COVID-19, MERS and SARS with Concomitant Liver Injury—Systematic Review of the Existing Literature
Abstract
1. Introduction
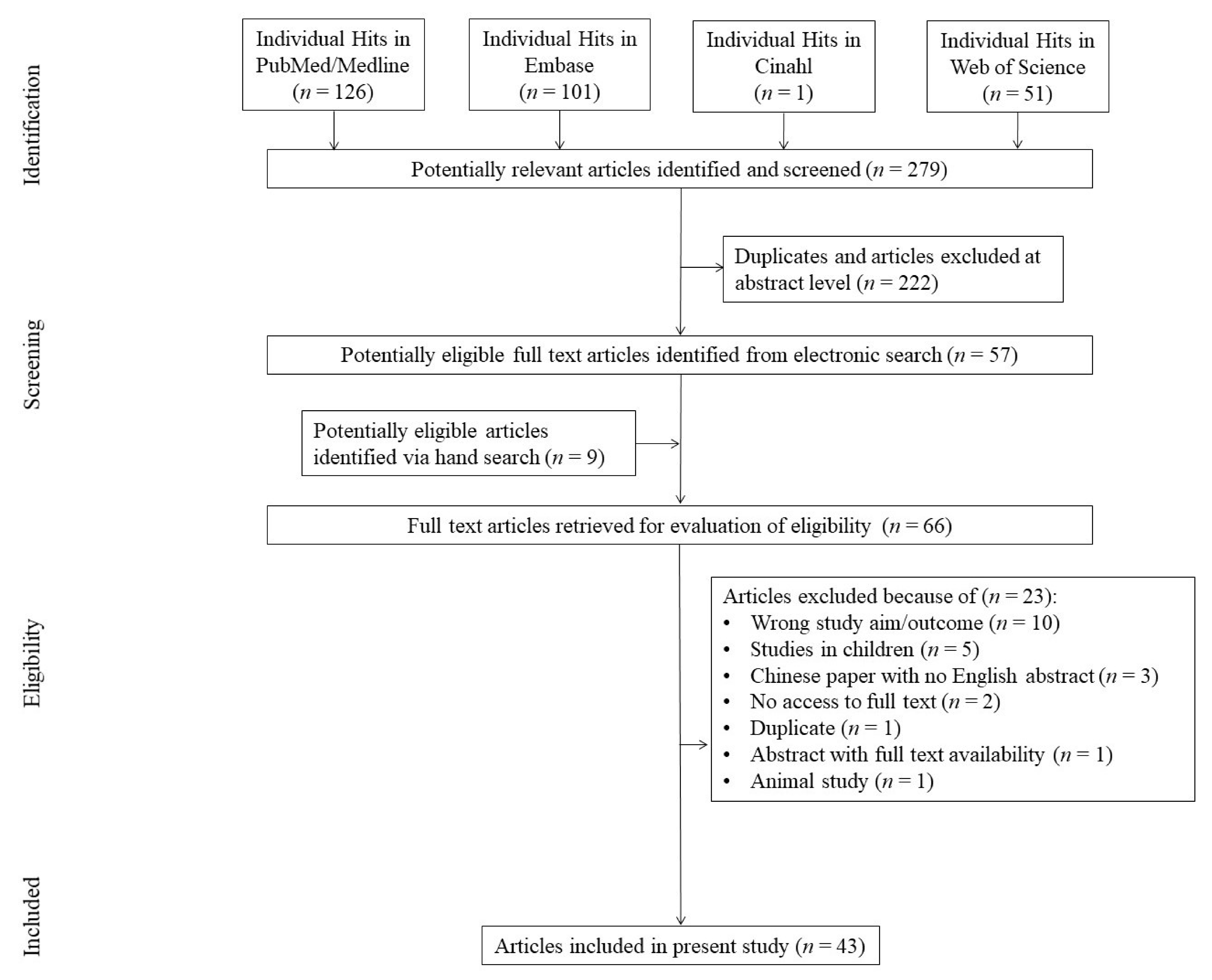
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Inclusion Criteria
- Human retrospective studies, including case reports and case series reporting clinical liver-related data in patients infected with SARC-CoV-1, SARS-CoV-2 and MERS;
- Any of the following clinical data: alanine aminotransferase (ALT)/aspartate aminotransferase (AST)/gamma-glutamyl-transpeptidase (GGTP)/alkaline phosphatase (ALP)/lactate dehydrogenase (LDH)/creatinine (Cr)/bilirubin (BIL)/total protein (TP)/albumin (ALB)/international normalised ratio (INR)/prothrombin time expressed either qualitatively (percent of abnormal results) or quantitatively (PT);
- Post-mortem studies reporting liver histopathology.
2.3. Data Extraction and Analysis
2.4. Risk of Bias Assessment
3. Results
3.1. Descriptive Data
3.2. Liver-Related Outcomes
3.3. Risk of Bias (ROB)
4. Discussion
4.1. COVID-19 Patients and Liver Injury
4.2. Hepatic Dysfunction Associated with ICU Procedures
4.3. Hepatotoxicity Related to COVID-19
4.4. Comorbidities and Liver Injury in COVID-19 Patients
4.5. SARS-CoV-1 and Liver Injury
4.6. MERS-CoV and Liver Injury
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Phelan, A.L.; Katz, R.; Gostin, L.O. The Novel Coronavirus Originating in Wuhan, China: Challenges for Global Health Governance. JAMA 2020. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection (SARI) when COVID-19 Disease is Suspected: Interim Guidance. Available online: https://apps.who.int/iris/handle/10665/331446 (accessed on 6 April 2020).
- Jin, X.; Lian, J.-S.; Hu, J.-H.; Gao, J.; Zheng, L.; Zhang, Y.-M.; Hao, S.-R.; Jia, H.-Y.; Cai, H.; Zhang, X.-L.; et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020. [Google Scholar] [CrossRef] [PubMed]
- Clinical Characteristics of COVID-19 Patients with Digestive Symptoms in Hubei, China. Available online: https://www.practiceupdate.com/content/clinical-characteristics-of-covid-19-patients-with-digestive-symptoms-in-hubei-china/98000 (accessed on 6 April 2020).
- Gu, J.; Han, B.; Wang, J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology 2020, 158, 1518–1519. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Skonieczna-Żydecka, K. COVID-19: Gastrointestinal symptoms and potential sources of 2019-nCoV transmission. Anaesthesiol. Intensive Ther. 2020, 52. [Google Scholar] [CrossRef]
- Alsaad, K.O.; Hajeer, A.H.; Al Balwi, M.; Al Moaiqel, M.; Al Oudah, N.; Al Ajlan, A.; AlJohani, S.; Alsolamy, S.; Gmati, G.E.; Balkhy, H.; et al. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection—clinicopathological and ultrastructural study. Histopathology 2018, 72, 516–524. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Liu, L.; Zhao, M.; Xiao, J.; Zhao, Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver Int. 2020. [Google Scholar] [CrossRef]
- Bangash, M.N.; Patel, J.; Parekh, D. COVID-19 and the liver: Little cause for concern. Lancet Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Xu, L.; Liu, J.; Lu, M.; Yang, D.; Zheng, X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Chau, T.-N.; Lee, K.-C.; Yao, H.; Tsang, T.-Y.; Chow, T.-C.; Yeung, Y.-C.; Choi, K.-W.; Tso, Y.-K.; Lau, T.; Lai, S.-T.; et al. SARS-associated viral hepatitis caused by a novel coronavirus: Report of three cases. Hepatology 2004, 39, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Chuan, L.; Zicheng, J.; Chuxiao, S.; Hongguang, Z.; Hongmei, Y.; Zhenhuai, C.; Baoyi, M.; Weiying, L.; Huihong, H.; Jie, Y.; et al. Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: A multicenter study. Chin. J. Hepatol. 2020, 28, 148–152. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, L.; Wang, F.-S. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020. [Google Scholar] [CrossRef]
- Xu, X.-W.; Wu, X.-X.; Jiang, X.-G.; Xu, K.-J.; Ying, L.-J.; Ma, C.-L.; Li, S.-B.; Wang, H.-Y.; Zhang, S.; Gao, H.-N.; et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ 2020, 368. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Zhao, X.; Liu, C.; Wang, W.; Wang, D.; Xu, W.; Zhang, C.; Yu, J.; Jiang, B.; et al. Clinical Characteristics of Imported Cases of COVID-19 in Jiangsu Province: A Multicenter Descriptive Study. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Chan, H.L.Y.; Leung, W.-K.; To, K.-F.; Chan, P.K.S.; Lee, N.; Wu, A.; Tam, J.S.L.; Sung, J.J.Y. Retrospective analysis of liver function derangement in severe acute respiratory syndrome. Am. J. Med. 2004, 116, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.-L.-Y.; Kwan, A.-C.-P.; To, K.-F.; Lai, S.-T.; Chan, P.K.-S.; Leung, W.-K.; Lee, N.; Wu, A.; Sung, J.J.-Y. Clinical significance of hepatic derangement in severe acute respiratory syndrome. World J. Gastroenterol. 2005, 11, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Xie, Y.; Wan, J.; Lu, Z.; Wang, D.; Wang, Q.; Xue, X.; Si, W.; Luo, Y.; et al. [Morphological study of severe acute respiratory syndrome (SARS)]. Zhonghua Bing Li Xue Za Zhi 2003, 32, 516–520. [Google Scholar]
- Cui, H.-J.; Tong, X.-L.; Li, P.; Hao, Y.-X.; Chen, X.-G.; Li, A.-G.; Zhang, Z.-Y.; Duan, J.; Zhen, M.; Zhang, B.; et al. Serum hepatic enzyme manifestations in patients with severe acute respiratory syndrome: Retrospective analysis. World J. Gastroenterol. 2004, 10, 1652–1655. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, H.; Shen, H.; Li, Z.; Geng, J.; Han, H.; Cai, J.; Li, X.; Kang, W.; Weng, D.; et al. The clinical pathology of severe acute respiratory syndrome (SARS): A report from China. J. Pathol. 2003, 200, 282–289. [Google Scholar] [CrossRef]
- Farcas, G.A.; Poutanen, S.M.; Mazzulli, T.; Willey, B.M.; Butany, J.; Asa, S.L.; Faure, P.; Akhavan, P.; Low, D.E.; Kain, K.C. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J. Infect. Dis. 2005, 191, 193–197. [Google Scholar] [CrossRef]
- Guan, Y.; Tang, X.; Yin, C.; Yi, Z. [Study on the damage of liver in patients with SARS]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2004, 16, 267–270. [Google Scholar] [PubMed]
- Han, Y.; Geng, H.; Feng, W.; Tang, X.; Ou, A.; Lao, Y.; Xu, Y.; Lin, H.; Liu, H.; Li, Y. A follow-up study of 69 discharged SARS patients. J. Tradit. Chin. Med. 2003, 23, 214–217. [Google Scholar]
- Hsiao, C.-H.; Wu, M.-Z.; Hsieh, S.-W.; Chien, L.-C.; Hwang, K.-C.; Su, I.-J. Clinicopathology of severe acute respiratory syndrome: An autopsy case report. J. Formos. Med. Assoc. 2004, 103, 787–792. [Google Scholar]
- Kumar, D.; Tellier, R.; Draker, R.; Levy, G.; Humar, A. Severe Acute Respiratory Syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am. J. Transplant. 2003, 3, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Lang, Z.; Zhang, L.; Zhang, S.; Meng, X.; Li, J.; Song, C.; Sun, L.; Zhou, Y. A clinicopathological study on 3 cases of severe acute respiratory syndrome. Zhonghua Bing Li Xue Za Zhi 2003, 32, 201–204. [Google Scholar] [PubMed]
- Liu, Z.; Li, T.; Wang, Z.; Xu, Z.; Wang, H.; Yu, Y.; Du, T.; Bai, Y.; Qiu, Z.; Lü, W.; et al. Clinical features and therapy of 106 cases of severe acute respiratory syndrome. Zhonghua Nei Ke Za Zhi 2003, 42, 373–377. [Google Scholar] [PubMed]
- Luo, H.-T.; Wu, M.; Wang, M.-M. Case report of the first Severe Acute Respiratory Syndrome patient in China: Successful application of extracorporeal liver support MARS therapy in multiorgan failure possibly induced by Severe Acute Respiratory Syndrome. Artif. Organs 2003, 27, 847–849. [Google Scholar] [CrossRef]
- Zhao, L.; Xing, H.; Xu, L. Effect of SARS-associated coronavirus on peripheral blood picture and liver function. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2004, 16, 660–663. [Google Scholar]
- Yin, C.; Wang, C.; Tang, Z.; Wen, Y.; Zhang, S.; Wang, B. Clinical analysis of multiple organ dysfunction syndrome in patients suffering from SARS. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2004, 16, 646–650. [Google Scholar]
- Yang, Z.; Xu, M.; Yi, J.-Q.; Jia, W.-D. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. HBPD INT 2005, 4, 60–63. [Google Scholar]
- Wu, K.-L.; Lu, S.-N.; Changchien, C.-S.; Chiu, K.-W.; Kuo, C.-H.; Chuah, S.-K.; Liu, J.-W.; Lin, M.-C.; Eng, H.-L.; Chen, S.-S.; et al. Sequential changes of serum aminotransferase levels in patients with severe acute respiratory syndrome. Am. J. Trop. Med. Hyg. 2004, 71, 125–128. [Google Scholar] [CrossRef][Green Version]
- Wong, W.-M.; Ho, J.C.; Ooi, G.C.; Mok, T.; Chan, J.; Hung, I.F.; Ng, W.; Lam, Y.-M.; Tam, W.-O.; Wong, B.C.Y.; et al. Temporal patterns of hepatic dysfunction and disease severity in patients with SARS. JAMA 2003, 290, 2663–2665. [Google Scholar] [CrossRef]
- Tong, Y.; Yin, C.; Tang, X.; Jia, W. [Changes of liver function in patients with serious acute respiratory syndrome]. Zhonghua Gan Zang Bing Za Zhi 2003, 11, 418–420. [Google Scholar]
- Shi, X.; Gong, E.; Gao, D.; Zhang, B.; Zheng, J.; Gao, Z.; Zhong, Y.; Zou, W.; Wu, B.; Fang, W.; et al. Severe acute respiratory syndrome associated coronavirus is detected in intestinal tissues of fatal cases. Am. J. Gastroenterol. 2005, 100, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zhao, C.; Dong, P.; Hu, Z.; Hou, W.; Zhang, K.; Liang, L.; Huang, C. Clinical features of severe acute respiratory syndrome in forty-one confirmed health care workers. Zhonghua Yu Fang Yi Xue Za Zhi 2003, 37, 236–239. [Google Scholar] [PubMed]
- Peiris, J.S.M.; Lai, S.T.; Poon, L.L.M.; Guan, Y.; Yam, L.Y.C.; Lim, W.; Nicholls, J.; Yee, W.K.S.; Yan, W.W.; Cheung, M.T.; et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003, 361, 1319–1325. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Hinedi, K.; Abbasi, S.; Babiker, M.; Sunji, A.; Eltigani, M. Hematologic, hepatic, and renal function changes in hospitalized patients with Middle East respiratory syndrome coronavirus. Int. J. Lab. Hematol. 2017, 39, 272–278. [Google Scholar] [CrossRef]
- Halim, A.A.; Alsayed, B.; Embarak, S.; Yaseen, T.; Dabbous, S. Clinical characteristics and outcome of ICU admitted MERS corona virus infected patients. Egypt. J. Chest Dis. Tuberc. 2016, 65, 81–87. [Google Scholar] [CrossRef]
- Ling, Y.; Qu, R.; Luo, Y. [Clinical analysis of the first patient with imported Middle East respiratory syndrome in China]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015, 27, 630–634. [Google Scholar] [CrossRef]
- Kapoor, M.; Pringle, K.; Kumar, A.; Dearth, S.; Liu, L.; Lovchik, J.; Perez, O.; Pontones, P.; Richards, S.; Yeadon-Fagbohun, J.; et al. Clinical and Laboratory Findings of the First Imported Case of Middle East Respiratory Syndrome Coronavirus to the United States. Clin. Infect. Dis. 2014, 59, 1511–1518. [Google Scholar] [CrossRef]
- Yousefi, M.; Dehesh, M.M.; Farokhnia, M. Epidemiological and Clinical Characteristics of Patients with Middle East Respiratory Syndrome Coronavirus in Iran in 2014. Jpn. J. Infect. Dis. 2017, 70, 115–118. [Google Scholar] [CrossRef]
- Sherbini, N.; Iskandrani, A.; Kharaba, A.; Khalid, G.; Abduljawad, M.; Al-Jahdali, H. Middle East respiratory syndrome coronavirus in Al-Madinah City, Saudi Arabia: Demographic, clinical and survival data. J. Epidemiol. Glob. Health 2017, 7, 29–36. [Google Scholar] [CrossRef]
- Saad, M.; Omrani, A.S.; Baig, K.; Bahloul, A.; Elzein, F.; Matin, M.A.; Selim, M.A.A.; Mutairi, M.A.; Nakhli, D.A.; Aidaroos, A.Y.A.; et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: A single-center experience in Saudi Arabia. Int. J. Infect. Dis. 2014, 29, 301–306. [Google Scholar] [CrossRef]
- Ng, D.L.; Hosani, F.A.; Keating, M.K.; Gerber, S.I.; Jones, T.L.; Metcalfe, M.G.; Tong, S.; Tao, Y.; Alami, N.N.; Haynes, L.M.; et al. Clinicopathologic, Immunohistochemical, and Ultrastructural Findings of a Fatal Case of Middle East Respiratory Syndrome Coronavirus Infection in the United Arab Emirates, April 2014. Am. J. Pathol. 2016, 186, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhao, J.; Lian, N.; Lin, S.; Xie, Q.; Zhuo, H. Clinical characteristics of Non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A Retrospective study. Liver Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- George Sakoulas, M.D. ACE2 Is the SARS-CoV-2 Receptor Required for Cell Entry. NEJM J. Watch 2020, 2020. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Paizis, G.; Tikellis, C.; Cooper, M.E.; Schembri, J.M.; Lew, R.A.; Smith, A.I.; Shaw, T.; Warner, F.J.; Zuilli, A.; Burrell, L.M.; et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut 2005, 54, 1790–1796. [Google Scholar] [CrossRef]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab. Clin. Exp. 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef]
- Sorribas, M.; Jakob, M.O.; Yilmaz, B.; Li, H.; Stutz, D.; Noser, Y.; de Gottardi, A.; Moghadamrad, S.; Hassan, M.; Albillos, A.; et al. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J. Hepatol. 2019, 71, 1126–1140. [Google Scholar] [CrossRef]
- Fine, R.L.; Manfredo Vieira, S.; Gilmore, M.S.; Kriegel, M.A. Mechanisms and consequences of gut commensal translocation in chronic diseases. Gut Microbes 2020, 11, 217–230. [Google Scholar] [CrossRef]
- Parohan, M.; Yaghoubi, S.; Seraj, A. Liver injury is associated with severe Coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of retrospective studies. MedRxiv 2020. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Acute Kidney Injury (AKI)—KDIGO. Available online: https://kdigo.org/guidelines/acute-kidney-injury/ (accessed on 7 April 2020).
- SCCM | COVID-19 Guidelines. Available online: https://sccm.org/SurvivingSepsisCampaign/Guidelines/COVID-19 (accessed on 7 April 2020).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA 2020. [Google Scholar] [CrossRef]
- Chai, X.; Hu, L.; Zhang, Y.; Han, W.; Lu, Z.; Ke, A.; Zhou, J.; Shi, G.; Fang, N.; Fan, J.; et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. BioRxiv 2020. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Krüger, N.; Müller, M.; Drosten, C.; Pöhlmann, S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv 2020. [Google Scholar] [CrossRef]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140. [Google Scholar] [CrossRef]
- Banales, J.M.; Huebert, R.C.; Karlsen, T.; Strazzabosco, M.; LaRusso, N.F.; Gores, G.J. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 269–281. [Google Scholar] [CrossRef]
- Selzner, N.; Selzner, M.; Tian, Y.; Kadry, Z.; Clavien, P.-A. Cold ischemia decreases liver regeneration after partial liver transplantation in the rat: A TNF-alpha/IL-6-dependent mechanism. Hepatology 2002, 36, 812–818. [Google Scholar] [CrossRef]
- Kukla, M.; Mazur, W.; Bułdak, R.J.; Zwirska-Korczala, K. Potential role of leptin, adiponectin and three novel adipokines--visfatin, chemerin and vaspin--in chronic hepatitis. Mol. Med. 2011, 17, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Dietz, W.; Santos-Burgoa, C. Obesity and its Implications for COVID-19 Mortality. Obesity 2020. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; He, L.; Zhang, Q.; Huang, Z.; Che, X.; Hou, J.; Wang, H.; Shen, H.; Qiu, L.; Li, Z.; et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J. Pathol. 2004, 203, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Tan, Y.-J.; Fielding, B.C.; Goh, P.-Y.; Shen, S.; Tan, T.H.P.; Lim, S.G.; Hong, W. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. J. Virol. 2004, 78, 14043–14047. [Google Scholar] [CrossRef]
- Duan, Z.; Chen, Y.; Zhang, J.; Zhao, J.; Lang, Z.; Meng, F.; Bao, X. Clinical characteristics and mechanism of liver injury in patients with severe acute respiratory syndrome. Zhonghua Gan Zang Bing Za Zhi 2003, 11, 493–496. [Google Scholar]
- Hwang, S.-M.; Na, B.-J.; Jung, Y.; Lim, H.-S.; Seo, J.-E.; Park, S.-A.; Cho, Y.-S.; Song, E.-H.; Seo, J.-Y.; Kim, S.-R.; et al. Clinical and Laboratory Findings of Middle East Respiratory Syndrome Coronavirus Infection. Jpn. J. Infect. Dis. 2019, 72, 160–167. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Al-Omari, A.; Mandourah, Y.; Al-Hameed, F.; Sindi, A.A.; Alraddadi, B.; Shalhoub, S.; Almotairi, A.; Al Khatib, K.; Abdulmomen, A.; et al. Critically Ill Patients with the Middle East Respiratory Syndrome: A Multicenter Retrospective Cohort Study. Crit. Care Med. 2017, 45, 1683–1695. [Google Scholar] [CrossRef]
- Assiri, A.; Al-Tawfiq, J.A.; Al-Rabeeah, A.A.; Al-Rabiah, F.A.; Al-Hajjar, S.; Al-Barrak, A.; Flemban, H.; Al-Nassir, W.N.; Balkhy, H.H.; Al-Hakeem, R.F.; et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect. Dis. 2013, 13, 752–761. [Google Scholar] [CrossRef]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.W.; Müller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.A.; Zaki, A.; Fouchier, R.A.M.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Boonacker, E.; Van Noorden, C.J.F. The multifunctional or moonlighting protein CD26/DPPIV. Eur. J. Cell Biol. 2003, 82, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Multi-Organ Damage in Human Dipeptidyl Peptidase 4 Transgenic Mice Infected with Middle East Respiratory Syndrome-Coronavirus. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0145561 (accessed on 9 April 2020).
- Cheung, K.S.; Hung, I.F.; Chan, P.P.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples from the Hong Kong Cohort and Systematic Review and Meta-analysis. Gastroenterology 2020. [Google Scholar] [CrossRef] [PubMed]
| Reference/Year/Country | n Total/ n Males | Age (Mean/SD) | Mechanical Ventilation/Suppl Oxygen | Hospitalization/ICU Stay | Hospital Mortality/ICU Mortality | Comorbidities (Diabetes/Hypertension/CVD/Other) | Preexisting Liver Disease (n)/Diagnosis | Intervention |
|---|---|---|---|---|---|---|---|---|
| SARS CoV-2 | ||||||||
| Chen et al. [16]/2020/China | 99/67 | 55.5/13.1 | 17/75 | 99/nd | 31/nd | nd/nd/nd/0 | nd/nd | antibiotic, antiviral treatment |
| Chuan et al. [17]/2020/China | 32/nd | nd/nd | nd/nd | 32/nd | nd/nd | nd/nd/nd/5 | nd/nd | nd |
| Guan et al. [2]/2020/China | 1099/640 | 47/35.58 | 81/454 | 1029/55 | 15/nd | 81/165/27/261 | 23/HBV | antibiotics (n = 637), oseltamivir (n = 393), antifungals (n = 31), systemic glucocorticoids (n = 204) |
| Wang et al. [12]/2020/China | 138/75 | 56/26 | 32/106 | 138/36 | 6/nd | 14/43/20/0 | 4/chronic liver disease | moxifloxacin (n = 89), ceftriaxone (n = 34), azithromycin (n = 25), glucocorticoids (n = 62) |
| Zhou et al. [18]/2020/China | 191/nd | nd/nd | 58/41 | 191/50 | 54/nd | 36/58/15/22 | nd/nd | antibiotics (n = 181), antivirals (n = 41), corticosteroids (n = 57), immunoglobulins (n = 46) |
| Zhang et al. [19]/2020/China | 56/nd | nd/nd | nd/nd | 56/nd | nd/nd | nd/nd/nd/0 | 2/nd | nd |
| Yang et al. [20]/2020/China | 52/35 | 59.7/13.2 | 37/33 | 52/52 | nd/32 | 9/nd/7/34 | nd/nd | vasoconstrictive agents (n = 18), antivirals (n = 23), antibacterials (n = 49),glucocorticoids (n = 30), immunoglobulin (n = 28) |
| Xu et al. [21]/2020/China | 62/35 | 41/20 | 1/nd | 61/1 | 0/0 | 1/5/nd/3 | 7/nd | antivirals (n = 55), antibiotics (n = 28), systematic corticosteroid (n = 16) |
| Wu et al. [22]/2020/China | 80/39 | 46.1/15.42 | 0/35 | 80/nd | 0/0 | nd/nd/25/12 | 1/nd | antibiotic treatment (n = 73), antivirals (n = 80), hormone therapy (n = 12), immunoglobulins (n = 16) |
| Shi et al. [23]/2020/China | 81/42 | 49.5/11 | nd/nd | 81/nd | 3/nd | 10/12/8/0 | 7/liver cirrhosis, hepatitis | nd |
| Jin et al. [4]/2020/China | 651/331 | 45.21/14.42 | 17/nd | nd/17 | nd/nd | 48/100/5/8 | 25/nd | antivirals (n = 546), antibiotics (n = 277), glucocorticoids (n = 74), |
| SARS CoV-1 | ||||||||
| Chan et al. [24]/2004/China | 118/55 | 33 */(20–18) # | 16/nd | nd/nd | 9/nd | nd/nd/nd/16 | 12/HBV | lamivudine |
| Chan et al. [25]/2005/China | 294/126 | 36 */(12–83) # | 33/nd | 194/141 | 27/nd | 5/12/6/18 | 30/HBV | cefotaxime, clarithromycin, oseltamivircorticosteroids, ribavirin, lamivudine |
| Chau et al. [13]/2004/China | 3/0 | 34.7/8.2 | nd/nd | 3/nd | 3/nd | nd/nd/nd/nd | nd/nd | ceftriaxone, clarithromycin, Kaletra, methylprednisolone or levofloxacin alone |
| Chen et al. [26]/2003/China | 7/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd/nd/nd | nd/nd | nd |
| Cui et al. [27]/2004/China | 182/103 | nd/(11–86) # | nd/nd | 57/nd | nd/nd | nd/nd/nd/nd | nd/nd | antibiotics (n = 160), ribavirin (n = 137),methylprednisolone (n = 115) |
| Ding et al. [28]/2003/China | 3/2 | 48/16.4 | nd/nd | nd/nd | nd/nd | nd/nd/nd/nd | nd/nd | nd |
| Farcas et al. [29]/2005/Canada | 21/9 | 68.8/15 | nd/nd | nd/nd | nd/nd | 6/9/3/16 | nd/nd | nd |
| Guan et al. [30]/2004/China | 110/nd | nd/nd | nd/nd | nd/nd | 8/nd | nd/nd/nd/nd | nd/nd | nd |
| Han et al. [31]/2003/China | 69/29 | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd |
| Hsiao et al. [32]/2004/Taiwan | 346/nd | nd/nd | nd/nd | nd/nd | 73/nd | nd/nd/nd/nd | nd/nd | nd |
| Kumar et al. [33]/2003/Canada | 1/1 | 74/0 | nd | 1/1 | 1/1 | nd/nd/nd/nd | nd/nd | cyclosporin, prednisone, insulin, trimethoprim/sulfamethoxazole prophylaxis |
| Lang et al. [34]/2003/China | 3/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd/nd/nd | nd/nd | nd |
| Liu et al. [35]/2003/China | 106/56 | 36/10 | nd/nd | nd/nd | nd/nd | nd/nd/nd/nd | nd/nd | steroids, antibiotics, antiviral drugs |
| Luo et al. [36]/2003/Germany | 1/1 | 54/nd | 1/nd | 1/1 | 0/0 | nd/nd/nd/nd | nd/nd | ribavirin |
| Zhao et al. [37]/2004/China | 106/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd/nd/nd | nd/nd | nd |
| Yin et al. [38]/2004/China. | 148 | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd/nd/nd | nd/nd | nd |
| Yang et al. [39]/2005/China | 168/72 | 42.8/18.6 | nd/nd | nd/nd | nd/nd | nd/nd/nd/nd | 17/HBV | quinolones, macrolides, floxacin, tetracycline, roxithromycin, ciprofloxacin |
| Wu et al. [40]/2004/ Taiwan | 52/20 | 45/20 | nd/nd | nd/21 | 16/nd | nd/nd/nd/nd | 8/HBV | nd |
| Wong et al. [41]/2003/China | 54/24 | 37.9/13 | nd/nd | nd/nd | nd/nd | nd/nd/nd/nd | nd/nd | Corticosteroids and oral (or iv) ribavirin, cefipime, oral clarithromycin, azithromycin |
| Tong et al. [42]/2003/China | 114/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd/nd/nd | nd/nd | nd |
| Shi et al. [43]/2005/China | 7/6 | 40.43/13.95 | nd/nd | nd/nd | nd/nd | nd/nd/nd/nd | nd/nd | nd |
| Peiris et al. [45]/2003/China | 50/22 | 42.99/12.58 | 19/nd | /nd19 | 1/nd | nd/nd/nd/nd | nd/nd | Oral levofloxacin (n = 9), amoxicillin-clavulanate (given intravenously n = 40), oseltamivir orally (n = 4), intravenous ceftriaxone, Azithromycin, oral amantadine (n = 1), intravenous ribavirin, steroid (n = 49) |
| Meng et al. [44]/2003/China | 41/8 | nd/nd | 27/11 | nd/nd | 1/nd | nd/nd/nd/nd | nd/nd | Steroids |
| MERS CoV | ||||||||
| Al Tawfiq et al. [46]/2017/USA | 16/nd | nd/nd | nd/nd | 15/nd | nd/nd | nd/nd/nd/nd | nd | nd |
| Alsaad et al. [8]/2018/Saudi Arabia | 1/1 | 33/nd | 1/nd | 1/1 | 1/1 | nd/nd/nd/1 | nd | Chemotherapy, methotrexate, antibiotics ifosfamide, etoposide, L-asparginase, prednisolone |
| Halim et al. [47]/2016/Egypt | 32/20 | 43.99/13.03 | 23/nd | 32/32 | 14/14 | nd/nd/nd/31 | nd | nd |
| Ling et al. [48]/2015/China | 1/nd | 43/nd | 1/nd | 1/nd | nd/nd | nd/nd/nd/nd | nd | Ribavirin, ceftriaxone, meropenem |
| Kapoor et al. [49]/2014/USA | 1/1 | 65/nd | 0/nd | 1/0 | 0/0 | nd/1/1/1 | nd | vancomycin, piperacillin/, ceftriaxone tazobactam, levofloxacin, linezolid, furosemide |
| Yousefi et al. [50]/2017/Iran | 5/1 | 49.6/10.52 | nd/nd | 4/nd | 3/3 | nd/1/nd/1 | nd | PT1: azithromycin, ceftriaxone, meropenem, vancomycin, oseltamivir; PT2: levofloxacin, ceftriaxone, azithromycin, oseltamivir; PT3: no drugs, P4: no data (pt. died in ICU), P5: meropenem and vancomycin, oseltamivir |
| Sherbini et al. [51]/2017/Saudi Arabia | 29/20 | 45.49/12.22 | 9/nd | nd/nd | 10/nd | 9/nd/nd/8 | nd | Meropenem (n = 20), linezolid (n = 17), levofloxacin (n = 15), piperacillin (n = 15), ribavirin (n = 10), azithromycin (n = 19), interferon (n = 19), steroids (n = 29) |
| Saad et al. [52]/2014/Saudi Arabia | 70/46 | 61 */(1–90)Z | 49/nd | nd/49 | 42/nd | nd/nd/nd/nd | nd | nd |
| Ng et al. [53]/2014/United Arab Emirates | 1/1 | 45 | 1/nd | nd/nd | nd/nd | nd/nd/nd/nd | nd | Prednisolone, paracetamol, levofloxacin, oseltamivir, ceftriaxone, azithromycin, hydrocortisone intravenously |
| Reference | n Total | AST (Mean) ALT (Mean) LDH (Mean) | Abnormal AST (n) Abnormal ALT (n) Abnormal LDH (n) | Bilirubin (Mean) ALP (Mean) Creatinine (Mean) | Abnormal Bilirubin (n) Abnormal ALP (n) Abnormal Creatinine (n) | Total Protein (Mean) Albumin (Mean) | Abnormal Tot. Protein (n) Abnormal Albumin (n) | Prothrombin Time (Mean) INR (Mean) | Abnormal Prothrombin Time (n) Abnormal INR (n) | Abnormal Liver Function (n/n Total) |
|---|---|---|---|---|---|---|---|---|---|---|
| SARS CoV-2 | ||||||||||
| Chen et al. [16]/2020/China | 99 | nd | AST: 35 ALT: 28 LDH: 75 | nd | BIL: 18 CR: 24 | nd | nd | nd | nd | nd |
| Chuan et al. [17]/2020/China | 32 | AST (U/L): 24.75 ALT (U/L): 26.98 | nd | BIL (mmol/L): 16.4 | nd | ALB (g/L): 39 | nd | nd | nd | nd |
| [2]/2020/China | 1099 | nd | AST: 168 ALT: 158 LDH: 277 | nd | BIL: 76 CR: 12 | nd | nd | nd | nd | nd |
| Wang et al. [12]/2020/China | 138 | AST (U/L):31 * ALT (U/L): 24 * LDH (U/L): 261 * | nd | BIL (mmol/L): 9.8 * CR (µmol/L): 72 * | nd | nd | nd | PT (s): 13 * | nd | nd |
| Zhou et al. [18]/2020/China | 191 | ALT (U/L): 30 * LDH (U/L): 300 * | ALT: 59 LDH: 123 | nd | CR: 8 | ALB (g/L): 32.3 * | nd | PT (s): 11.6 * | PT: 182 | nd |
| Zhang et al. [19]/2020/China | 56 | nd | nd | nd | ALP: 1 | nd | nd | nd | nd | 16/56 |
| Yang et al. [20]/2020/China | 52 | nd | nd | BIL (µmol/L): 17.04 CR (µmol/L): 79 | nd | nd | nd | PT (s): 12.3 | nd | nd |
| Xu et al. [21]/2020/China | 62 | AST(U/L): 26 * ALT (U/L):22* LDH (U/L): 205 * | AST: 10 LDH: 17 | CR (µmol/L): 72 | CR: 3 | nd | nd | nd | nd | nd |
| Wu et al. [22]/2020/China | 80 | AST (U/L): 30 * ALT (U/L): 24 * LDH (U/L): 226 * | AST: 3 ALT: 3 LDH: 17 | BIL (µmol/L): 6.6 CR (µmol/L): 78 | BIL: 1 CR: 2 | ALB (g/L): 38.3 * | ALB: 2 | PT (s): 10.8 | nd | nd |
| Shi et al. [23]/2020/China | 81 | AST (U/L): 40.8 ALT (U/L): 46.2 | AST: 43 | BIL (µmol/L): 11.9 CR (µmol/L): 75.4 | nd | ALB (g/L): 32.9 | nd | PT (s): 10.7 | nd | nd |
| Jin et al. [4]/2020/China | 651 | AST (U/L): 29.35 (g)/24.2 (ng) * ALT (U/L): 25(g)/21.5 (ng) LDH (U/L): 229(g)/210 (ng) | nd | BIL (µmol/L): 10 (g)/9.6 (ng) CR (µmol/L):66.0 (g)/66.0 (ng) | nd | ALB (g/L): 40.13 (g)/41.5 (ng) | nd | nd | INR: 1.03 (g)/1.02 (ng) | nd |
| SARS CoV-1 | ||||||||||
| Chan et al. [24]/2004/China | 118 | ALT (U/L): 25.5 | ALT: 25 | CR (µmol/L): 85 | nd | nd | nd | PT (s): 11.2 * | nd | nd |
| Chan et al. [25]/2005/China | 294 | nd | ALT: 52 | nd | ALP: 40 | nd | nd | nd | nd | nd |
| Chau et al. [13]/2004/China | 3 | ALT (U/L): 165X | nd | BIL (µmol/L): 7.3X | nd | ALB (g/L): 32Y | nd | nd | nd | nd |
| Chen et al. [26]/2003/China | 7 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Cui et al. [27]/2004/China | 182 | nd | ALT: 89 AST: 89 LDH: 76 | nd | nd | nd | nd | nd | nd | nd |
| Ding et al. [28]/2003/China | 3 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Farcas et al. [29]/2005/Canada | 21 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Guan et al. [30]/2004/China | 110 | ALT (U/L): 91.61X AST (U/L): 78.68X LDH (U/L): 429.69X | nd | BIL (µmol/L): 11.67X | ALP: 0 | ALB (g/L): 34.4 | nd | nd | nd | nd |
| Han et al. [31]/2003/China | 69 | nd | nd | nd | nd | nd | nd | nd | nd | 37/nd |
| Hsiao et al. [32]/2004/Taiwan | 346 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Kumar et al. [33]/2003/Canada | 1 | AST (U/L):51 | ALT: 1 | nd | BIL: 1 | nd | nd | nd | nd | nd |
| Lang et al. [34]/2003/China | 3 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Liu et al. [35]/2003/China | 106 | nd | ALT: 8 | nd | nd | nd | nd | nd | nd | nd |
| Luo et al. [36]/2003/Germany | 1 | ALT (U/L): 425.5X AST (U/L): 319X | LDH: 0 | nd | CR: 0 | nd | nd | nd | nd | nd |
| Zhao et al. [37]/2004/China | 106 | nd | ALT: 106 AST: 68 | nd | nd | nd | TP: 0 ALB: 122 | nd | nd | nd |
| Yin et al. [38]/2004/China | 148 | nd | nd | nd | nd | nd | nd | nd | nd | 148 |
| Yang et al. [39]/2005/China | 168 | ALT (U/L): 111.32X AST (U/L): 48.95X | ALT:118 | BIL: (µmol/L): 10.41X | nd | ALB (mg/L): 34.26Y | nd | nd | nd | nd |
| Wu et al. [40]/2004/ Taiwan | 52 | ALT (U/L): 86.19X AST (U/L): 69.05 X | ALT: 28 AST: 28 | nd | nd | nd | nd | nd | nd | nd |
| Wong et al. [41]/2003/China | 54 | ALT (U/L): 95.7X AST (U/L): 62.8X | ALT:41X | BIL: (µmol/L): 11.1X ALP (U/L): 72.6X | nd | ALB (g/L): 33.2Y | nd | nd | 61 | nd |
| Tong et al. [42]/2003/China | 114 | nd | nd | nd | nd | nd | nd | nd | nd | 84/nd |
| Shi et al. [43]/2005/China | 7 | nd | AST: 4 | nd | nd | nd | nd | nd | nd | nd |
| Peiris et al. [45]/2003/China | 50 | ALT (U/L): 63 * | ALT: 17 | nd | nd | ALB (g/L): 37 | ALB: 34 | nd | nd | 17/nd |
| Meng et al. [44]/2003/China | 41 | nd | nd | nd | nd | nd | nd | nd | nd | 27/nd |
| MERS CoV | ||||||||||
| Al Tawfiq et al. [46]/2017/USA | 16 | AST (U/L): 661X ALT (U/L): 476X LDH (U/L): 1825.8X | nd | BIL (µmol/L): 21X ALP (U/L): 257.3X CR (mg/dL): 3.8X | nd | nd | nd | nd | nd | nd |
| Alsaad et al. [8]/2018/Saudi Arabia | 1 | nd | nd | nd | nd | nd | nd | nd | nd | 0/1 |
| Halim et al. [47]/2016/Egypt | 32 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Ling et al. [48]/2015/China | 1 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Kapoor et al. [49]/2014/USA | 1 | AST (U/L): 95 ALT (U/L): 80 | nd | BIL (mg/dL): 1 ALP (U/L) 270 CR (mg/dL): 0.75 | nd | nd | nd | nd | nd | nd |
| Yousefi et al. [50]/2017/Iran | 5 | AST (U/L): 60.7 ALT (U/L): 35.75 | AST: 3 ALT: 2 | CR (mg/dL): 0.77 | nd | nd | nd | PT: 13.65 INR: 1.1 | PT: 2 INR: 1 | nd |
| Sherbini et al. [51]/2017/Saudi Arabia | 29 | AST (U/L): 86.3 ALT (U/L): 98.4 | nd | BIL (µmol/L): 16.64 CR (µmol/L): 225 | nd | nd | nd | nd | nd | nd |
| Saad et al. [52]/2014/Saudi Arabia | 70 | AST (U/L): 112 * XALT (U/L): 54 *X | nd | BIL (µmol/L): 17 * XALP (U/L): 145 * XCR (µmol/L): 251.5 *X | nd | ALB (mg/dL): 21 *Y | nd | nd | nd | 22/70 |
| Ng et al. [53]/2014/United Arab Emirates | 1 | AST (U/L): 51 ALT (U/L): 28 | nd | CR (mg/dL): 0.9 | nd | nd | nd | PT: 12 INR: 1.1 | nd | nd |
| Reference | Post-Mortem Study (Y/N) | Type of Coronavirus | Histopathology Cases (in Words) |
|---|---|---|---|
| [24] | N | SARS-CoV-1 | • no acute changes, no necrosis |
| [13] | N | SARS-CoV-1 | • mild lobular activities with occasional acidophilic bodies and prominent Kupffer cell • smildly inflamed portal tracts with lymphocytic infiltration |
| [26] | Y | SARS-CoV-1 | • massive necrosis (1 case) • nodular cirrhosis (1 case) |
| [28] | Y | SARS-CoV-1 | • dissociation of hepatocyte cords, together with fatty degeneration and focal necrosis (1 case) • massive central necrosis of hepatocytes (2 cases) • the vascular walls with edema and infiltration of monocytes and lymphocytes |
| [29] | Y | SARS-CoV-1 | • minor inflammatory changes observed in the liver on microscopic examination |
| [30] | N | SARS-CoV-1 | • non-specific inflammation in the liver in biopsy • non-specific hepatitis in postmortem biopsy |
| [32] | N | SARS-CoV-1 | • no specific pathological change in the gastrointestinal tract |
| [34] | Y | SARS-CoV-1 | • hydropic degeneration • fatty degeneration • interstitial cell proliferation |
| [39] | N | SARS-CoV-1 | • hydropic degeneration • steatosis • focal necrosis (n = 4) |
| [43] | Y | SARS-CoV-1 | • mild fatty-acid degeneration • mild congestion • central lobular necrosis |
| [8] | Y | MERS-CoV | • mild chronic lymphocytic portal inflammation • reactive parenchyma with mild cellular hydropic degeneration • rare multinucleated hepatocytes and mild disarray of the hepatic plates • mild sinusoidal lymphocytosis and small necroinflammatory foci in the hepatic lobules • congestion, hemorrhage and focal perivenular loss of hepatocytes • macrovesicular perivenular steatotic change, sinusoidal congestion, hemorrhage and focal perivenular loss of hepatocytes |
| [53] | Y | MERS-CoV | • moderate steatosis • scattered calcifications • mild portal tract and lobular lymphocytic inflammation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukla, M.; Skonieczna-Żydecka, K.; Kotfis, K.; Maciejewska, D.; Łoniewski, I.; Lara, L.F.; Pazgan-Simon, M.; Stachowska, E.; Kaczmarczyk, M.; Koulaouzidis, A.; et al. COVID-19, MERS and SARS with Concomitant Liver Injury—Systematic Review of the Existing Literature. J. Clin. Med. 2020, 9, 1420. https://doi.org/10.3390/jcm9051420
Kukla M, Skonieczna-Żydecka K, Kotfis K, Maciejewska D, Łoniewski I, Lara LF, Pazgan-Simon M, Stachowska E, Kaczmarczyk M, Koulaouzidis A, et al. COVID-19, MERS and SARS with Concomitant Liver Injury—Systematic Review of the Existing Literature. Journal of Clinical Medicine. 2020; 9(5):1420. https://doi.org/10.3390/jcm9051420
Chicago/Turabian StyleKukla, Michał, Karolina Skonieczna-Żydecka, Katarzyna Kotfis, Dominika Maciejewska, Igor Łoniewski, Luis. F. Lara, Monika Pazgan-Simon, Ewa Stachowska, Mariusz Kaczmarczyk, Anastasios Koulaouzidis, and et al. 2020. "COVID-19, MERS and SARS with Concomitant Liver Injury—Systematic Review of the Existing Literature" Journal of Clinical Medicine 9, no. 5: 1420. https://doi.org/10.3390/jcm9051420
APA StyleKukla, M., Skonieczna-Żydecka, K., Kotfis, K., Maciejewska, D., Łoniewski, I., Lara, L. F., Pazgan-Simon, M., Stachowska, E., Kaczmarczyk, M., Koulaouzidis, A., & Marlicz, W. (2020). COVID-19, MERS and SARS with Concomitant Liver Injury—Systematic Review of the Existing Literature. Journal of Clinical Medicine, 9(5), 1420. https://doi.org/10.3390/jcm9051420










