D-Chiro-Inositol Improves Sperm Mitochondrial Membrane Potential: In Vitro Evidence
Abstract
1. Introduction
2. Patients and Methods
2.1. In Vitro Study
2.2. Sperm Preparation
2.3. Chemicals
2.4. Experimental Design
2.5. Flow Cytometric Analysis
2.6. Evaluation of the Mitochondrial Membrane Potential
2.7. Statistical Analysis
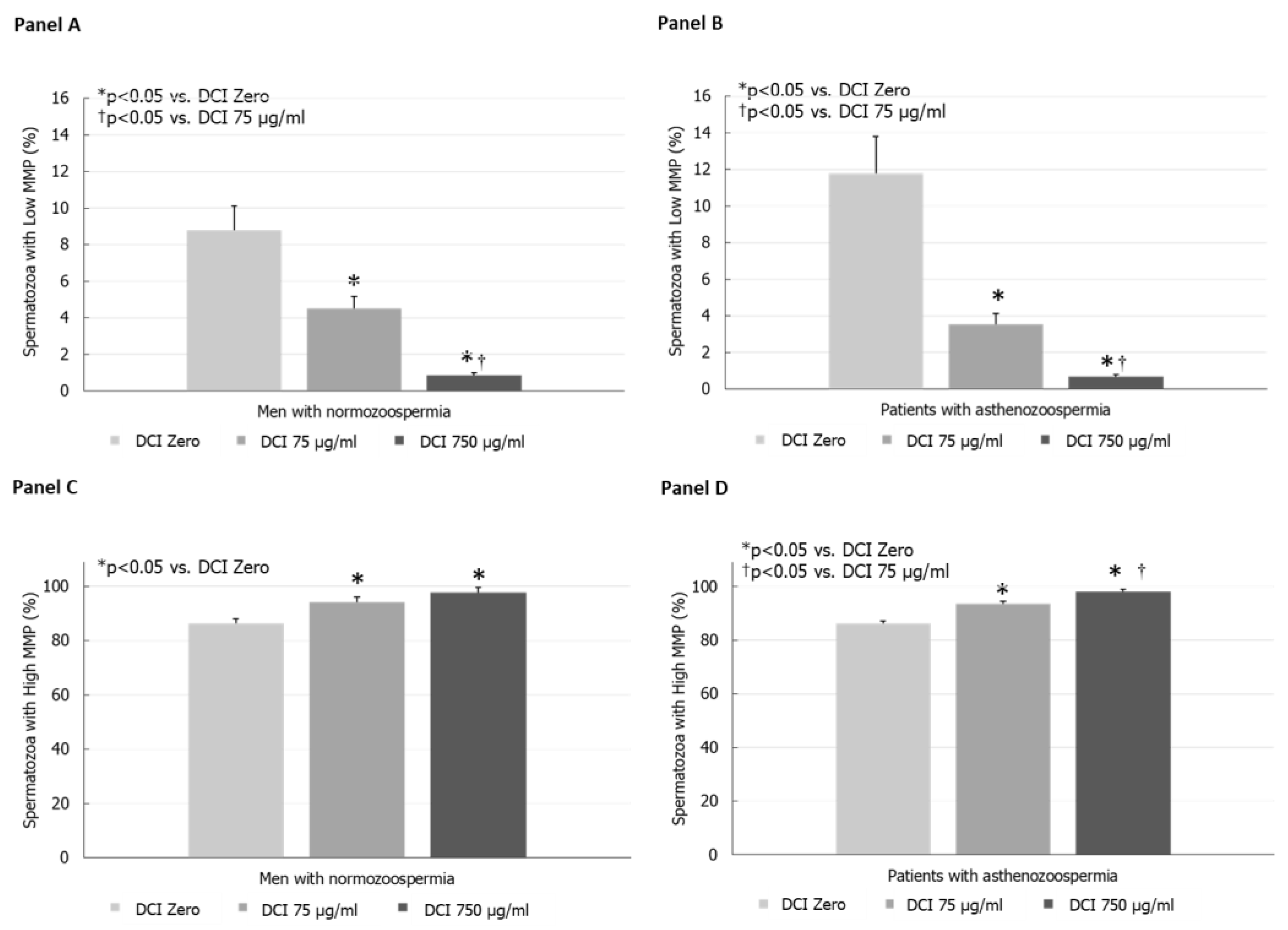
3. Results
In Vitro Study
4. Discussion
5. Main limitations of the Study
Author Contributions
Funding
Conflicts of Interest
References
- Morley, L.C.; Tang, T.; Yasmin, E.; Norman, R.J.; Balen, A.H. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst. Rev. 2017, 11, CD003053. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, P.; Ferrari, S.M.; Elia, G.; Ragusa, F.; Paparo, S.R.; Caruso, C.; Guglielmi, G.; Antonelli, A. Myo-inositol in autoimmune thyroiditis, and hypothyroidism. Rev. Endocr. Metab. Disord. 2018, 19, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Fuso, A.; Dinicola, S.; Cucina, A.; Bevilacqua, A. Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Larner, J.; Brautigan, D.L.; Thorner, M.O. D-chiro-inositol glycans in insulin signaling and insulin resistance. Mol. Med. 2010, 16, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Yoshida, K. Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol. Hum. Reprod. 2011, 17, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.D.; Bai, F.; Yu, L.; Sivitz, W.I. Regulation of ATP production: Dependence on calcium concentration and respiratory state. Am. J. Physiol. Cell Physiol. 2017, 313, C146–C153. [Google Scholar] [CrossRef] [PubMed]
- Monastra, G.; Unfer, V.; Harrath, A.H.; Bizzarri, M. Combining treatment with myo-inositol and D-chiro-inositol (40:1) is effective in restoring ovary function and metabolic balance in PCOS patients. Gynecol. Endocrinol. 2017, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; La Vignera, S.; Di Bari, F.; Unfer, V.; Calogero, A.E. Effects of myoinositol on sperm mitochondrial function in-vitro. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 129–134. [Google Scholar] [PubMed]
- Condorelli, R.A.; La Vignera, S.; Bellanca, S.; Vicari, E.; Calogero, A.E. Myoinositol: Does it improve sperm mitochondrial function and sperm motility? Urology 2012, 79, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Montanino Oliva, M.; Minutolo, E.; Lippa, A.; Iaconianni, P.; Vaiarelli, A. Effect of myoinositol and antioxidants on sperm quality in men with metabolic sSyndrome. Int. J. Endocrinol. 2016, 2016, 1674950. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; La Vignera, S.; Mongioì, L.M.; Vitale, S.G.; Laganà, A.S.; Cimino, L.; Calogero, A.E. Myo-inositol as a male fertility molecule: Speed them up! Eur. Rev. Med. Pharmacol. Sci. 2017, 21 (Suppl. S2), 30–35. [Google Scholar] [PubMed]
- Dinkova, A.; Martinov, D.; Konova, E. Efficacy of myo-inositol in the clinical management of patients with asthenozoospermia. Eur. Rev. Med. Pharmacol. Sci. 2017, 21 (Suppl. S2), 62–65. [Google Scholar] [PubMed]
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of sperm mitochondrial function: A key organelle for sperm motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory. Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Marchetti, C.; Obert, G.; Deffosez, A.; Formstecher, P.; Marchetti, P. Study of mitochondrial membrane potential reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum. Reprod. 2002, 17, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.G.; Martins, A.D.; Cavaco, J.E.; Socorro, S.; Oliveira, P.F. Diabetes, insulin-mediated glucose metabolism and Sertoli/blood-testis barrier function. Tissue Barriers 2013, 1, e23992. [Google Scholar] [CrossRef] [PubMed]
- Ortmeyer, H.K.; Bodkin, N.L.; Hansen, B.C.; Larner, J. In vivo D-chiroinositol activates skeletal muscle glycogen synthase and inactivates glycogen phosphorylase in rhesus monkeys. J. Nutr. Biochem. 1995, 6, 499–503. [Google Scholar] [CrossRef]
- Mancini, M.; Andreassi, A.; Salvioni, M.; Pelliccione, F.; Mantellassi, G.; Banderali, G. Myoinositol and D-Chiro Inositol in Improving Insulin Resistance in Obese Male Children: Preliminary Data. Int. J. Endocrinol. 2016, 2016, 8720342. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; Mongioi, L.; Favilla, V.; Morgia, G.; Cimino, S.; Russo, G.; La Vignera, S. The gonadal function in obese adolescents: Review. J. Endocrinol. Investig. 2014, 37, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
| Men with Normozoospermia | Patients with Asthenozoospermia | p-Value | |
|---|---|---|---|
| Volume (mL) | 2.6 ± 0.26 | 2.3 ± 0.36 | NS |
| Sperm concentration (106/mL) | 39.6 ± 5.3 | 25.7 ± 8 | NS |
| Total sperm count (106/ejaculate) | 104.7 ± 18.6 | 48.7 ± 16.5 | <0.05 |
| Progressive motility (%) | 33.1 ± 0.64 | 19.7 ± 1.87 | <0.01 |
| Total motility (%) | 52 ± 3.5 | 41.2 ± 4.5 | NS |
| Normal forms (%) | 6.8 ± 1.2 | 8.0 ± 2.7 | NS |
| Leukocytes (106/mL) | 0.5 ± 0.2 | 1.0 ± 0.2 | NS |
| High MMP (%) | 86.2 ± 3.1 | 86.2 ± 0.8 | NS |
| Low MMP (%) | 8.8 ± 1.4 | 11.8 ± 0.5 | <0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Condorelli, R.A.; Barbagallo, F.; Calogero, A.E.; Cannarella, R.; Crafa, A.; La Vignera, S. D-Chiro-Inositol Improves Sperm Mitochondrial Membrane Potential: In Vitro Evidence. J. Clin. Med. 2020, 9, 1373. https://doi.org/10.3390/jcm9051373
Condorelli RA, Barbagallo F, Calogero AE, Cannarella R, Crafa A, La Vignera S. D-Chiro-Inositol Improves Sperm Mitochondrial Membrane Potential: In Vitro Evidence. Journal of Clinical Medicine. 2020; 9(5):1373. https://doi.org/10.3390/jcm9051373
Chicago/Turabian StyleCondorelli, Rosita A., Federica Barbagallo, Aldo E. Calogero, Rossella Cannarella, Andrea Crafa, and Sandro La Vignera. 2020. "D-Chiro-Inositol Improves Sperm Mitochondrial Membrane Potential: In Vitro Evidence" Journal of Clinical Medicine 9, no. 5: 1373. https://doi.org/10.3390/jcm9051373
APA StyleCondorelli, R. A., Barbagallo, F., Calogero, A. E., Cannarella, R., Crafa, A., & La Vignera, S. (2020). D-Chiro-Inositol Improves Sperm Mitochondrial Membrane Potential: In Vitro Evidence. Journal of Clinical Medicine, 9(5), 1373. https://doi.org/10.3390/jcm9051373








