Optimal Control Theory for Personalized Therapeutic Regimens in Oncology: Background, History, Challenges, and Opportunities
Abstract
1. Introduction
2. Current Approaches for Establishing Therapeutic Regimens
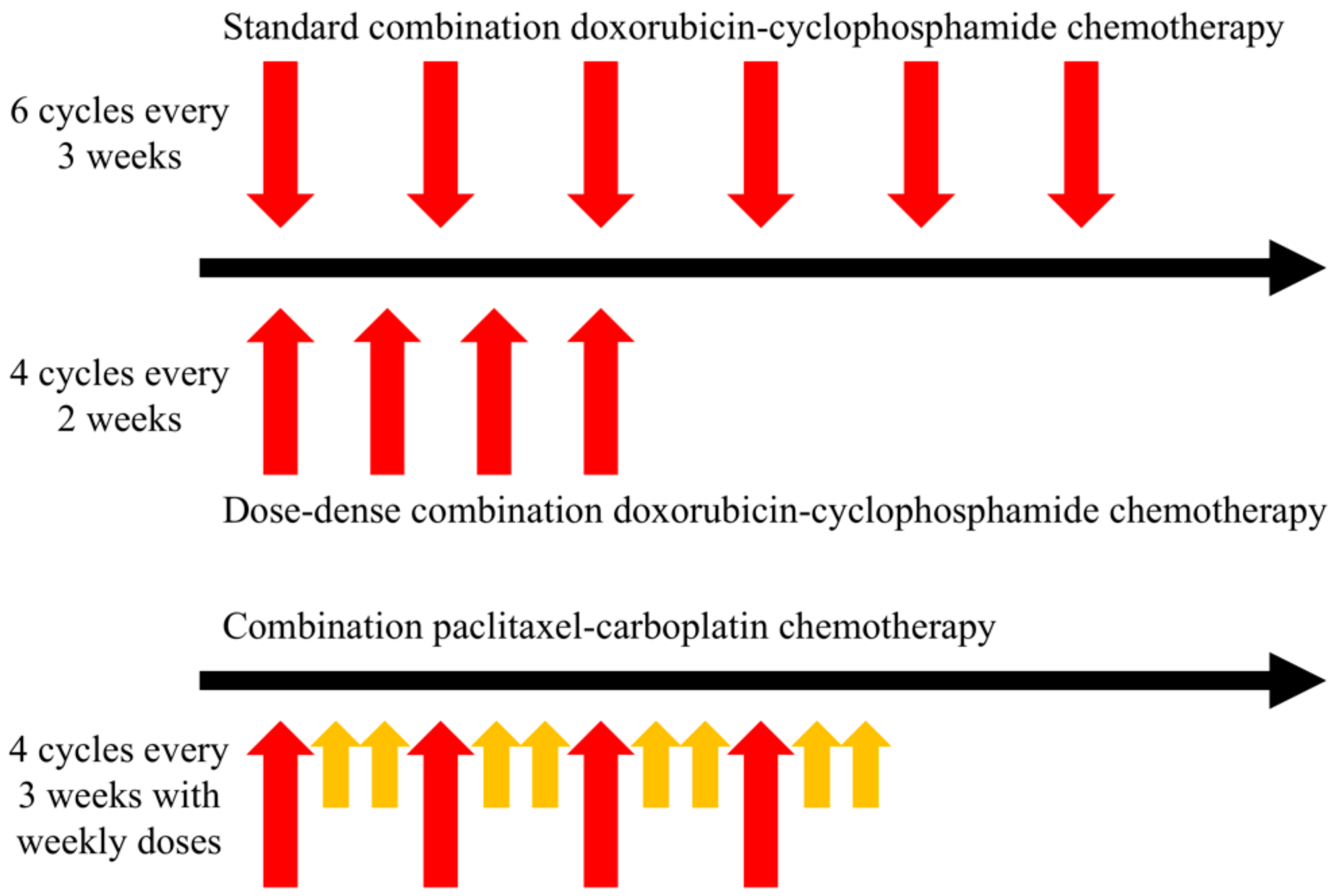
2.1. Systemic Therapy
2.2. Radiation Therapy
3. Fundamentals of OCT
3.1. What is an Objective Function?
3.2. Why and How Should Constraints be Applied to the Cost Function?
3.3. How do We Solve Optimal Control Systems?
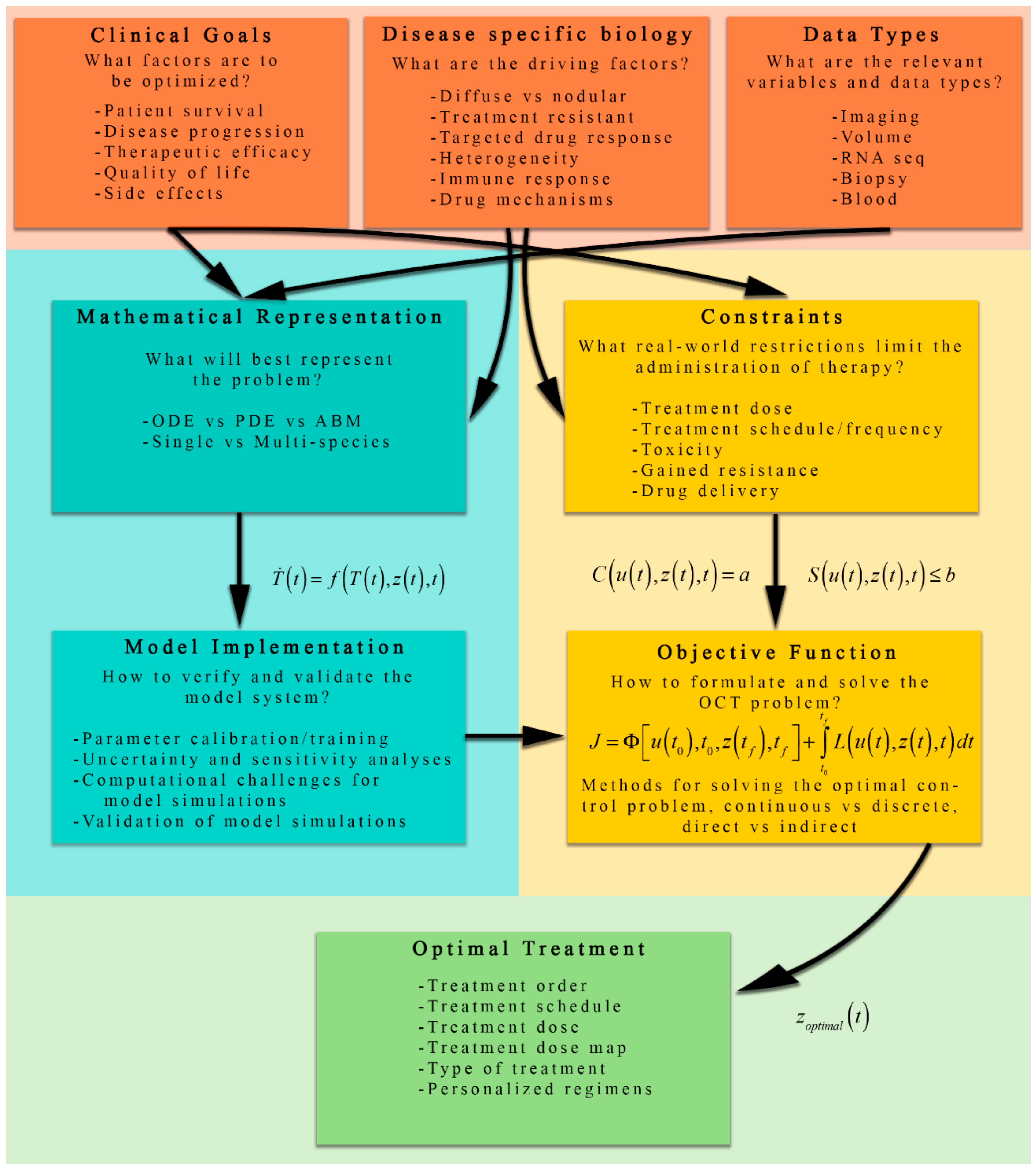
4. Formulating the Optimal Control Problem for Cancer Therapy
4.1. Overview of Mathematical Models of Tumor Growth for Optimization
4.2. Qualitative Discussion of the Cost Functional and Control Constraints
5. Applications of OCT in Cancer Therapy
5.1. Examples of OCT Applied to Chemotherapy
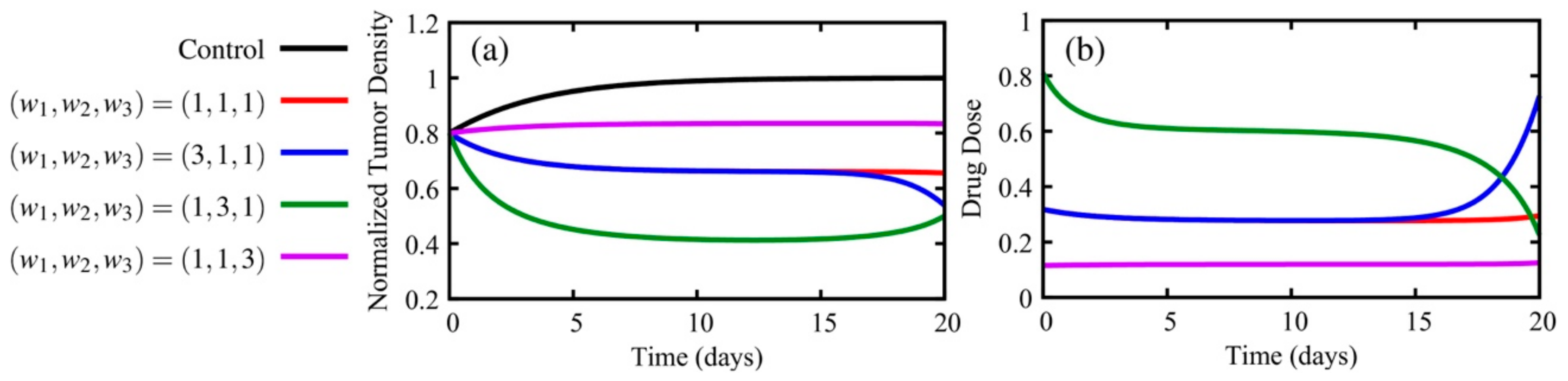
5.1.1. How Do the Weights of the Cost Functional Affect the Optimal Solutions?
5.1.2. What Clinically Relevant Constraints Might Be Considered?
5.1.3. What If the OCT Problem is Dependent on More Than One Variable? What about Drug Resistance?
5.2. Examples for Radiation Therapy
5.2.1. How Do Effects of Therapy on Both Healthy and Tumor Cells Affect the OCT Problem?
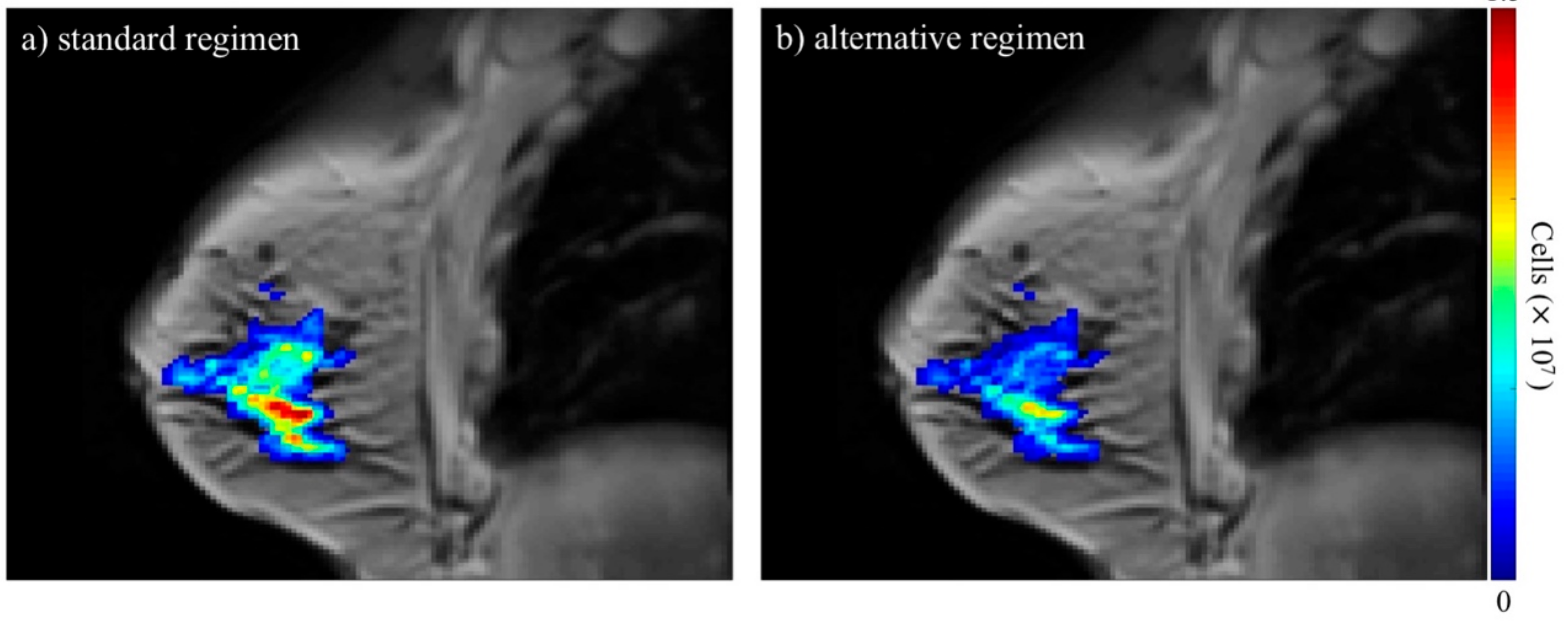
5.2.2. How Do We Account for the Spatial Distributions of Radiation Dose on Normal Tissue?
6. Challenges and Opportunities
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Connell, P.P.; Hellman, S. Advances in Radiotherapy and Implications for the Next Century: A Historical Perspective. Cancer Res. 2009, 69, 383–392. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Sorace, A.G.; Quarles, C.C.; Whisenant, J.G.; Hanker, A.B.; McIntyre, J.O.; Sanchez, V.; E Yankeelov, T. Trastuzumab improves tumor perfusion and vascular delivery of cytotoxic therapy in a murine model of HER2+ breast cancer: Preliminary results. Breast Cancer Res. Treat. 2016, 155, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Norton, L.; Albanell, J.; Kim, Y.M.; Mendelsohn, J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998, 58, 2825–2831. [Google Scholar]
- Modlin, J.; Mancini, R. Chemotherapy administration sequence: A review of the literature and creation of a sequencing chart. J. Hematol. Oncol. Pharm. 2011, 1, 1–21. [Google Scholar]
- Govindan, S.V.; Griffiths, G.L.; Hansen, H.J.; Horak, I.D.; Goldenberg, D.M. Cancer therapy with radiolabeled and drug/toxin-conjugated antibodies. Technol. Cancer Res. Treat. 2005, 4, 375–391. [Google Scholar] [CrossRef]
- Bonadonna, G.; Valagussa, P.; Moliterni, A.; Zambetti, M.; Brambilla, C. Adjuvant Cyclophosphamide, Methotrexate, and Fluorouracil in Node-Positive Breast Cancer — The Results of 20 Years of Follow-up. New Engl. J. Med. 1995, 332, 901–906. [Google Scholar] [CrossRef]
- Budman, D.R.; Cirrincione, C.T.; Henderson, I.C.; Wood, W.C.; Ferree, C.R.; Muss, H.B.; Green, M.R.; Frei, E.; Berry, D.A.; Weiss, R.B. Dose and Dose Intensity as Determinants of Outcome in the Adjuvant Treatment of Breast Cancer. J. Natl. Cancer Inst. 1998, 90, 1205–1211. [Google Scholar] [CrossRef]
- Sachs, J.R.; Mayawala, K.; Gadamsetty, S.; Kang, S.P.; De Alwis, D.P. Optimal Dosing for Targeted Therapies in Oncology: Drug Development Cases Leading by Example. Clin. Cancer Res. 2015, 22, 1318–1324. [Google Scholar] [CrossRef]
- Pinkel, D. The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Cancer Res. 1958, 18, 853–856. [Google Scholar]
- Redlarski, G.; Palkowski, A.; Krawczuk, M. Body surface area formulae: An alarming ambiguity. Sci. Rep. 2016, 6, 27966. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, S.; Sewell, G. Chemotherapy dosing part I: Scientific basis for current practice and use of body surface area. Clin. Oncol. 2007, 19, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Desoize, B.; Robert, J. Individual dose adaptation of anticancer drugs. Eur. J. Cancer 1994, 30, 844–851. [Google Scholar] [CrossRef]
- Cardoso, F.; Veer, L.V.T.; Bogaerts, J.; Slaets, L.; Viale, G.; Delaloge, S.; Pierga, J.-Y.; Brain, E.; Causeret, S.; Delorenzi, M.; et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N. Engl. J. Med. 2016, 375, 717–729. [Google Scholar] [CrossRef]
- A Habel, L.; Shak, S.; Jacobs, M.K.; Capra, A.; Alexander, C.; Pho, M.; Baker, J.B.; Walker, M.; Watson, D.; Hackett, J.; et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006, 8, R25. [Google Scholar] [CrossRef]
- Dos Reis, F.J.C.; Wishart, G.; Dicks, E.; Greenberg, D.; Rashbass, J.; Schmidt, M.K.; Broek, A.J.V.D.; Ellis, I.; Green, A.R.; Rakha, E.A.; et al. An updated PREDICT breast cancer prognostication and treatment benefit prediction model with independent validation. Breast Cancer Res. 2017, 19, 58. [Google Scholar] [CrossRef]
- Bernier, J.; Hall, E.J.; Giaccia, A. Radiation oncology: A century of achievements. Nat. Rev. Cancer 2004, 4, 737–747. [Google Scholar] [CrossRef]
- Smith, B.D.; Bellon, J.R.; Blitzblau, R.; Freedman, G.; Haffty, B.; Hahn, C.; Halberg, F.; Hoffman, K.; Horst, K.; Moran, J.; et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pr. Radiat. Oncol. 2018, 8, 145–152. [Google Scholar] [CrossRef]
- Roger Stupp, M.D.; Warren, P.; Mason, M.D.; Martin, J.; van den Bent, M.D.; Michael Weller, M.D.; Barbara Fisher, M.D.; Martin, J.B.; Taphoorn, M.D.; Karl Belanger, M.D.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Morgan, S.; Hoffman, K.; Loblaw, D.A.; Buyyounouski, M.K.; Patton, C.; Barocas, D.; Bentzen, S.; Chang, M.; Efstathiou, J.; Greany, P.; et al. Hypofractionated Radiation Therapy for Localized Prostate Cancer: Executive Summary of an ASTRO, ASCO and AUA Evidence-Based Guideline. J. Urol. 2019, 201, 528–534. [Google Scholar] [CrossRef]
- Curran, W.J.; Paulus, R.; Langer, C.J.; Komaki, R.; Lee, J.S.; Hauser, S.; Movsas, B.; Wasserman, T.; Rosenthal, S.A.; Gore, E.; et al. Sequential vs Concurrent Chemoradiation for Stage III Non–Small Cell Lung Cancer: Randomized Phase III Trial RTOG 9410. J. Natl. Cancer Inst. 2011, 103, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G. The Rationale for Fractionation in Radiotherapy. Clin. J. Oncol. Nurs. 2013, 17, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Eisbruch, A.; Harris, J.; Garden, A.S.; Chao, C.K.; Straube, W.; Harari, P.M.; Sanguineti, G.; Jones, C.U.; Bosch, W.R.; Ang, K.K. Multi-Institutional Trial of Accelerated Hypofractionated Intensity-Modulated Radiation Therapy for Early-Stage Oropharyngeal Cancer (RTOG 00-22). Int. J. Radiat. Oncol. 2009, 76, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.; Pignol, J.-P.; Levine, M.N.; Julian, J.A.; MacKenzie, R.; Parpia, S.; Shelley, W.; Grimard, L.; Bowen, J.; Lukka, H.; et al. Long-Term Results of Hypofractionated Radiation Therapy for Breast Cancer. N. Engl. J. Med. 2010, 362, 513–520. [Google Scholar] [CrossRef]
- Fogh, S.E.; Andrews, D.W.; Glass, J.; Curran, W.; Glass, C.; Champ, C.; Evans, J.J.; Hyslop, T.; Pequignot, E.; Downes, B.; et al. Hypofractionated Stereotactic Radiation Therapy: An Effective Therapy for Recurrent High-Grade Gliomas. J. Clin. Oncol. 2010, 28, 3048–3053. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Pei, X.; Chou, J.F.; Schechter, M.; Kollmeier, M.; Cox, B.; Yamada, Y.; Fidaleo, A.; Sperling, D.; Happersett, L.; et al. Dose escalation for prostate cancer radiotherapy: Predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur. Urol. 2011, 60, 1133–1139. [Google Scholar] [CrossRef]
- Kong, F.; Haken, R.T.; Schipper, M.; Sullivan, M.; Chen, M.; Lopez, C.; Kalemkerian, G.; Hayman, J. P-624 High dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small cell lung cancer: Long-term results of a radiation dose escalation study. Lung Cancer 2005, 49, S283. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C.A. The Concentration of Oxygen Dissolved in Tissues at the Time of Irradiation as a Factor in Radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef]
- Tatum, J.L. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int. J. Radiat. Boil. 2006, 82, 699–757. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Birer, S.R. Oxygen-Enhanced MRI Is a Major Advance in Tumor Hypoxia Imaging. Cancer Res. 2016, 76, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.A.; Link, J.M.; Mason, R. Molecular Imaging of Hypoxia. J. Nucl. Med. 2008, 49, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.; Mortensen, L.S.; Petersen, J.B.; Busk, M.; Overgaard, J. Imaging hypoxia to improve radiotherapy outcome. Nat. Rev. Clin. Oncol. 2012, 9, 674–687. [Google Scholar] [CrossRef]
- De Figueiredo, B.H.; Zacharatou, C.; Galland-Girodet, S.; Benech, J.; De Clermont-Gallerande, H.; Lamare, F.; Hatt, M.; Digue, L.; Pujol, E.D.M.D.; Fernandez, P. Hypoxia imaging with [18F]-FMISO-PET for guided dose escalation with intensity-modulated radiotherapy in head-and-neck cancers. Strahlenther. und Onkol. 2014, 191, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Batabyal, A.A. Optimal Control Theory with Applications in Economics. Interfaces 2013, 43, 108–109. [Google Scholar]
- Becker, R.A.; Sierstad, A.; Sydsæter, K.; Sydsaeter, K. Optimal Control Theory with Economic Applications. Scand. J. Econ. 1989, 91, 175. [Google Scholar] [CrossRef]
- Longuski, J.M.; Guzmán, J.J.; Prussing, J.E. Optimal Control. with Aerospace Applications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Ben-Asher, J.Z. Optimal Control. Theory with Aerospace Applations; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2010. [Google Scholar]
- Lee, E.B.; Markus, L. Foundations of Optimal Control. Theory; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1967. [Google Scholar]
- Kirk, D.E. Optimal Control. Theory: An. Introduction; Dover Publications Inc.: Mineola, NY, USA, 2012. [Google Scholar]
- Lenhart, S.; Workman, J.T. Optimal Control. Applied to Biological Models; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2007. [Google Scholar]
- Pistikopoulos, E.N.; Nacscu, I.; Velliou, E.G. Modelling Optimization and Control. of Biomedical Systems; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Schättler, H.; Ledzewicz, U. Optimal Control for Mathematical Models of Cancer Therapies; Springer Science and Business Media LLC: New York, NY, USA, 2015; Volume 42. [Google Scholar]
- Aniţa, S.; Arnăutu, V.; Capasso, V. An Introduction to Optimal Control Problems in Life Sciences and Economics from Mathematical Models to Numerical Simulation with MATLAB®; Birkhauser: Boston, MA, USA, 2011. [Google Scholar]
- Jarrett, A.M.; Lima, E.A.; Hormuth, D.A.; McKenna, M.T.; Feng, X.; Ekrut, D.A.; Resende, A.C.M.; Brock, A.; E Yankeelov, T.; Ii, M.T.M. Mathematical models of tumor cell proliferation: A review of the literature. Expert Rev. Anticancer Ther. 2018, 18, 1271–1286. [Google Scholar] [CrossRef]
- Sargent, R.W.H. Optimal control. J. Comput. Appl. Math. 2000, 124, 361–371. [Google Scholar] [CrossRef]
- Trélat, E. Optimal Control and Applications to Aerospace: Some Results and Challenges. J. Optim. Theory Appl. 2012, 154, 713–758. [Google Scholar] [CrossRef]
- Jarrett, A.M.; A Hormuth, D.; Barnes, S.L.; Feng, X.; Huang, W.; E Yankeelov, T.; Ii, D.A.H. Incorporating drug delivery into an imaging-driven, mechanics-coupled reaction diffusion model for predicting the response of breast cancer to neoadjuvant chemotherapy: Theory and preliminary clinical results. Phys. Med. Biol. 2018, 63, 105015. [Google Scholar] [CrossRef]
- Hormuth, D.A.; Jarrett, A.M.; Feng, X.; Yankeelov, T.E. Calibrating a Predictive Model of Tumor Growth and Angiogenesis with Quantitative MRI. Ann. Biomed. Eng. 2019, 47, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Weis, J.; Miga, M.I.; Arlinghaus, L.R.; Li, X.; Abramson, V.; Chakravarthy, A.B.; Pendyala, P.; E Yankeelov, T. Predicting the Response of Breast Cancer to Neoadjuvant Therapy Using a Mechanically Coupled Reaction-Diffusion Model. Cancer Res. 2015, 75, 4697–4707. [Google Scholar] [CrossRef]
- Clatz, O.; Sermesant, M.; Bondiau, P.-Y.; Delingette, H.; Warfield, S.K.; Malandain, G.; Ayache, N. Realistic simulation of the 3-D growth of brain tumors in MR images coupling diffusion with biomechanical deformation. IEEE Trans. Med. Imaging 2005, 24, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Rockne, R.C.; Trister, A.D.; Jacobs, J.; Hawkins-Daarud, A.J.; Neal, M.L.; Hendrickson, K.; Mrugala, M.M.; Rockhill, J.K.; Kinahan, P.; Krohn, K.A.; et al. A patient-specific computational model of hypoxia-modulated radiation resistance in glioblastoma using 18F-FMISO-PET. J. R. Soc. Interface 2015, 12, 20141174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sadowski, S.M.; Weisbrod, A.B.; Kebebew, E.; Summers, R.M.; Yao, J. Patient specific tumor growth prediction using multimodal images. Med. Image Anal. 2014, 18, 555–566. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, K.C.L.; Summers, R.M.; Kebebew, E.; Yao, J. Pancreatic Tumor Growth Prediction With Elastic-Growth Decomposition, Image-Derived Motion, and FDM-FEM Coupling. IEEE Trans. Med. Imaging 2016, 36, 111–123. [Google Scholar] [CrossRef]
- Neittaanmaki, P.; Tiba, D. Optimal Control. of Nonlinear Parabolic Systems: Theory: Algorithms and Applications; CRC Press: Boca Raton, FL, USA, 1994; Volume 179. [Google Scholar]
- Ramirez, W.F. Process Control and Identification; Academic Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Lions, J.L. Some Aspects of the Optimal Control of Distributed Parameter Systems; Society for Industrial & Applied Mathematics (SIAM): Philadelphia, PA, USA, 1972; Volume 6. [Google Scholar]
- Lions, J.L. Optimal Control of Systems Governed by Partial Differential Equations Problèmes Aux Limites; Springer: Berlin/Heidelberg, Germany, 1971. [Google Scholar]
- Bellman, R. Dynamic Programming. Science 1966, 153, 34. [Google Scholar] [CrossRef]
- Pontryagin, L. Mathematical Theory of Optimal Processes; Gordon and Breach Science Publishers: Montreux, Switzerland, 1986; Volume 4. [Google Scholar]
- Betts, J.T. Survey of Numerical Methods for Trajectory Optimization. J. Guid. Control. Dyn. 1998, 21, 193–207. [Google Scholar] [CrossRef]
- De Jager, B.; Van Keulen, T.; Kessels, J. Optimal Control. of Hybrid. Vehicles; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Betts, J.T. Practical Methods for Optimal Control. and Estimation Using Nonlinear Programming; Society for Industrial & Applied Mathematics (SIAM): Philadelphia, PA, USA, 2010; Volume 19. [Google Scholar]
- Yankeelov, T.E.; Atuegwu, N.; Hormuth, D.A.; Weis, J.; Barnes, S.L.; Miga, M.I.; Rericha, E.C.; Quaranta, V. Clinically Relevant Modeling of Tumor Growth and Treatment Response. Sci. Transl. Med. 2013, 5, 187ps9. [Google Scholar] [CrossRef]
- Yankeelov, T.E.; Quaranta, V.; Evans, K.J.; Rericha, E.C. Toward a science of tumor forecasting for clinical oncology. Cancer Res. 2015, 75, 918–923. [Google Scholar] [CrossRef]
- Rocha, H.L.; Almeida, R.C.; Lima, E.A.B.F.; Resende, A.C.M.; Oden, J.T.; Yankeelov, T.E. A hybrid three-scale model of tumor growth. Math. Model. Methods Appl. Sci. 2017, 28, 61–93. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.M.; Lowengrub, J.S.; Frieboes, H.B.; Cristini, V. Three-dimensional multispecies nonlinear tumor growth - I - Model and numerical method. J. Theor. Biol. 2008, 253, 524–543. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.A.B.F.; Oden, J.T.; Hormuth, D.A.; Yankeelov, T.E.; Almeida, R.C. Selection, calibration, and validation of models of tumor growth. Math. Model. Methods Appl. Sci. 2016, 26, 2341–2368. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Martin, J.D.; Chauhan, V.; Jain, S.R.; Diop-Frimpong, B.; Bardeesy, N.; Smith, B.L.; Ferrone, C.R.; Hornicek, F.J.; Boucher, Y.; et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 15101–15108. [Google Scholar] [CrossRef]
- Rodriguez, E.K.; Hoger, A.; McCulloch, A.D. Stress-dependent finite growth in soft elastic tissues. J. Biomech. 1994, 27, 455–467. [Google Scholar] [CrossRef]
- Weis, J.; Miga, M.I.; Arlinghaus, L.R.; Li, X.; Chakravarthy, A.B.; Abramson, V.; Farley, J.; E Yankeelov, T. A mechanically coupled reaction–diffusion model for predicting the response of breast tumors to neoadjuvant chemotherapy. Phys. Med. Biol. 2013, 58, 5851–5866. [Google Scholar] [CrossRef]
- Mendoza-Juez, B.; González, A.M.; Calvo, G.F.; Pérez-García, V.M. A Mathematical Model for the Glucose-Lactate Metabolism of in Vitro Cancer Cells. Bull. Math. Biol. 2011, 74, 1125–1142. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gawlinski, E.T. The glycolytic phenotype in carcinogenesis and tumor invasion: Insights through mathematical models. Cancer Res. 2003, 63, 3847–3854. [Google Scholar]
- Cai, Y.; Xu, S.; Wu, J.; Long, Q. Coupled modelling of tumour angiogenesis, tumour growth and blood perfusion. J. Theor. Biol. 2011, 279, 90–101. [Google Scholar] [CrossRef]
- Lima, E.A.B.F.; Oden, J.T.; Haeckel, M.; Shahmoradi, A.; Hormuth, D.A.; E Yankeelov, T.; Scarabosio, L.; Horger, T. Selection and validation of predictive models of radiation effects on tumor growth based on noninvasive imaging data. Comput. Methods Appl. Mech. Eng. 2017, 327, 277–305. [Google Scholar] [CrossRef]
- Jackson, T.L.; Byrne, H. A mathematical model to study the effects of drug resistance and vasculature on the response of solid tumors to chemotherapy. Math. Biosci. 2000, 164, 17–38. [Google Scholar] [CrossRef]
- Yankeelov, T.E.; Abramson, R.G.; Quarles, C.C. Quantitative multimodality imaging in cancer research and therapy. Nat. Rev. Clin. Oncol. 2014, 11, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Corwin, D.; Holdsworth, C.; Rockne, R.C.; Trister, A.D.; Mrugala, M.M.; Rockhill, J.K.; Stewart, R.D.; Phillips, M.; Swanson, K.R. Toward Patient-Specific, Biologically Optimized Radiation Therapy Plans for the Treatment of Glioblastoma. PLoS ONE 2013, 8, e79115. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, C.H.; Corwin, D.; Stewart, R.D.; Rockne, R.; Trister, A.D.; Swanson, K.R.; Phillips, M. Adaptive IMRT using a multiobjective evolutionary algorithm integrated with a diffusion-invasion model of glioblastoma. Phys. Med. Biol. 2012, 57, 8271–8283. [Google Scholar] [CrossRef] [PubMed]
- Metzcar, J.; Wang, Y.; Heiland, R.; Macklin, P. A Review of Cell-Based Computational Modeling in Cancer Biology. JCO Clin. Cancer Inform. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Swan, G.W.; Vincent, T.L. Optimal control analysis in the chemotherapy of IgG multiple myeloma. Bull. Math. Biol. 1977, 39, 317–337. [Google Scholar] [CrossRef]
- Bahrami, K.; Kim, M. Optimal control of multiplicative control systems arising from cancer therapy. IEEE Trans. Autom. Control. 1975, 20, 537–542. [Google Scholar] [CrossRef]
- Ledzewicz, U.; Schättler, H. Analysis of a class of optimal control problems arising in cancer chemotherapy. In Proceedings of the 2002 American Control Conference (IEEE Cat. No.CH37301), Anchorage, AK, USA, 8–10 May 2002; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2002; Volume 5, pp. 3460–3465. [Google Scholar]
- Ledzewicz, U.; Schättler, H. Optimal Bang-Bang Controls for a Two-Compartment Model in Cancer Chemotherapy. J. Optim. Theory Appl. 2002, 114, 609–637. [Google Scholar] [CrossRef]
- Świerniak, A.; Śmieja, J. Cancer chemotherapy optimization under evolving drug resistance. Nonlinear Anal. Theory Methods Appl. 2001, 47, 375–386. [Google Scholar] [CrossRef]
- Martin, R.; Teo, K.L. Optimal Control of Drug Administration in Cancer Chemotherapy; World Scientific: Singapore, 1994. [Google Scholar]
- Florian, J.A.; Eiseman, J.L.; Parker, R.S. A Nonlinear Model Predictive Control Algorithm for Breast Cancer Treatment. IFAC Proc. Vol. 2004, 37, 929–940. [Google Scholar] [CrossRef]
- Pereira, F.L.; Pedreira, C.E.; Pinho, M.R.; Fernandes, M.H.; Sousa, J.B. An Optimal Control Algorithm For Multidrug Cancer Chemotherapy Design. In Proceedings of the Twelfth Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Hong Kong, China, 29 October–1 November 1998; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 1998; pp. 1021–1022. [Google Scholar]
- Murray, J. Optimal control for a cancer chemotheraphy problem with general growth and loss functions. Math. Biosci. 1990, 98, 273–287. [Google Scholar] [CrossRef]
- Miettinen, K. Nonlinear Multiobjective Optimization; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 12. [Google Scholar]
- Almquist, K.; Banks, H. A theoretical and computational method for determining optimal treatment schedules in fractionated radiation therapy. Math. Biosci. 1976, 29, 159–179. [Google Scholar] [CrossRef]
- Badri, H.; Leder, K. Optimal treatment and stochastic modeling of heterogeneous tumors. Biol. Direct 2016, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- De Pillis, L.G.; Fister, K.R.; Gu, W.; Head, T.; Maples, K.; Neal, T.; Murugan, A.; Kozai, K. Optimal control of mixed immunotherapy and chemotherapy of tumors. J. Biol. Syst. 2008, 16, 51–80. [Google Scholar] [CrossRef]
- De Pillis, L.; Radunskaya, A. The dynamics of an optimally controlled tumor model: A case study. Math. Comput. Model. 2003, 37, 1221–1244. [Google Scholar] [CrossRef]
- Engelhart, M.; Lebiedz, D.; Sager, S. Optimal control for selected cancer chemotherapy ODE models: A view on the potential of optimal schedules and choice of objective function. Math. Biosci. 2011, 229, 123–134. [Google Scholar] [CrossRef]
- Ergun, A.; Camphausen, K.; Wein, L.M. Optimal Scheduling of Radiotherapy and Angiogenic Inhibitors. Bull. Math. Biol. 2003, 65, 407–424. [Google Scholar] [CrossRef]
- Garcke, H.; Lam, K.F.; Rocca, E. Optimal Control of Treatment Time in a Diffuse Interface Model of Tumor Growth. Appl. Math. Optim. 2017, 78, 495–544. [Google Scholar] [CrossRef]
- Garrido, M.-L.K.; Breitenbach, T.; Chudej, K.; Borzì, A. Modeling and Numerical Solution of a Cancer Therapy Optimal Control Problem. Appl. Math. 2018, 9, 985–1004. [Google Scholar] [CrossRef][Green Version]
- Hamdache, A.; Elmouki, I.; Saadi, S. Optimal Control with an Isoperimetric Constraint Applied to Cancer Immunotherapy. Int. J. Comput. Appl. 2014, 94, 31–37. [Google Scholar] [CrossRef]
- Hethcote, H.W.; Waltman, P. Theoretical Determination of Optimal Treatment Schedules for Radiation Therapy. Radiat. Res. 1973, 56, 150. [Google Scholar] [CrossRef] [PubMed]
- Arana, I.I.; Janda, Á.; Ardanza-Trevijano, S.; Trocóniz, I.F. Optimal dynamic control approach in a multi-objective therapeutic scenario: Application to drug delivery in the treatment of prostate cancer. PLoS Comput. Biol. 2018, 14, e1006087. [Google Scholar] [CrossRef]
- Itik, M.; Salamci, M.U.; Banks, S.P. Optimal control of drug therapy in cancer treatment. Nonlinear Anal. Theory Methods Appl. 2009, 71, e1473–e1486. [Google Scholar] [CrossRef]
- Ledzewicz, U.; Schättler, H.; D’Onofrio, A. Optimal control for combination therapy in cancer. In Proceedings of the 2008 47th IEEE Conference on Decision and Control, Cancun, Mexico, 9–11 December 2008; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2008; pp. 1537–1542. [Google Scholar]
- Ledzewicz, U.; Schättler, H. Multi-input Optimal Control Problems for Combined Tumor Anti-angiogenic and Radiotherapy Treatments. J. Optim. Theory Appl. 2011, 153, 195–224. [Google Scholar] [CrossRef]
- Ledzewicz, U.; Schättler, H. An Optimal Control Approach to Cancer Chemotherapy with Tumor–Immune System Interactions. In Graduate Texts in Mathematics; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; Volume 107, pp. 157–196. [Google Scholar]
- Ledzewicz, U.; Schättler, H.; Gahrooi, M.R.; Dehkordi, S.M. On the MTD paradigm and optimal control for multi-drug cancer chemotherapy. Math. Biosci. Eng. 2013, 10, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Ledzewicz, U.; Maurer, H.; Schättler, H. Optimal Combined Radio- and Anti-Angiogenic Cancer Therapy. J. Optim. Theory Appl. 2018, 180, 321–340. [Google Scholar] [CrossRef]
- Oke, S.; Matadi, M.B.; Xulu, S.S. Optimal Control Analysis of a Mathematical Model for Breast Cancer. Math. Comput. Appl. 2018, 23, 21. [Google Scholar] [CrossRef]
- Panetta, J.C.; Fister, K.R. Optimal Control Applied to Competing Chemotherapeutic Cell-Kill Strategies. SIAM J. Appl. Math. 2003, 63, 1954–1971. [Google Scholar] [CrossRef]
- Sbeity, H.; Younes, R. Review of Optimization Methods for Cancer Chemotherapy Treatment Planning. J. Comput. Sci. Syst. Biol. 2015, 8, 074–095. [Google Scholar] [CrossRef]
- Sharp, J.A.; Browning, A.P.; Mapder, T.; Burrage, K.; Simpson, M.J. Optimal control of acute myeloid leukaemia. J. Theor. Biol. 2019, 470, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Schättler, H. Optimal control of a mathematical model for cancer chemotherapy under tumor heterogeneity. Math. Biosci. Eng. 2016, 13, 1223–1240. [Google Scholar] [CrossRef] [PubMed]
- Swan, G.W. Cancer chemotherapy: Optimal control using the Verhulst-Pearl equation. Bull. Math. Biol. 1986, 48, 381–404. [Google Scholar] [CrossRef]
- Glick, A.; Mastroberardino, A. An Optimal Control Approach for the Treatment of Solid Tumors with Angiogenesis Inhibitors. Mathematics 2017, 5, 49. [Google Scholar] [CrossRef]
- Pouchol, C.; Clairambault, J.; Lorz, A.; Trélat, E. Asymptotic analysis and optimal control of an integro-differential system modelling healthy and cancer cells exposed to chemotherapy. J. Mathématiques Pures Appliquées 2018, 116, 268–308. [Google Scholar] [CrossRef]
- Almeida, L.; Bagnerini, P.; Fabrini, G.; Hughes, B.; Lorenzi, T. Evolution of cancer cell populations under cytotoxic therapy and treatment optimisation: Insight from a phenotype-structured model. ESAIM: Math. Model. Numer. Anal. 2019, 53, 1157–1190. [Google Scholar] [CrossRef]
- Scharovsky, O.; Mainetti, L.E.; Rozados, V.R. Metronomic chemotherapy: Changing the paradigm that more is better. Curr. Oncol. 2009, 16, 7–15. [Google Scholar] [CrossRef]
- Hahnfeldt, P.; Panigrahy, D.; Folkman, J.; Hlatky, L. Tumor development under angiogenic signaling: A dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res. 1999, 59, 4770–4775. [Google Scholar]
- Gorski, D.H.; Mauceri, H.J.; Salloum, R.M.; Gately, S.; Hellman, S.; A Beckett, M.; Sukhatme, V.P.; Soff, G.A.; Kufe, D.W.; Weichselbaum, R. Potentiation of the antitumor effect of ionizing radiation by brief concomitant exposures to angiostatin. Cancer Res. 1998, 58, 5686–5689. [Google Scholar]
- E Yankeelov, T.; An, G.; Saut, O.; Luebeck, E.G.; Popel, A.S.; Ribba, B.; Vicini, P.; Zhou, X.; Weis, J.; Ye, K.; et al. Multi-scale Modeling in Clinical Oncology: Opportunities and Barriers to Success. Ann. Biomed. Eng. 2016, 44, 2626–2641. [Google Scholar] [CrossRef]
- D’Esposito, A.; Sweeney, P.W.; Ali, M.; Saleh, M.; Ramasawmy, R.; Roberts, T.A.; Agliardi, G.; Desjardins, A.E.; Lythgoe, M.F.; Pedley, R.B.; et al. Computational fluid dynamics with imaging of cleared tissue and of in vivo perfusion predicts drug uptake and treatment responses in tumours. Nat. Biomed. Eng. 2018, 2, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hormuth, D.A.; Oliver, T.A.; Pineda, F.; Lorenzo, G.; Karczmar, G.S.; Moser, R.D.; Yankeelov, T.E. Patient-Specific Characterization of Breast Cancer Hemodynamics Using Image-Guided Computational Fluid Dynamics. IEEE Trans. Med. Imaging 2020. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- E Yankeelov, T.; Mankoff, D.A.; Schwartz, L.H.; Lieberman, F.S.; Buatti, J.M.; Mountz, J.M.; Erickson, B.J.; Fennessy, F.M.; Huang, W.; Kalpathy-Cramer, J.; et al. Quantitative Imaging in Cancer Clinical Trials. Clin. Cancer Res. 2016, 22, 284–290. [Google Scholar] [CrossRef]
- Huang, W.; Li, X.; Chen, Y.; Li, X.; Chang, M.-C.; Oborski, M.J.; Malyarenko, D.I.; Muzi, M.; Jajamovich, G.; Fedorov, A.Y.; et al. Variations of Dynamic Contrast-Enhanced Magnetic Resonance Imaging in Evaluation of Breast Cancer Therapy Response: A Multicenter Data Analysis Challenge. Transl. Oncol. 2014, 7, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Bane, O.; Hectors, S.J.; Wagner, M.; Arlinghaus, L.L.; Aryal, M.P.; Cao, Y.; Chenevert, T.L.; Fennessy, F.; Huang, W.; Hylton, N.M.; et al. Accuracy, repeatability, and interplatform reproducibility of T1 quantification methods used for DCE-MRI: Results from a multicenter phantom study. Magn. Reson. Med. 2017, 79, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Newitt, D.C.; Malyarenko, D.; Chenevert, T.L.; Quarles, C.C.; Bell, L.; Fedorov, A.Y.; Fennessy, F.; Jacobs, M.A.; Solaiyappan, M.; Hectors, S.; et al. Multisite concordance of apparent diffusion coefficient measurements across the NCI Quantitative Imaging Network. J. Med. Imaging 2017, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.C.; Semmineh, N.; An, H.; Eldeniz, C.; Wahl, R.; Schmainda, K.M.; Prah, M.A.; Erickson, B.J.; Korfiatis, P.; Wu, C.; et al. Evaluating Multisite rCBV Consistency from DSC-MRI Imaging Protocols and Postprocessing Software Across the NCI Quantitative Imaging Network Sites Using a Digital Reference Object (DRO). Tomography 2019, 5, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hormuth, D.A.; Jarrett, A.M.; Lima, E.A.; McKenna, M.T.; Fuentes, D.T.; Yankeelov, T.E. Mechanism-Based Modeling of Tumor Growth and Treatment Response Constrained by Multiparametric Imaging Data. JCO Clin. Cancer Inform. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Jarrett, A.M.; A Hormuth, D.; Wu, C.; Virostko, J.; Sorace, A.G.; Dicarlo, J.C.; Patt, D.; Goodgame, B.; Avery, S.; E Yankeelov, T. Abstract P2-16-17: Optimizing neoadjuvant regimens for individual breast cancer patients generated by a mathematical model utilizing quantitative magnetic resonance imaging data: Preliminary results. Poster Sess. Abstr. 2020, 80, 2–16. [Google Scholar] [CrossRef]
- Hormuth, D.A.; Sorace, A.G.; Virostko, J.; Abramson, R.G.; Bhujwalla, Z.M.; Enriquez-Navas, P.; Gillies, R.; Hazle, J.D.; Mason, R.P.; Quarles, C.C.; et al. Translating preclinical MRI methods to clinical oncology. J. Magn. Reson. Imaging 2019, 50, 1377–1392. [Google Scholar] [CrossRef]
- Koh, D.-M.; Collins, D. Diffusion-Weighted MRI in the Body: Applications and Challenges in Oncology. Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- E Yankeelov, T.; Gore, J.C. Dynamic Contrast Enhanced Magnetic Resonance Imaging in Oncology: Theory, Data Acquisition, Analysis, and Examples. Curr. Med. Imaging Formerly Curr. Med. Imaging Rev. 2007, 3, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Soloviev, D.; Lewis, D.Y.; Honess, D.; Aboagye, E. [18F]FLT: An imaging biomarker of tumour proliferation for assessment of tumour response to treatment. Eur. J. Cancer 2012, 48, 416–424. [Google Scholar] [CrossRef]
- Zhao, D.; Pacheco-Torres, J.; Hallac, R.R.; White, D.; Peschke, P.; Cerdan, S.; Mason, R. Dynamic oxygen challenge evaluated by NMR T1 and T2*--insights into tumor oxygenation. NMR Biomed. 2015, 28, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Quarles, C.; Bell, L.C.; Stokes, A.M. Imaging vascular and hemodynamic features of the brain using dynamic susceptibility contrast and dynamic contrast enhanced MRI. NeuroImage 2019, 187, 32–55. [Google Scholar] [CrossRef]
- Imam, S.K. Review of Positron Emission Tomography Tracers for Imaging of Tumor Hypoxia. Cancer Biotherapy Radiopharm. 2010, 25, 365–374. [Google Scholar] [CrossRef]
- Brady, A.; Laoide, R.Ó.; McCarthy, P.; McDermott, R. Discrepancy and Error in Radiology: Concepts, Causes and Consequences. Ulst. Med. J. 2012, 81, 3–9. [Google Scholar]
- Begley, C.G.; Ellis, L.M. Raise standards for preclinical cancer research. Nature 2012, 483, 531–533. [Google Scholar] [CrossRef]
- Hamby, D.M. A review of techniques for parameter sensitivity analysis of environmental models. Environ. Monit. Assess. 1994, 32, 135–154. [Google Scholar] [CrossRef]
- Campolongo, F.; Tarantola, S.; Saltelli, A.; Ratto, M. Sensitivity Analysis in Practice: A Guide to Assessing Scientific Models; John Wiley & Sons Ltd.: West Sussex, England, 2004. [Google Scholar]
- Babuška, I.; Nobile, F.; Tempone, R. A Stochastic Collocation Method for Elliptic Partial Differential Equations with Random Input Data. SIAM J. Numer. Anal. 2007, 45, 1005–1034. [Google Scholar] [CrossRef]
- Cohen, A.; Devore, R. Approximation of high-dimensional parametric PDEs. Acta Numer. 2015, 24, 1–159. [Google Scholar] [CrossRef]
- Le Maître, O.P.; Knio, O.M.; Le Maître, O. Spectral Methods for Uncertainty Quantification; Springer Science and Business Media: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Martinez-Frutos, J.; Herrero-Perez, D.; Kessler, M.; Periago, F. Robust shape optimization of continuous structures via the level set method. Comput. Methods Appl. Mech. Eng. 2016, 305, 271–291. [Google Scholar] [CrossRef]
- Gunzburger, M.D.; Lee, H.-C.; Lee, J. Error Estimates of Stochastic Optimal Neumann Boundary Control Problems. SIAM J. Numer. Anal. 2011, 49, 1532–1552. [Google Scholar] [CrossRef]
- Alexanderian, A.; Petra, N.; Stadler, G.; Ghattas, O. Mean-Variance Risk-Averse Optimal Control of Systems Governed by PDEs with Random Parameter Fields Using Quadratic Approximations. SIAM/ASA J. Uncertain. Quantif. 2017, 5, 1166–1192. [Google Scholar] [CrossRef]
- Chen, P.; Villa, U.; Ghattas, O. Taylor approximation for PDE-constrained optimization under uncertainty: Application to turbulent jet flow. PAMM 2018, 18, e201800466-4. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y.-X. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, W.; Li, N.; Neri, S.; Sharma, A.; Jiang, W.; Lin, S.H. Combining Immunotherapy and Radiotherapy for Cancer Treatment: Current Challenges and Future Directions. Front. Pharmacol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Lazzari, C.; Karachaliou, N.; Bulotta, A.; Viganò, M.; Mirabile, A.; Brioschi, E.; Santarpia, M.; Gianni, L.; Rosell, R.; Gregorc, V. Combination of immunotherapy with chemotherapy and radiotherapy in lung cancer: Is this the beginning of the end for cancer? Ther. Adv. Med. Oncol. 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Rocco, D.; Della Gravara, L.; Battiloro, C.; Gridelli, C. The role of combination chemo-immunotherapy in advanced non-small cell lung cancer. Expert Rev. Anticancer. Ther. 2019, 19, 561–568. [Google Scholar] [CrossRef]
- Zahnd, D.; Aebi, S.; Rusterholz, S.; Fey, M.F.; Borner, M.M. A randomized crossover trial assessing patient preference for two different types of portable infusion-pump devices. Ann. Oncol. 1999, 10, 727–729. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarrett, A.M.; Faghihi, D.; Hormuth, D.A., II; Lima, E.A.B.F.; Virostko, J.; Biros, G.; Patt, D.; Yankeelov, T.E. Optimal Control Theory for Personalized Therapeutic Regimens in Oncology: Background, History, Challenges, and Opportunities. J. Clin. Med. 2020, 9, 1314. https://doi.org/10.3390/jcm9051314
Jarrett AM, Faghihi D, Hormuth DA II, Lima EABF, Virostko J, Biros G, Patt D, Yankeelov TE. Optimal Control Theory for Personalized Therapeutic Regimens in Oncology: Background, History, Challenges, and Opportunities. Journal of Clinical Medicine. 2020; 9(5):1314. https://doi.org/10.3390/jcm9051314
Chicago/Turabian StyleJarrett, Angela M., Danial Faghihi, David A. Hormuth, II, Ernesto A. B. F. Lima, John Virostko, George Biros, Debra Patt, and Thomas E. Yankeelov. 2020. "Optimal Control Theory for Personalized Therapeutic Regimens in Oncology: Background, History, Challenges, and Opportunities" Journal of Clinical Medicine 9, no. 5: 1314. https://doi.org/10.3390/jcm9051314
APA StyleJarrett, A. M., Faghihi, D., Hormuth, D. A., II, Lima, E. A. B. F., Virostko, J., Biros, G., Patt, D., & Yankeelov, T. E. (2020). Optimal Control Theory for Personalized Therapeutic Regimens in Oncology: Background, History, Challenges, and Opportunities. Journal of Clinical Medicine, 9(5), 1314. https://doi.org/10.3390/jcm9051314




