Detection of Circulating Tumor Cells in Renal Cell Carcinoma: Disease Stage Correlation and Molecular Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. CTCs Enrichment and Culture
2.2. Cytomorphological Analysis
2.3. Immunohistochemistry Analysis
3. Results
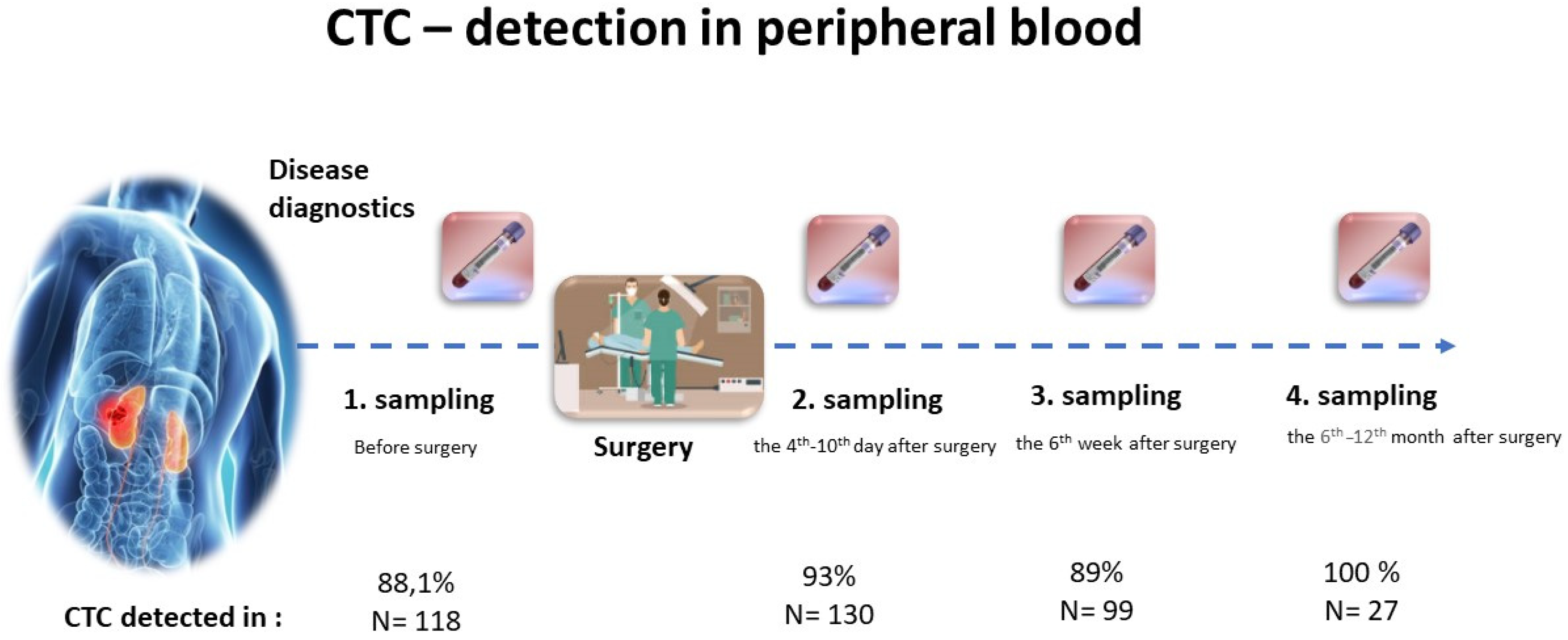
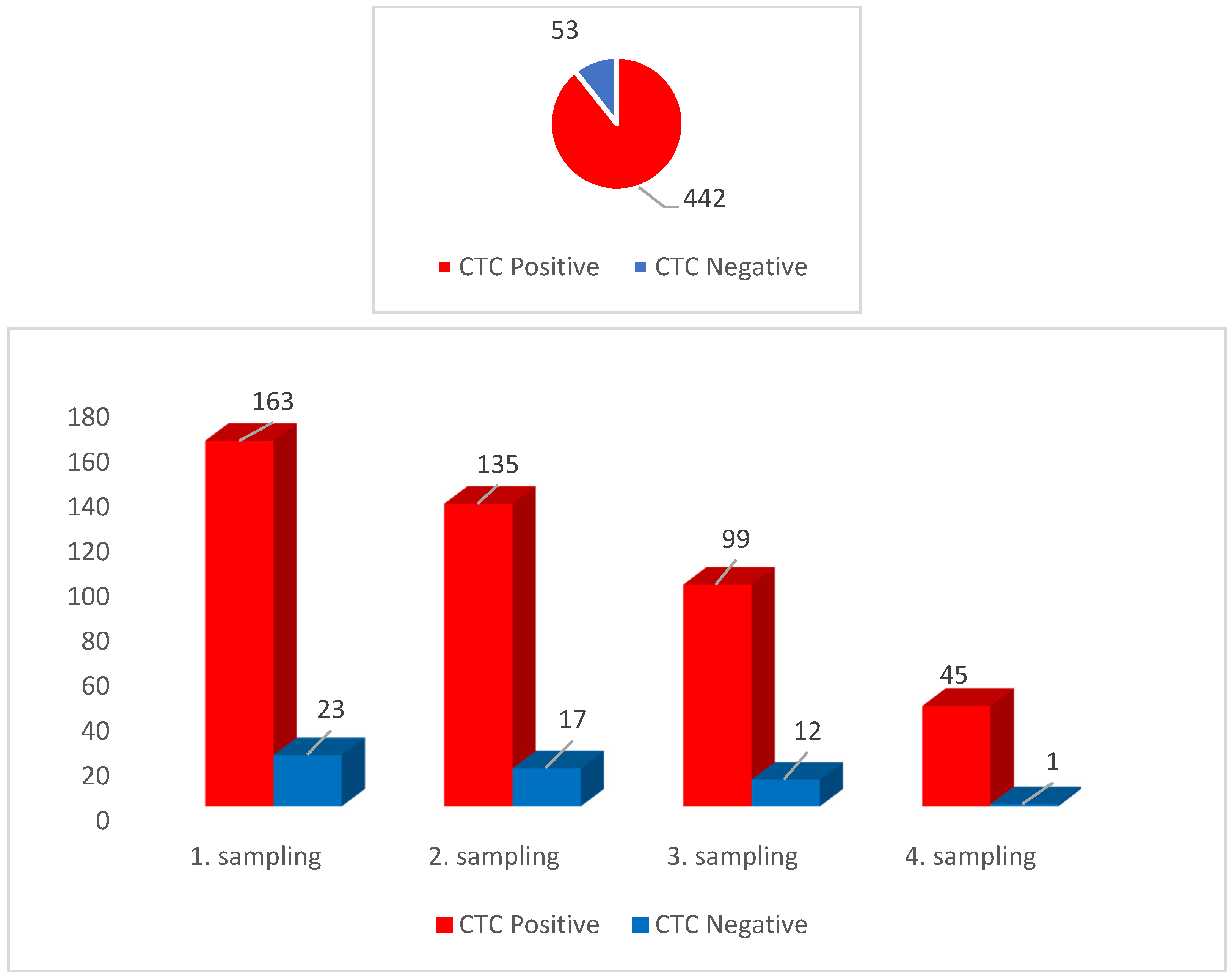
3.1. CTCs Prevalence
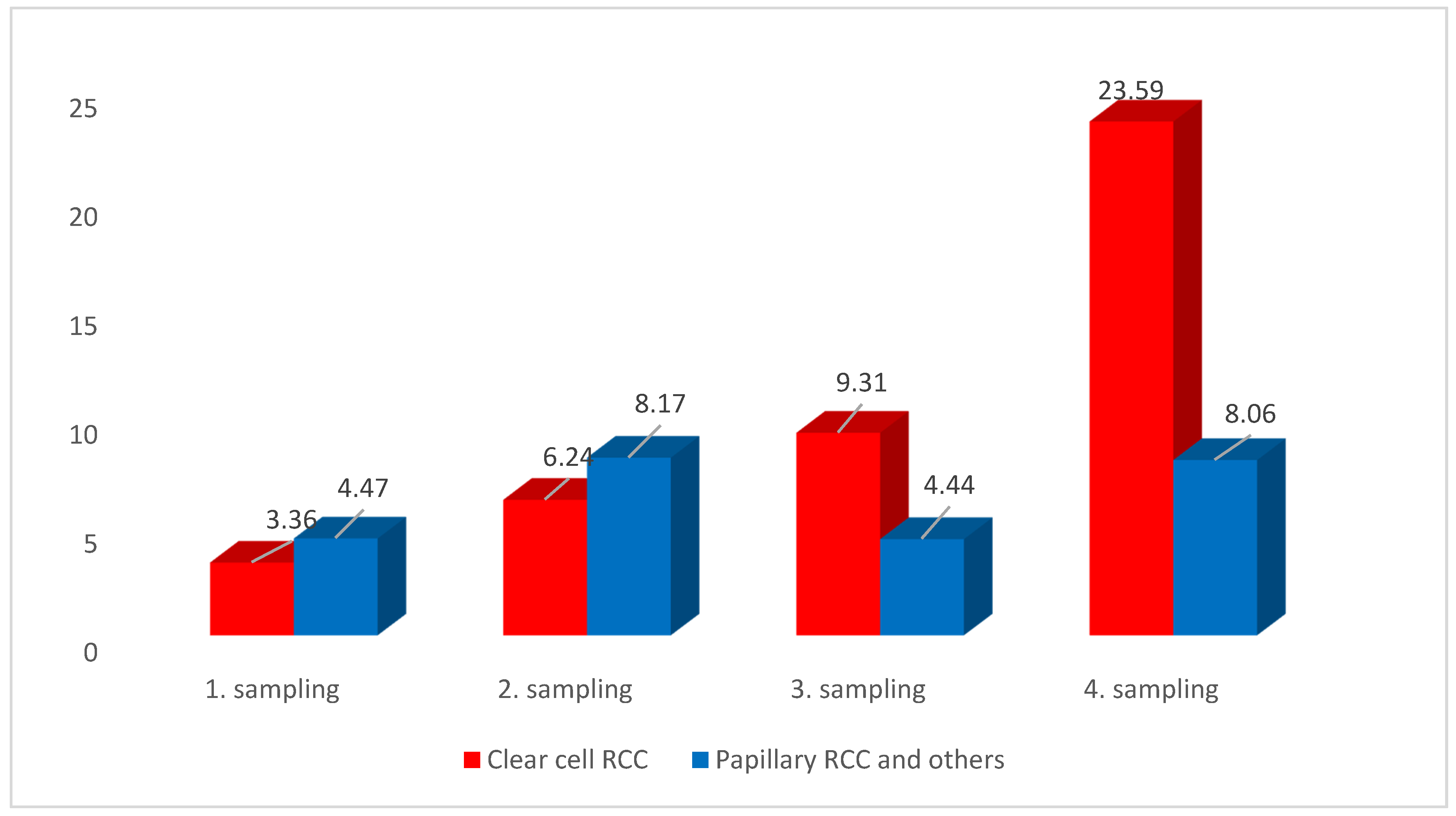
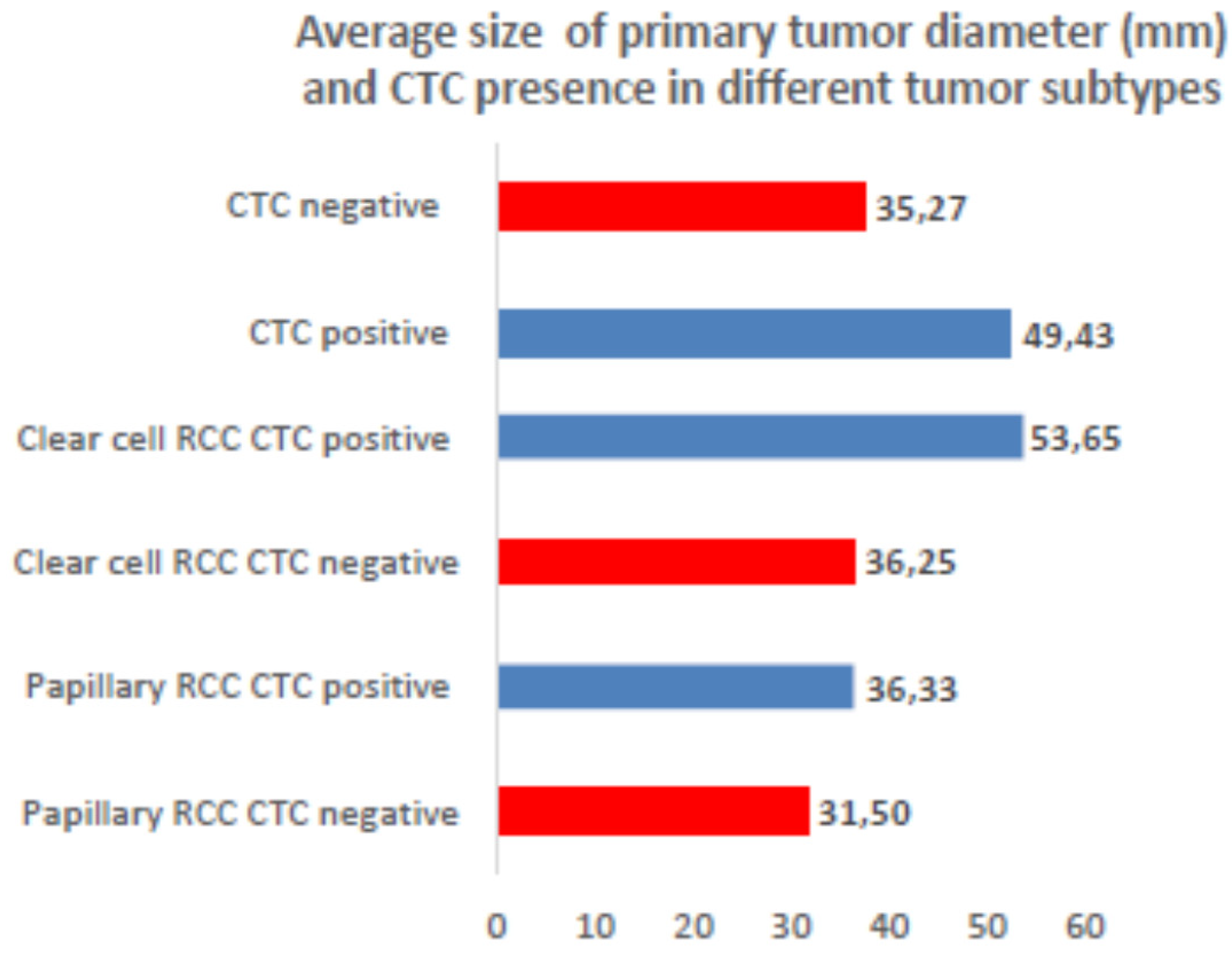
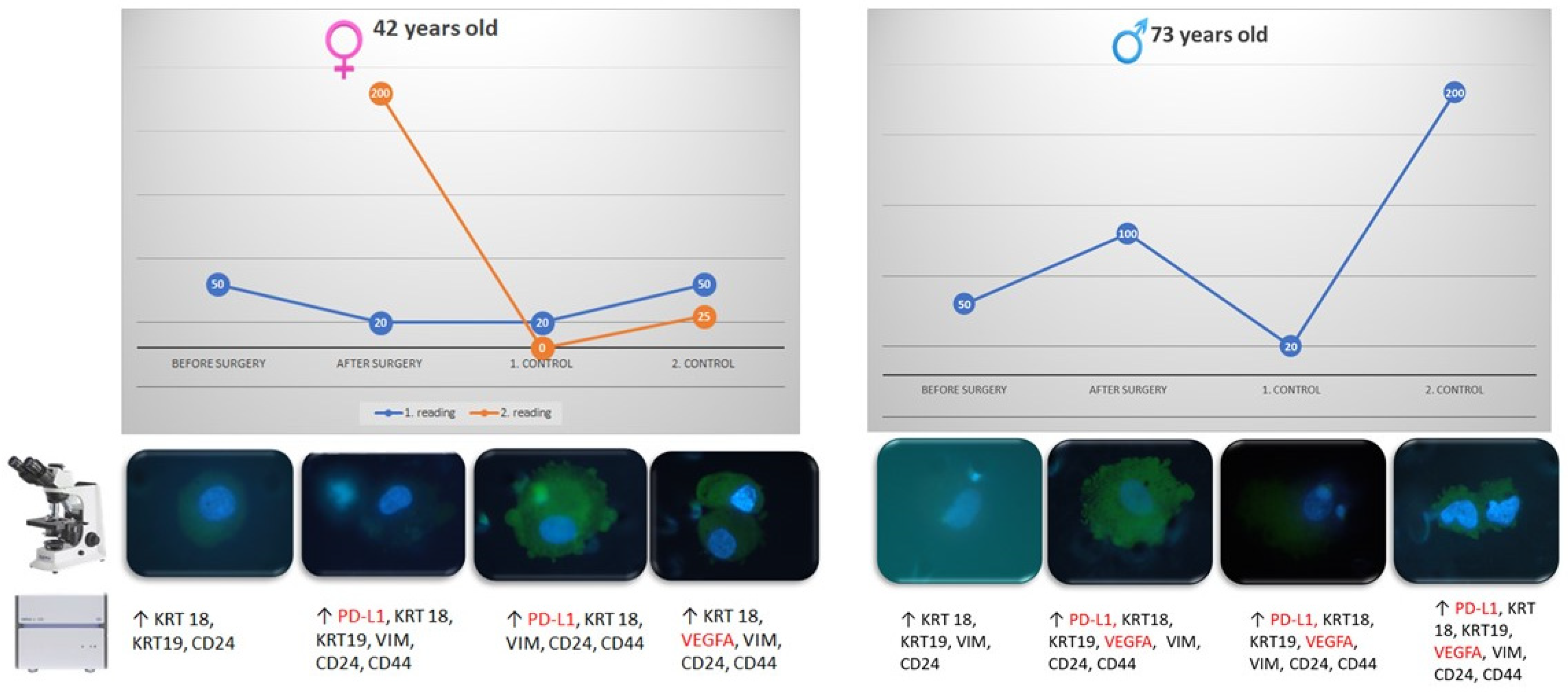
3.2. CTCs Cytomorphological Parameters
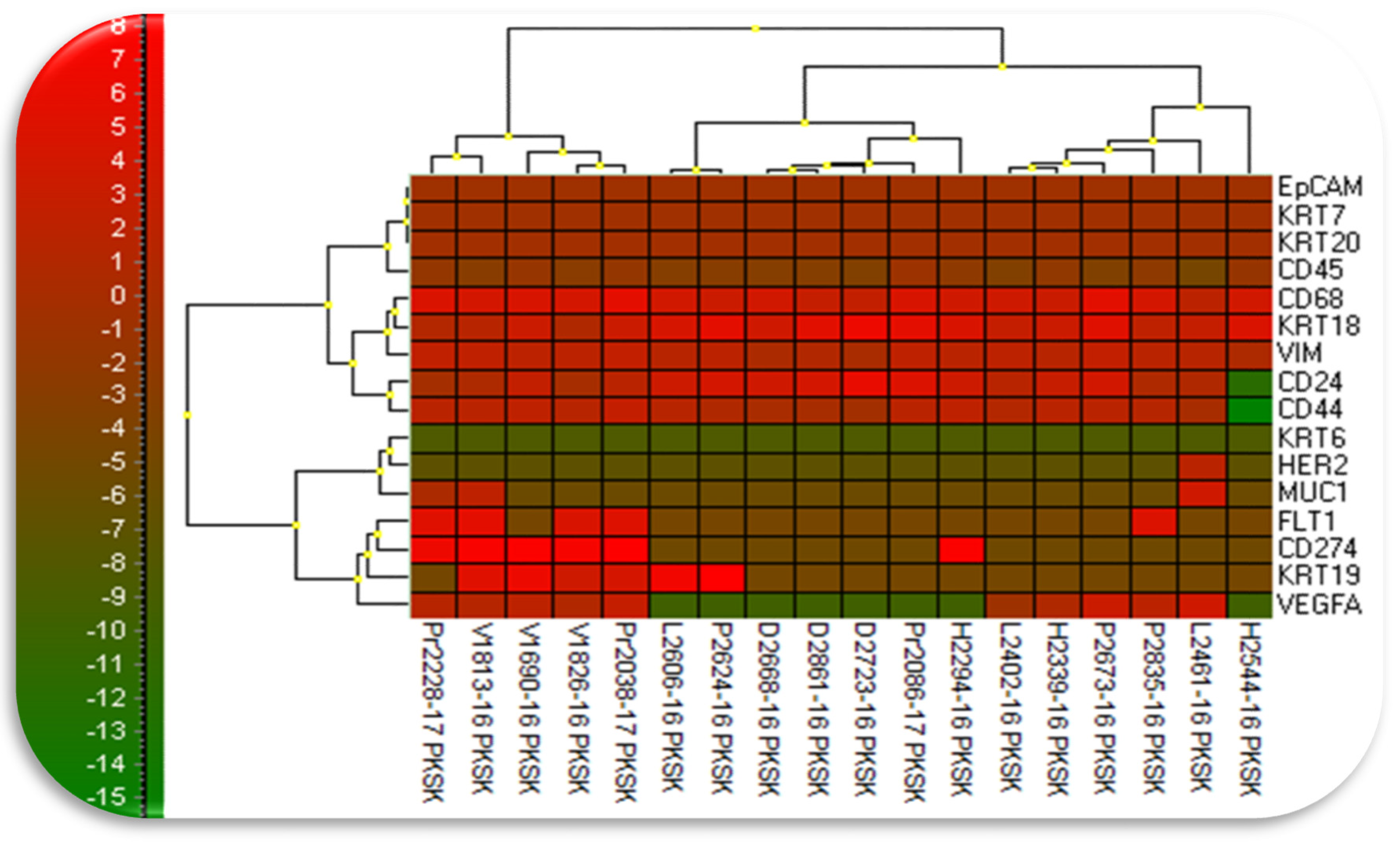
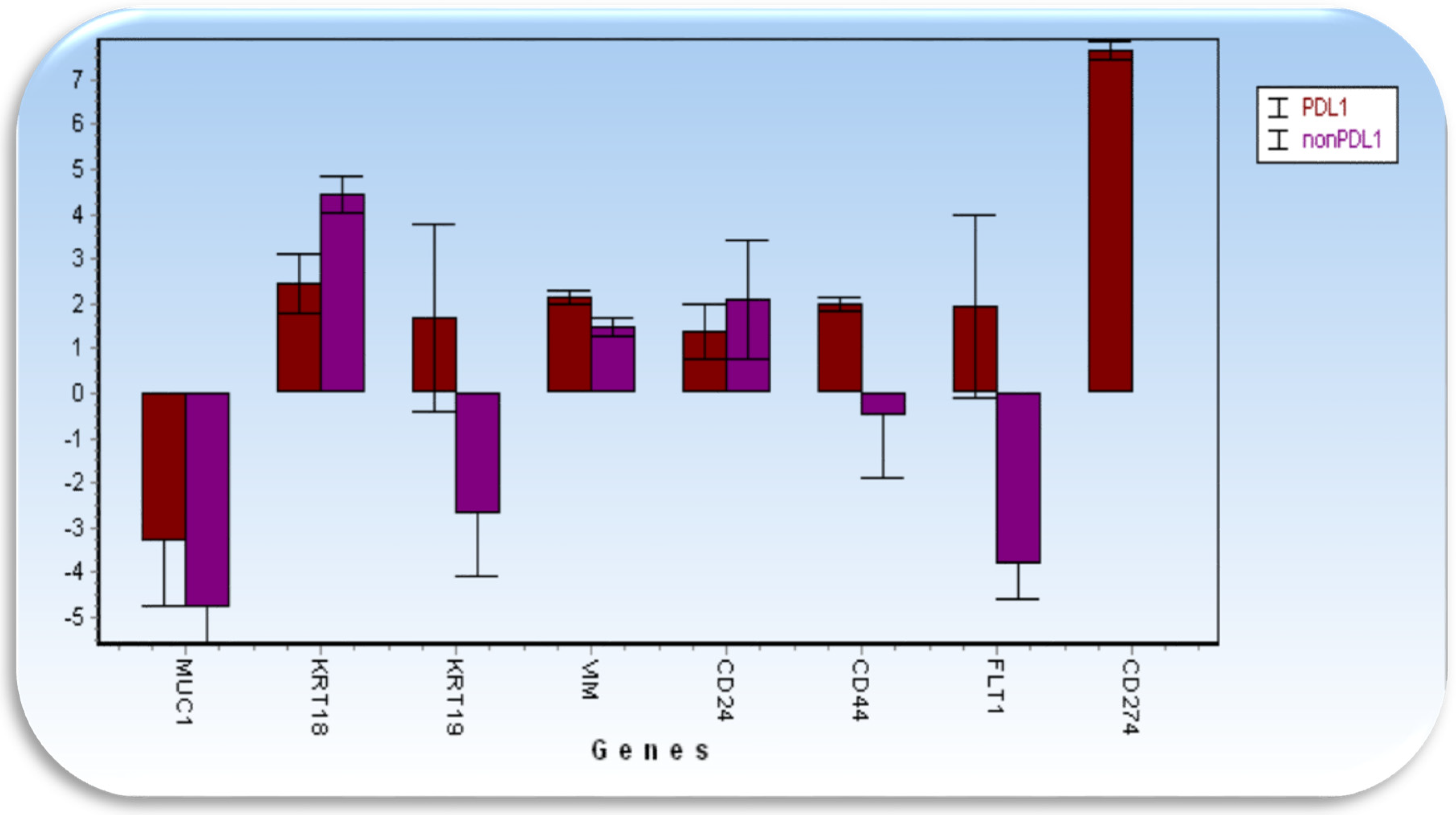
3.3. Gene Epression
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Motzer, R.J.; Bander, N.H.; Nanus, D.M. Renal-cell carcinoma. N. Engl. J. Med. 1996, 335, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Gore, M.; Griffin, C.L.; Hancock, B.; Patel, P.M.; Pyle, L.; Aitchison, M.; James, N.; Oliver, R.T.D.; Mardiak, J.; Hussain, T.; et al. Interferon alfa-2a versus combination therapy with interferon alfa-2a, interleukin-2, and fluorouracil in patients with untreated metastatic renal cell carcinoma (MRC RE04/EORTC GU 30012): An open-label randomised trial. Lancet 2010, 375, 641–648. [Google Scholar] [CrossRef]
- Motzer, R.J.; Russo, P. Systemic therapy for renal cell carcinoma. J. Urol. 2000, 163, 408–417. [Google Scholar] [CrossRef]
- Silvestris, N.; Ciliberto, G.; De Paoli, P.; Apolone, G.; Lavitrano, M.L.; Pierotti, M.A.; Stanta, G. On the behalf of the “dynamic medicine OECI group”. Liquid dynamic medicine and N-of-1 clinical trials: A change of perspective in oncologyresearch. J. Exp. Clin. Cancer Res. 2017, 36, 128. [Google Scholar] [CrossRef] [PubMed]
- Kolostova, K.; Matkowski, M.; Jędryka, M.; Soter, K.; Cegan, M.; Pinkas, M.; Pavlasek, J.; Spicka, J.; Bobek, V. The added value of circulating tumor cells examination in ovarian cancer staging of ovarian cancer. Am. J. Cancer Res. 2015, 5, 3363–3375. [Google Scholar] [PubMed]
- Kolostova, K.; Matkowski, R.; Gürlich, R.; Grabowski, K.; Soter, K.; Lischke, R.; Schützner, J.; Bobek, V. Detection and cultivation of circulating tumor cells in gastric cancer. Cytotechnology 2016, 68, 1095–1102. [Google Scholar] [CrossRef]
- Cegan, M.; Kolostova, K.; Matkowski, R.; Broul, M.; Schraml, J.; Fiutowski, M.; Bobek, V. In vitro culturing of viable circulating tumor cells of urinary bladder cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 7164–7171. [Google Scholar]
- Kolostova, K.; Pinkas, M.; Jakabova, A.; Pospisilova, E.; Svobodova, P.; Spicka, J.; Cegan, M.; Matkowski, R.; Bobek, V. Molecular characterization of circulating tumor cells in ovarian cancer. Am. J. Cancer Res. 2016, 6, 973–980. [Google Scholar] [PubMed]
- Broncy, L.; Paterlini-Bréchot, P. Circulating tumor cells for the management of renal cell carcinoma. Diagnostics 2018, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Gerstung, M.; Beisel, C.; Rechsteiner, M.; Wild, P.; Schraml, P.; Moch, H.; Beerenwinkel, N. Reliable detection of subclonal single-nucleotide variants in tumour cell populations. Nat. Commun. 2012, 3, 811. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Horswell, S.; Larkin, J.; Rowan, A.J.; Salm, M.P.; Varela, I.; Fisher, R.; McGranahan, N.; Matthews, N.; Santos, C.R.; et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat. Genet. 2014, 46, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Mandriota, S.J.; Turner, K.J.; Davies, D.R.; Murray, P.G.; Morgan, N.V.; Sowter, H.M.; Wykoff, C.C.; Maher, E.R.; Harris, A.L.; Ratcliffe, P.J.; et al. HIF activation identifies early lesions in VHL kidneys: Evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 2002, 1, 459–468. [Google Scholar] [CrossRef]
- Harten, S.K.; Shukla, D.; Barod, R.; Hergovich, A.; Balda, M.S.; Matter, K.; Esteban, M.A.; Maxwell, P.H. Regulation of renal epithelial tight junctions by the von Hippel-Lindau tumor suppressor gene involves occludin and claudin 1 and is independent of E-cadherin. Mol. Biol. Cell 2009, 20, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef] [PubMed]
- Farace, F.; Massard, C.; Vimond, N.; Drusch, F.; Jacques, N.; Billiot, F.; Laplanche, A.; Chauchereau, A.; Lacroix, L.; Planchard, D.; et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br. J. Cancer 2011, 105, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Zou, B.; Wang, Z.; Li, P.; Wang, H.; Ou, Y.; Cui, K.; Bian, J.; Li, S.; Xu, X. Comparison of two detection systems for circulating tumor cells among patients with renal cell carcinoma. Int. Urol. Nephrol. 2018, 50, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Bankó, P.; Lee, S.Y.; Nagygyörgy, V.; Zrínyi, M.; Chae, C.H.; Cho, D.H.; Telekes, A. Technologies for circulating tumor cell separation from whole blood. J. Hematol. Oncol. 2019, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Yanez, M.; Heymach, J.V.; Zurita, A.J. Circulating biomarkers in advanced renal cell carcinoma: Clinical applications. Curr. Oncol. Rep. 2012, 14, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus everolimus in advanced renal cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
| All | Primary Tumor Diameter (mm) | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 49.3 | 168 | |||||||||||
| CTC positive | 50.08 | 137 | |||||||||||
| CTC negative | 34.9 | 20 | |||||||||||
| Not evaluable | 65.81 | 11 | |||||||||||
| CTC | CTC Positive | CTC Negative | Not evaluable | Total Samples | Patients | ||||||||
| Patients | In % | Patients | In % | ||||||||||
| Clear cell RCC | 270 | 85.99 | 33 | 10.51 | 11 | 314 | 119 | ||||||
| Papillary RCC | 56 | 84.85 | 7 | 10.61 | 3 | 66 | 22 | ||||||
| Clear cell RCC and Papillary RCC | 11 | 84.62 | 2 | 15.38 | 13 | 4 | |||||||
| Chromophobe RCC | 19 | 76.00 | 4 | 16.00 | 2 | 25 | 8 | ||||||
| CTC | Type | CTC Positive | CTC Negative | Not Evaluable | Total | ||||||||
| Patients | In % | Patients | In % | ||||||||||
| 1. sampling | Clear cell RCC | 21 | 75.00 | 5 | 17.86 | 2 | 28 | ||||||
| 1. sampling | Papillary RCC and others | 13 | 68.42 | 4 | 21.05 | 2 | 19 | ||||||
| 2. sampling | Clear cell RCC | 27 | 96.43 | 1 | 3.57 | 28 | |||||||
| 2. sampling | Papillary RCC and others | 18 | 94.74 | 1 | 5.26 | 19 | |||||||
| 3. sampling | Clear cell RCC | 24 | 85.71 | 4 | 14.29 | 28 | |||||||
| 3. sampling | Papillary RCC and others | 18 | 94.74 | 1 | 19 | ||||||||
| 4. sampling | Clear cell RCC | 28 | 100.00 | 28 | |||||||||
| 4. sampling | Papillary RCC and others | 18 | 94.74 | 1 | 5.26 | 19 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klezl, P.; Pospisilova, E.; Kolostova, K.; Sonsky, J.; Maly, O.; Grill, R.; Pawlak, I.; Bobek, V. Detection of Circulating Tumor Cells in Renal Cell Carcinoma: Disease Stage Correlation and Molecular Characterization. J. Clin. Med. 2020, 9, 1372. https://doi.org/10.3390/jcm9051372
Klezl P, Pospisilova E, Kolostova K, Sonsky J, Maly O, Grill R, Pawlak I, Bobek V. Detection of Circulating Tumor Cells in Renal Cell Carcinoma: Disease Stage Correlation and Molecular Characterization. Journal of Clinical Medicine. 2020; 9(5):1372. https://doi.org/10.3390/jcm9051372
Chicago/Turabian StyleKlezl, Petr, Eliska Pospisilova, Katarina Kolostova, Jindrich Sonsky, Ondrej Maly, Robert Grill, Ireneusz Pawlak, and Vladimir Bobek. 2020. "Detection of Circulating Tumor Cells in Renal Cell Carcinoma: Disease Stage Correlation and Molecular Characterization" Journal of Clinical Medicine 9, no. 5: 1372. https://doi.org/10.3390/jcm9051372
APA StyleKlezl, P., Pospisilova, E., Kolostova, K., Sonsky, J., Maly, O., Grill, R., Pawlak, I., & Bobek, V. (2020). Detection of Circulating Tumor Cells in Renal Cell Carcinoma: Disease Stage Correlation and Molecular Characterization. Journal of Clinical Medicine, 9(5), 1372. https://doi.org/10.3390/jcm9051372




