinology 2005, 30, 846–856. [Google Scholar] [CrossRef]
- Ciufolini, S.; Dazzan, P.; Kempton, M.J.; Pariante, C.; Mondelli, V. HPA axis response to social stress is attenuated in schizophrenia but normal in depression: Evidence from a meta-analysis of existing studies. Neurosci. Biobehav. Rev. 2014, 47, 359–368. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Pirke, K.M.; Hellhammer, D.H. The Trier Social Stress Test: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Rao, U.; Wang, L.; Garber, J. Cortisol reactivity to experimentally manipulated psychosocial stress in young adults at varied risk for depression. Depress. Anxiety 2014, 31, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Burke, H.M.; Fernald, L.C.; Gertler, P.J.; Adler, N.E. Depressive symptoms are associated with blunted cortisol stress responses in very low income women. Psychosom. Med. 2005, 67, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Peeters, F.; Nicholson, N.A.; Berkhof, J. Cortisol responses to daily events in major depressive disorder. Psychosom. Med. 2003, 65, 836–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Duran, N.L.; Kovacs, M.; George, C.J. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology 2009, 34, 1272–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, F.; Nicolson, N.A.; Berkhof, J. Levels and variability of daily life cortisol secretion in major depression. Psychiatry Res. 2004, 126, 1–13. [Google Scholar] [CrossRef]
- Von Dawans, B.; Heinrichs, M. Physiologische Stressreaktionen. In Stressregulation und Sport; Fuchs, R., Gerber, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Mücke, M.; Ludyga, S.; Colledge, F.; Gerber, M. Influence of regular physical activity and fitness on stress reactivity as measured with the Trier Social Stress Test protocol: A systematic review. Sports Med. 2018, 48, 2607–2622. [Google Scholar] [CrossRef]
- Sothmann, M.S. The cross-stressor adaptation hypothesis and exercise training. In Psychobiology of Physical Activity; Acevedo, E.O., Ekkekakis, P., Eds.; Human Kinetics: Champaign, IL, USA, 2006; pp. 149–160. [Google Scholar]
- Klaperski, S.; von Dawans, B.; Heinrichs, M.; Fuchs, R. Effects of a 12-week endurance training program on the physiological response to psychosocial stress in men: A randomized controlled trial. J. Behav. Med. 2014, 37, 1118–1133. [Google Scholar] [CrossRef]
- Schuch, F.B.; Dunn, A.L.; Kanitz, A.C.; Delevatti, R.S.; Fleck, M.P. Moderators of response in exercise treatment for depression: A systematic review. J. Affect. Disord. 2016, 195, 40–49. [Google Scholar] [CrossRef]
- Rethorst, C.D.; Wipfli, B.M.; Landers, D.M. The antidepressive effects of exercise: A meta-analysis of randomized trials. Sports Med. 2009, 39, 491–511. [Google Scholar] [CrossRef]
- Krogh, J.; Hjorthøj, C.; Speyer, H.; Gluud, C.; Nordentoft, M. Exercise for patients with major depression: A systematic review with meta-analysis and trial sequential analysis. BMJ Open 2017, 7, e014820. [Google Scholar] [CrossRef] [PubMed]
- Nebiker, L.; Lichtenstein, E.; Minghetti, A.; Zahner, L.; Gerber, M.; Faude, O.; Donath, L. Moderating effects of exercise duration and intensity in neuromuscular versus endurance exercise interventions for the treatment of depression: A meta-analytical review with meta-regression and sensitivity analyses. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Cooney, G.M.; Dwan, K.; Greig, C.A.; Lawlor, D.A.; Rimer, J.; Waugh, F.R.; McMurdo, M.E.T.; Mead, G.E. Exercise for depression. Cochrane Database Syst. Rev. 2013, 9. [Google Scholar] [CrossRef]
- Josefsson, T.; Lindwall, M.; Archer, T. Physical exercise intervention in depressive disorders: Meta-analysis and systematic review. Scand. J. Med. Sci. Sports 2013, 24, 259–272. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stubbs, B.; Rosenbaum, S.; Vancampfort, D.; Ward, P.B.; Schuch, F.B. Exercise improves cardiorespiratory fitness in people with depression: A meta-analysis of randomized controlled trials. J. Affect. Disord. 2016, 190, 249–253. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Michael, A.; Babjak, A. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom. Med. 2007, 69, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Schuch, F.B.; Vasconcelos-Moreno, M.P.; Borowsky, C.; Fleck, M.P. Exercise and severe depression: Preliminary results of an add-on study. J. Affect. Disord. 2011, 133, 615–618. [Google Scholar] [CrossRef]
- Mota-Pereira, J.; Silverio, J.; Carvalho, S.; Ribeiro, J.C.; Fonte, D.; Ramos, J. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J. Psychiatr. Res. 2011, 45, 1005–1011. [Google Scholar] [CrossRef]
- Schuch, F.B.; Camaz Deslandes, A.; Stubbs, B.; Pereira Gosmann, N.; Tschiedel Belem da Silva, C.; de Almeida Fleck, M.P. Neurobiological effects of exercise on major depressive disorder: A systematic review. Neurosci. Biobehav. Rev. 2016, 61, 1–11. [Google Scholar] [CrossRef]
- Kandola, A.; Vancampfort, D.; Herring, M.P.; Rebar, A.L.; Hallgren, M.; Firth, J.; Stubbs, B. Moving to beat anxiety: Epidemiology and therapeutic issues with physical activity for anxiety. Curr. Psychiatry Rep. 2018, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matta Mello Portugal, E.; Cevada, T.; Sobral Monteiro-Junior, R.; Teixeira Guimarães, T.; da Cruz Rubini, E.; Lattari, E.; Blois, C.; Camaz Deslandes, A. Neuroscience of exercise: From neurobiology mechanisms to mentalhealth. Neuropsychobiology 2013, 68, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wegner, M.; Helmich, I.; Machado, S.; Nardi, A.E.; Arias-Carrion, O.; Budde, H. Effects of exercise on anxiety and depression disorders: Review of meta-analyses and neurobiological mechanisms. CNS Neurol. Disord. Drug Targets 2014, 13, 1002–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiive, E.; Maaroos, J.; Shlik, J.; Toru, I.; Harro, J. Growth hormone, cortisol and prolactin responses to physical exercise: Higher prolactin response in depressed patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 1007–1013. [Google Scholar] [CrossRef]
- Krogh, J.; Nordentoft, M.; Mohammad-Nezhad, M.; Westrin, A. Growth hormone, prolactin and cortisol response to exercise in patients with depression. J. Affect. Disord. 2010, 125, 189–197. [Google Scholar] [CrossRef]
- Ida, M.; Ida, I.; Wada, N.; Sohmiya, M.; Tazawa, M.; Shirakura, K. A clinical study of the efficacy of a single session of individual exercise for depressive patients, assessed by the change in saliva free cortisol level. Biopsychosoc. Med. 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Foley, L.S.; Prapavessis, H.; Osuch, E.A.; De Pace, J.A.; Murphy, B.A.; Podolinsky, N.J. An examination of potential mechanisms for exercise as a treatment fordepression: A pilot study. Ment. Health Phys. Act. 2008, 1, 69–73. [Google Scholar] [CrossRef]
- Katan, M.; Christ-Crain, M. The stress hormone copeptin: A new prognostic biomarker in acute illness. Swiss Med. Wkly. 2010, 140. [Google Scholar] [CrossRef]
- Krogh, J.; Gøtze, J.P.; Jørgensen, M.B.; Kristensen, L.Ø.; Kistorp, C.; Nordentoft, M. Copeptin during rest and exercise in major depression. J. Affect. Disord. 2013, 151, 284–290. [Google Scholar] [CrossRef]
- Pariante, C.; Miller, A.H. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol. Psychiatry 2001, 49, 391–404. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Hartman, M.L. Cortsiol and growth hormone responses to exercise. Endocrinologist 2002, 12, 421–435. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Lee, K.; Mattson, M.P. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromolecular Med. 2008, 10, 118–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Nakagawa, S.; An, Y.; Ito, K.; Kitaichi, Y.; Kusumi, I. The exercise glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front. Neuroendocrinol. 2017, 44, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.M.; Shaikh, M.A.K.; Shaikh, I.B. Exercise as a treatment modality for depression: A narrative review. Alex. J. Med. 2018, 54, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Kandola, A.; Ashdown-Franks, G.; Hendrikse, J.; Sabiston, C.M.; Stubbs, B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019, 107, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Kennedy, P.J.; Dockray, S.; Cryan, J.F.; Dinan, T.G.; Clarke, G. The Trier Social Stress Test: Principles and practice. Neurobiol. Stress 2016, 6, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Imboden, C.; Gerber, M.; Beck, J.; Eckert, A.; Pühse, U.; Holsboer-Trachsler, E.; Hatzinger, M. Effects of aerobic exercise as add-on treatment for inpatients with moderate to severe depression on depression severity, sleep, cognition, psychological well-being, and biomarkers: Study protocol, description of study population, and manipulation-check. Front. Psychiatry 2019. [Google Scholar] [CrossRef]
- Imboden, C.; Gerber, M.; Beck, J.; Holsboer-Trachsler, E.; Pühse, U.; Hatzinger, M. Aerobic exercise or stretching as add-on to inpatient treatment of depression: Similar antidepressant effects on depressive symptoms and larger effects on working memory for aerobic exercise alone. In revision.
- Dunn, A.L.; Trivedi, M.H.; Kampert, J.B.; Clark, C.G.; Chambliss, H.O. Exercise treatment for depression: Efficacy and dose response. Am. J. Prev. Med. 2005, 28, 1–8. [Google Scholar] [CrossRef]
- Holsboer-Trachsler, E.; Hättenschwiler, J.; Beck, J.; Brand, S.; Hemmeter, U.M.; Keck, M.E.; Rennhard, S.; Hatzinger, M.; Merlo, M.; Bondolfi, G.; et al. Die somatische Behandlung der unipolaren depressiven Störung-Teil 1. Schweiz. Med. Forum 2010, 10, 802–809. [Google Scholar]
- Kudielka, B.M.; Schommer, N.C.; Hellhammer, D.H.; Kirschbaum, C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TST) in humans at different times of day. Psychoneuroendocrinology 2004, 29, 983–992. [Google Scholar] [CrossRef]
- Taylor, A.C. Physical activity, anxiety, and stress. In Physical Activity and Psychological Well-being; Biddle, S.J., Fox, K.R., Boutcher, S.H., Eds.; Routledge: London, UK, 2000; pp. 10–45. [Google Scholar]
- Kanaley, J.A.; Weltman, J.Y.; Pieper, K.S.; Weltman, A.; Hartman, M.L. Cortisol and growth hormone responses to exercise at different times of day. J. Clin. Endocrinol. Metab. 2001, 86, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Boesch, M.; Sefidan, S.; Ehlert, U.; Annen, H.; Wyss, T.; Steptoe, A.; La Marca, R. Mood and autonomic responses to repeated exposure to the Trier Social Stress Test for Groups (TSST-G). Psychoneuroendocrinology 2014, 43, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrowski, K.; Winteramnn, G.B.; Siepmann, M. Cortisol responses to repeated psychosocial stress. Appl. Psychophysiol. Feedback 2012, 37, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Mazurka, R.; Wynne-Edwards, K.E.; Harkness, K.L. Sex differences in the cortisol response to the Trier Social Stress Test in depressed and nondepressed adolescents. Clin. Psychol. Sci. 2018, 6, 301–314. [Google Scholar] [CrossRef]
- Dressendörfer, R.A.; Kirschbaum, C.; Rohde, W.; Stahl, F.; Strasburger, C.J. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. Clin. Psychol. Sci. 1992, 43, 683–692. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. J. Steroid Biochem. Mol. Boil. 1961, 4, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Beck, A.T.; Steer, R.A.; Carbin, M.G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 1988, 8, 77–100. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.P.; et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 81. [Google Scholar] [CrossRef] [Green Version]
- Petrowski, K.; Herold, U.; Joraschky, P.; Wittchen, H.U.; Kirschbaum, C. A striking pattern of cortisol non-responsiveness to psychosocial stress in patients with panic disorder with concurrent normal cortisol awakening responses. Psychoneuroendocrinology 2010, 35, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve rpresent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, W.W.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, A.; Bauman, A. Physical activity and public health. Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sport Exerc. 2007, 39, 1423–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Dawans, B.; Kirschbaum, C.; Heinrichs, M. The Trier Social Stress Test for Groups (TSST-G): A new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology 2011, 36, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Belden, A.C.; Spitznagel, E.; Dietrich, R.; Luby, J.L. Blunted stress cortisol reactivity and failure to acclimate to familiar stress in depressed and sub-syndromal children. Psychiatry Res. 2013, 210, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Gunnar, M.R.; Vazquez, D.M. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev. Psychopathol. 2001, 13, 515–538. [Google Scholar] [CrossRef]
- McEwen, B.S.; Stellar, E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef]
- Juster, R.P.; McEwen, B.S.; Lupien, S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef]
- Lambert, G.; Schlaich, M.; Lambert, E.; Dawood, T.; Esler, M. Stress reactivity and its association with increased cardiovascular risk: A role for the sympathetic nervous system? Hypertension 2010, 55, e20. [Google Scholar] [CrossRef] [Green Version]
- Chida, Y.; Steptoe, A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension 2010, 55, 1026–1032. [Google Scholar] [CrossRef] [Green Version]
- Lovallo, W.R. Do low levels of stress reactivity signal poor states of helath. Biol. Psychol. 2011, 86, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Schuch, F.B.; Vancampfort, D.; Firth, J.; Rosenbaum, S.; Ward, P.B.; Reichert, T.; Bagatini, N.; Bgeginski, R.; Stubbs, B. Physical activity and sedentary behavior in people with major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2017, 210, 139–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, S.; Colledge, F.; Beeler, N.; Pühse, U.; Kalak, N.; Sadeghi Bahmani, D.; Mikoteit, T.; Holsboer-Trachsler, E.; Gerber, M. The current state of physical activity and exercise programs in German-speaking, Swiss psychiatric hospitals: Results from a brief online survey. Neuropsychiatr. Dis. Treat. 2016, 12, 1309–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrbar, J.; Brand, S.; Colledge, F.; Donath, L.; Egger, S.T.; Hatzinger, M.; Holsboer-Trachsler, E.; Imboden, C.; Schweinfurth, N.; Vetter, S.; et al. Psychiatric in-patients are more likely to meet recommended levels of health-enhancing physical activity if they engage in exercise and sport therapy programs. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Holsboer-Trachsler, E.; Pühse, U.; Brand, S. Exercise is medicine for patients with major depressive disorders. But only if the “pill” is taken! Neuropsychiatr. Dis. Treat. 2016, 12, 1977–1981. [Google Scholar] [CrossRef] [Green Version]
- Chalder, M.; Wiles, N.J.; Campbell, J.; Hollinghurst, S.P.; Haase, A.M.; Taylor, A.H.; Fox, K.R.; Costelloe, C.; Searle, A.; Baxter, H.; et al. Facilitated physical activity as a treatment for depressed adults: Randomized controlled trial. BMJ 2012, 344, e2758. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, B.M.; Babyak, M.A.; Craighead, E.; Sherwood, A.; Doraiswamy, P.M.; Coons, M.J.; Blumenthal, J.A. Exercise and pharmacotherapy in patients with major depression: One-year follow-up of the SMILE study. Psychosom. Med. 2011, 73, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Lindegård, A.; Jonsdottir, I.H.; Börjesson, M.; Lindwall, M.; Gerber, M. Changes in mental health in compliers and non-compliers with physical activity recommendations in patients with stress-related exhaustion. BMC Psychiatry 2015, 15. [Google Scholar] [CrossRef] [Green Version]
- Dockray, S.; Susman, E.J.; Dorn, L.D. Depression, cortisol reactivity and obesity in childhood and adolescence. J. Adolesc. Health 2009, 45, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Hilt, L.M.; Nolen-Hoeksema, S. Gender differences in depression. In Handbook of Depression; Gotlib, I.H., Hammen, C.L., Eds.; The Quilford Press: New York, NY, USA, 2014; pp. 355–373. [Google Scholar]
- Liu, J.J.W.; Ein, N.; Peck, K.; Huang, V.; Pruessner, J.C.; Vickers, K. Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): A meta-analysis. Psychoneuroendocrinology 2017, 82, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Childs, E.; de Wit, H. Regular exercise is associated with emotional resilience to acute stress in healthy adults. Front. Physiol. 2014, 5, 161. [Google Scholar] [CrossRef]
- Kudielka, B.M.; Federenko, I.S.; Hellhammer, D.H.; Wüst, S. Morningness and eveningness: The free cortisol rise after awakening in “early birds” and “night owls”. Biol. Psychol. 2006, 72, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Ludyga, S.; Mücke, M.; Colledge, F.; Brand, S.; Pühse, U. Low vigorous physical activity is associated with increased adrenocortical reactivity to psychosocial stress in students with high stress perceptions. Psychoneuroendocrinology 2017, 80, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Chopra, K.K.; Ravindran, A.; Kennedy, S.H.; Mackenzie, B.; Matthews, S.; Anisman, H.; Bagby, R.M.; Farvolden, P.; Levitan, R.D. Sex differences in hormonal responses to a social stressor in chronic major depression. Psychoneuroendocrinology 2009, 34, 1235–1241. [Google Scholar] [CrossRef]
- Murck, H. Atypical depression spectrum disorder – Neurobiology and treatment. Acta Neuropsychiatr. 2003, 15, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Zanstra, Y.J.; Johnston, D.W. Cardiovascular reactivity in real life settings: Measurement, mechanisms and meaning. Biol. Psychol. 2011, 86, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.; Ehlert, U. Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneruoendocrinology 2012, 37, 1111–1134. [Google Scholar] [CrossRef]
| Total Sample (n = 25) | Intervention Group (n = 14) | Control Group (n = 11) | Test of between-Group Differences (ANOVAs) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metric variables (normally distributed) | M | SD | M | SD | M | SD | F | p | η2 | ||
| Age | 38.1 | 12.0 | 39.4 | 9.7 | 36.4 | 14.8 | 0.39 | 0.539 | 0.017 | ||
| Depressive symptom severity (BDI) at baseline | 26.4 | 8.6 | 27.6 | 10.0 | 24.9 | 6.6 | 0.61 | 0.443 | 0.026 | ||
| Depressive symptom severity (BDI) at post-intervention | 15.7 | 11.1 | 17.1 | 12.5 | 14.0 | 9.2 | 0.46 | 0.503 | 0.020 | ||
| Age at onset of depression | 33.1 | 12.9 | 33.8 | 12.1 | 32.2 | 14.5 | 0.09 | 0.766 | 0.004 | ||
| Resting heart rate (bpm) | 75.0 | 12.8 | 78.1 | 14.3 | 71.0 | 9.8 | 1.95 | 0.176 | 0.078 | ||
| Systolic blood pressure (mmHg) | 123.9 | 18.2 | 128.4 | 18.9 | 118.3 | 16.3 | 1.97 | 0.173 | 0.079 | ||
| Diastolic blood pressure (mmHg) | 75.9 | 9.9 | 78.6 | 7.0 | 72.4 | 12.1 | 1.95 | 0.176 | 0.078 | ||
| Height (cm) | 172.1 | 8.1 | 171.6 | 7.4 | 172.7 | 9.3 | 0.12 | 0.732 | 0.005 | ||
| Weight (kg) | 71.6 | 20.3 | 74.1 | 22.2 | 68.4 | 18.2 | 0.47 | 0.500 | 0.020 | ||
| BMI (kg/m2) | 23.7 | 4.8 | 24.7 | 5.4 | 22.6 | 3.8 | 1.20 | 0.285 | 0.050 | ||
| Metric variables (non-normally distributed) | Mdn | min; max | Mdn | min; max | Mdn | min; max | Independent Samples MedianTest (p-Value) | Independent Samples Kruskal-Wallis Test (p-Value) | |||
| Duration of current depressive episode | 12 | 3; 52 | 10 | 3; 52 | 13 | 5; 50 | 0.202 | 0.703 | |||
| Number of prior depressive episodes | 1 | 0; 5 | 1 | 0; 5 | 1 | 0; 5 | 0.666 | 0.649 | |||
| Moderate-to-vigorous physical activity (min/week) | 60 | 0; 540 | 60 | 0; 360 | 45 | 0; 540 | 0.609 | 0.582 | |||
| Cortisol level –20 min prior to TSST onset (nmol/L) at baseline | 3.2 | 1.2; 8.8 | 3.0 | 2.1; 8.8 | 4.3 | 1.2; 7.9 | 0.609 | 0.582 | |||
| Cortisol level +20 min after completion of TSST (nmol/L) at baseline | 5.2 | 1.1; 31.9 | 4.2 | 1.7; 31.9 | 8.1 | 1.1; 16.1 | 0.344 | 0.324 | |||
| Overall cortisol response (AUCG) at baseline | 380.8 | 114.5; 1225.6 | 299.2 | 157.4; 1225.6 | 521.0 | 114.8; 805.0 | 0.267 | 0.250 | |||
| Cortisol level –20 min prior to TSST onset (nmol/L) at post-intervention | 5.0 | 2.0; 13.9 | 4.7 | 2.0; 13.9 | 5.9 | 2.4; 9.1 | 0.687 | 0.661 | |||
| Cortisol level + 20 min after completion of TSST (nmol/L) at post-intervention | 4.7 | 2.2; 21.4 | 3.0 | 2.2; 16.1 | 5.2 | 2.5; 21.4 | 0.095 | 0.090 | |||
| Overall cortisol response (AUCG) at post-intervention | 392.3 | 200.2; 1252.0 | 351.1 | 200.2; 992.5 | 429.6 | 217.6; 1252.0 | 0.202 | 0.189 | |||
| Categorial variables | n | % | n | % | n | % | χ2 | p | |||
| Sex (females) | 13 | 52 | 6 | 43 | 7 | 64 | 1.07 | 0.302 | |||
| Smoking status (smokers) | 9 | 36 | 4 | 29 | 5 | 46 | 0.76 | 0.383 | |||
| Educational background | |||||||||||
| Compulsory school | 3 | 13 | 2 | 18 | 1 | 9 | 3.30 | 0.194 | |||
| High school | 14 | 64 | 5 | 46 | 9 | 82 | |||||
| Higher education | 5 | 23 | 4 | 36 | 1 | 9 | |||||
| Diagnoses | |||||||||||
| F31 (Bipolar affective disorder) | 1 | 4 | 1 | 7 | 0 | 0 | 0.90 | 0.625 | |||
| F32 (Unipolar depressive episode) | 10 | 40 | 5 | 36 | 5 | 46 | |||||
| F33 (Recurrent depressive disorder) | 14 | 56 | 8 | 57 | 6 | 54 | |||||
| Bivariate Correlations with AUCG a | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metric Variables | Baseline | Post-Intervention | |||||||||||
| r/Rho | p | r/Rho | p | ||||||||||
| Age b | 0.09 | 0.671 | −0.07 | 0.744 | |||||||||
| Depressive symptom severity (BDI) at baseline b | 0.17 | 0.430 | −0.17 | 0.419 | |||||||||
| Duration of current depressive episode c | 0.155 | 0.458 | −0.10 | 0.631 | |||||||||
| Number of prior depressive episodes c | 0.11 | 0.601 | −0.35 | 0.095 | |||||||||
| Age at onset of depression b | −0.04 | 0.849 | −0.05 | 0.817 | |||||||||
| Resting heart rate (bpm) b | −0.22 | 0.301 | 0.14 | 0.514 | |||||||||
| Systolic blood pressure (mmHg) b | 0.09 | 0.662 | 0.04 | 0.856 | |||||||||
| Diastolic blood pressure (mmHg) b | 0.28 | 0.184 | 0.26 | 0.214 | |||||||||
| Height (cm) b | 0.34 | 0.091 | 0.20 | 0.337 | |||||||||
| Weight (kg) b | 0.33 | 0.104 | 0.22 | 0.300 | |||||||||
| BMI (kg/m2) b | 0.27 | 0.185 | 0.17 | 0.410 | |||||||||
| Moderate-to-vigorous physical activity (min/week) c | |||||||||||||
| Group differences in AUCG d | |||||||||||||
| Categorial variables | Baseline | Post-intervention | |||||||||||
| M | SD | F | p | η2 | M | SD | F | p | η2 | ||||
| Sex | 2.0 | 0.174 | 0.100 | 0.0 | 0.937 | 0.000 | |||||||
| Males | 538 | 312 | 413 | 135 | |||||||||
| Females | 373 | 192 | 466 | 315 | |||||||||
| Smoking status | 1.3 | 0.268 | 0.053 | 0.0 | 0.836 | 0.002 | |||||||
| Smokers | 506 | 223 | 425 | 201 | |||||||||
| Non-smokers | 421 | 224 | 468 | 315 | |||||||||
| Educational background | 0.1 | 0.942 | 0.006 | 0.1 | 0.939 | 0.007 | |||||||
| Compulsory school | 421 | 274 | 405 | 172 | |||||||||
| High school | 467 | 197 | 480 | 300 | |||||||||
| Higher education | 573 | 440 | 413 | 153 | |||||||||
| Diagnoses | 0.6 | 0.537 | 0.055 | 0.1 | 0.909 | 0.009 | |||||||
| F31 (Bipolar affective disorder) | 720 | 0.0 | 440 | 0.0 | |||||||||
| F32 (Unipolar depressive episode) | 443 | 340 | 433 | 142 | |||||||||
| F33 (Recurrent depressive disorder) | 439 | 209 | 445 | 242 | |||||||||
| Baseline | Post-Intervention | Time | Group | Time × Group Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stress reactivityas assessed via … | n | M | SD | M | SD | F | p | η2 | F | p | η2 | F | p | η2 |
| AUCG a in the … | 0.1 | 0.798 | 0.003 | 1.7 | 0.209 | 0.068 | 0.0 | 0.860 | 0.001 | |||||
| Total sample | 25 | 452 | 265 | 441 | 242 | |||||||||
| Intervention group | 14 | 423 | 301 | 393 | 215 | |||||||||
| Control group | 11 | 489 | 219 | 501 | 272 | |||||||||
| Depressive symptoms as assessed via the … | n | M | SD | M | SD | F | p | η2 | F | p | η2 | F | p | η2 |
| BDI in the … | 34.8 | 0.000 | 0.602 | 0.6 | 0.429 | 0.027 | 0.0 | 0.927 | 0.000 | |||||
| Total sample | 25 | 26.4 | 8.6 | 15.7 | 11.1 | |||||||||
| Intervention group | 14 | 27.6 | 10.0 | 17.1 | 12.5 | |||||||||
| Control group | 11 | 24.9 | 6.6 | 14.0 | 9.2 | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerber, M.; Imboden, C.; Beck, J.; Brand, S.; Colledge, F.; Eckert, A.; Holsboer-Trachsler, E.; Pühse, U.; Hatzinger, M. Effects of Aerobic Exercise on Cortisol Stress Reactivity in Response to the Trier Social Stress Test in Inpatients with Major Depressive Disorders: A Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1419. https://doi.org/10.3390/jcm9051419
Gerber M, Imboden C, Beck J, Brand S, Colledge F, Eckert A, Holsboer-Trachsler E, Pühse U, Hatzinger M. Effects of Aerobic Exercise on Cortisol Stress Reactivity in Response to the Trier Social Stress Test in Inpatients with Major Depressive Disorders: A Randomized Controlled Trial. Journal of Clinical Medicine. 2020; 9(5):1419. https://doi.org/10.3390/jcm9051419
Chicago/Turabian StyleGerber, Markus, Christian Imboden, Johannes Beck, Serge Brand, Flora Colledge, Anne Eckert, Edith Holsboer-Trachsler, Uwe Pühse, and Martin Hatzinger. 2020. "Effects of Aerobic Exercise on Cortisol Stress Reactivity in Response to the Trier Social Stress Test in Inpatients with Major Depressive Disorders: A Randomized Controlled Trial" Journal of Clinical Medicine 9, no. 5: 1419. https://doi.org/10.3390/jcm9051419
APA StyleGerber, M., Imboden, C., Beck, J., Brand, S., Colledge, F., Eckert, A., Holsboer-Trachsler, E., Pühse, U., & Hatzinger, M. (2020). Effects of Aerobic Exercise on Cortisol Stress Reactivity in Response to the Trier Social Stress Test in Inpatients with Major Depressive Disorders: A Randomized Controlled Trial. Journal of Clinical Medicine, 9(5), 1419. https://doi.org/10.3390/jcm9051419







