The Multispecies Probiotic Effectively Reduces Homocysteine Concentration in Obese Women: A Randomized Double-Blind Placebo-Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Probiotic and Placebo
2.4. Biochemical Analyses
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 December 2019).
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metabol. 2017, 14, 78. [Google Scholar] [CrossRef]
- Lai, W.K.; Kan, M.Y. Homocysteine-inducted endothelial dysfunction. Ann Nutr. Metabol. 2015, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Yang, H.; Wang, H. Hyperhomocysteinemia and Endothelial Dysfunction. Curr. Hypertens. Rev. 2009, 5, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Pushpakumar, S.; Kundu, S.; Sen, U. Endothelial Dysfunction: The link between homocysteine and hydrogen sulfide. Curr. Med. Chem. 2014, 21, 3662–3672. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Young, C.; Mshvildadze, M.; Neu, J. Intestinal microbiota: Does it play a role in diseases of the neonate? Neo. Rev. 2009, 10, 166–179. [Google Scholar] [CrossRef]
- Mu, Q.; Kirby, J.; Christopher, M.; Reilly, C.h.M.; Luo, X.M. Leaky gut as a danger signal for autoimmune diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef]
- Miele, L.; Giorgio, V.; Alberelli, M.A.; De Candia, E.; Gasbarrini, A.; Grieco, A. Impact of gut microbiota on obesity, diabetes and cardiovascular disease risk. Curr. Cardiol. Rep. 2015, 17, 120–127. [Google Scholar] [CrossRef]
- Lau, E.; Carvalho, D.; Pina-Vaz, C.; Barbosa, J.A.; Freitas, P. Beyond gut microbiota: Understanding obesity and type 2 diabetes. Hormones 2015, 14, 358–369. [Google Scholar] [CrossRef]
- Dibaise, J.K.; Zhang, H.; Crowell, M.D.; Krajmalnik-Brown, R.; Decker, G.A.; Rittmann, B.E. Gut microbiota and its possible relationship with obesity. Mayo Clin. Proc. 2008, 83, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Le Barz, M.; Anhe, F.F.; Varin, T.V.; Desjardins, Y.; Levv, E.; Roy, D.; Urdaci, M.C.; Marette, A. Probiotics as complementary treatment for metabolic disorders. Diabetes Metab. 2015, 39, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Kleniewska, P.; Hoffmann, A.; Pniewska, E.; Pawliczak, R. The influence of probiotic Lactobacillus casei in combination with prebiotic inulin on the antioxidant capacity of human plasma. Oxid. Med. Cell. Longev. 2016, 1340903. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Szulinska, M.; Loniewski, I.; Van Hemert, S.; Sobieska, M.; Bogdanski, P. Dose-dependent effects of multispecies probiotic supplementation on the lipopolysaccharide (LPS) level and cardiometabolic profile in obese postmenopausal women: A 12-week randomized clinical trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef]
- Szulinska, M.; Loniewski, I.; Skrypnik, K.; Sobieska, M.; Korybalska, K.; Suliburska, J.; Bogdański, P. Multispecies probiotic supplementation favorably affects vascular function and reduces arterial stiffness in obese postmenopausal women—A 12-week placebo-controlled and randomized clinical study. Nutrients 2018, 10, 1672. [Google Scholar] [CrossRef]
- Barreto, F.M.; Simao, A.N.C.; Morimoto, H.K.; Batisti Lozovoy, M.A.; Dichi, I.; Silva Miglioranza, H.D.L. Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition 2014, 30, 939–942. [Google Scholar] [CrossRef]
- Alihosseini, N.; Moahboob, S.A.; Farrin, N.; Mobasseri, M.; Taghizadeh, A.; Ostadrahimi, A.R. Effect of probiotic fermented milk (kefir) on serum level of insulin and homocysteine in type 2 diabetes patients. Acta Endocrinol. 2017, 13, 431–436. [Google Scholar] [CrossRef]
- Jarosz, M. Dietary Suplements and Health; PZWL Medical Publishing: Warsaw, Poland, 2015; pp. 36–45. [Google Scholar]
- Cani, P.D. Gut microbiota and obesity: Lessons from the microbiome. Brief. Funct. Genomics 2013, 12, 381–387. [Google Scholar] [CrossRef]
- Bindels, L.B.; Dewulf, E.M.; Delzenne, N.M. GPR43/FFA2: Physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 2013, 34, 226–232. [Google Scholar] [CrossRef]
- Sybesma, W.; Starrenburg, M.; Tijsseling, L.; Hoefnagel, M.H.N.; Hugenholtz, J. Effects of cultivation conditions on folateproduction by lactic acid bacteria. Appl. Environ. Microbiol. 2003, 69, 4542–4548. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate production by probiotic bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Thompson, P.L.; Stojceski, B.; Prince, R.L. The effect of youghurt and its probiotics on blood pressure and serum lipid profile, a randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Qujeq, D.; Omran, T.S.; Hosini, L. Correlation between total homocysteine, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol in the serum of patients with myocardial infarction. Clin. Biochem. 2001, 34, 97–101. [Google Scholar] [CrossRef]
- Obeid, R.; Herrmann, W. Homocysteine and lipids: S-Adenosyl methionine as a key intermediate. FEBS Letters 2009, 583, 1215–1225. [Google Scholar] [CrossRef]
- Chernyavskiy, I.; Veeranki, S.; Sen, U. Atherogenesis: Hyperhomocysteinemia interactions with LDL, macrophage function, paraoxonase 1, and exercise. Ann. N.Y. Acad. Sci. 2016, 1363, 138–154. [Google Scholar] [CrossRef]
- Liao, D.; Tan, H.; Hui, R.; Li, Z.; Jiang, X.; Gaubatz, J.; Yang, F.; Durante, W.; Chan, L.; Schafer, A.I.; et al. Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipoprotein A-I protein synthesis and enhancing HDL cholesterol clearance. Circ. Res. 2006, 99, 598–606. [Google Scholar] [CrossRef]
- Guéant-Rodriguez, R.M.; Spada, R.; Moreno-Garcia, M.; Anello, G.; Bosco, P.; Lagrost, L.; Romano, A.; Elia, M.; Guéant, J.L. Homocysteine is a determinant of ApoA-I and both are associated with ankle brachial index, in an ambulatory elderly population. Atherosclerosis 2011, 214, 480–485. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Bogdanski, P.; Pupek-Musialik, D.; Dytfeld, J. Plasma homocysteinevis a determinant of tissue necrosis factor-alfa in hypertensive patients. Biomed. Pharmacother. 2008, 62, 360–365. [Google Scholar] [CrossRef]
- Valentini, L.; Pinto, A.; Bourdel-Marchasson, I.; Ostan, R.; Brigidi, P.; Turroni, S.; Hrelia, S.; Hrelia, P.; Bereswill, S.; Fischer, A.; et al. Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota–The “RISTOMED project”: Randomized controlled trial in healthy older people. Clin. Nutr. 2015, 34, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: A randomized, controlled trial. Mediat. Inflamm. 2014, 348959. [Google Scholar] [CrossRef]
- Vaghef-Mehrabany, E.; Alipour, B.; Homayouni-Rad, A.; Sharif, S.K.; Asghari-Jafarabadi, M.; Zavvari, S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 2014, 30, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Probiotic(n = 25) | Placebo (n = 25) | p-Value |
|---|---|---|---|
| Age (years) | 55.2 ± 6.9 | 58.7 ± 7.3 | NS |
| Weight (kg) | 94.5 ± 16.6 | 92.8 ± 11.9 | NS |
| Height (cm) | 160.8 ± 6.2 | 160.4 ± 6.4 | NS * |
| BMI (kg/m2) | 36.6 ± 6.0 | 36.1 ± 4.4 | NS |
| Waist circumference (cm) | 109.8 ± 11.7 | 109.9 ± 8.3 | NS * |
| SBP (mmHg) | 134.8 ± 10.1 | 133.6 ± 12.2 | NS |
| DBP (mmHg) | 79.9 ± 8.1 | 83.8 ± 7.3 | NS * |
| Variables | Probiotic (n = 25) | Placebo (n = 25) | ||||
|---|---|---|---|---|---|---|
| Before | After | p-Value | Before | After | p-Value | |
| Hcy (µmol/L) | 11.32 ± 2.23 | 10.11 ±1.57 | <0.0001 | 11.63 ± 2.08 | 11.13 ± 2.13 | NS * |
| TNF-α (ng/L) | 1.04 ± 0.34 | 0.85 ± 0.23 | 0.0001 | 1.05 ± 0.34 | 1.04 ± 0.31 | NS |
| TAS (mmol/L) | 1.65 ± 0.20 | 1.76 ± 0.14 | 0.0076 | 1.65 ± 0.20 | 1.64 ± 0.12 | NS |
| TC (mmol/L) | 5.65 ± 0.85 | 5.24 ± 0.80 | 0.0020 | 5.27 ± 0.91 | 5.12 ± 0.98 | NS * |
| LDL (mmol/L) | 3.09 ± 0.82 | 2.97 ± 0.96 | 0.0149 | 3.00 ± 0.87 | 2.93 ± 0.91 | NS * |
| HDL (mmol/L) | 1.36 ± 0.28 | 1.41 ± 0.22 | NS * | 1.35 ± 0.26 | 1.44 ± 0.28 | NS * |
| TG (mmol/L) | 1.86 ± 0.88 | 1.73 ± 0.63 | 0.0140 | 1.60 ± 0.71 | 1.53 ± 0.78 | NS |
| Probiotic vs. Placebo | |||
|---|---|---|---|
| Variables | p-Value | ||
| Before | After | Δ | |
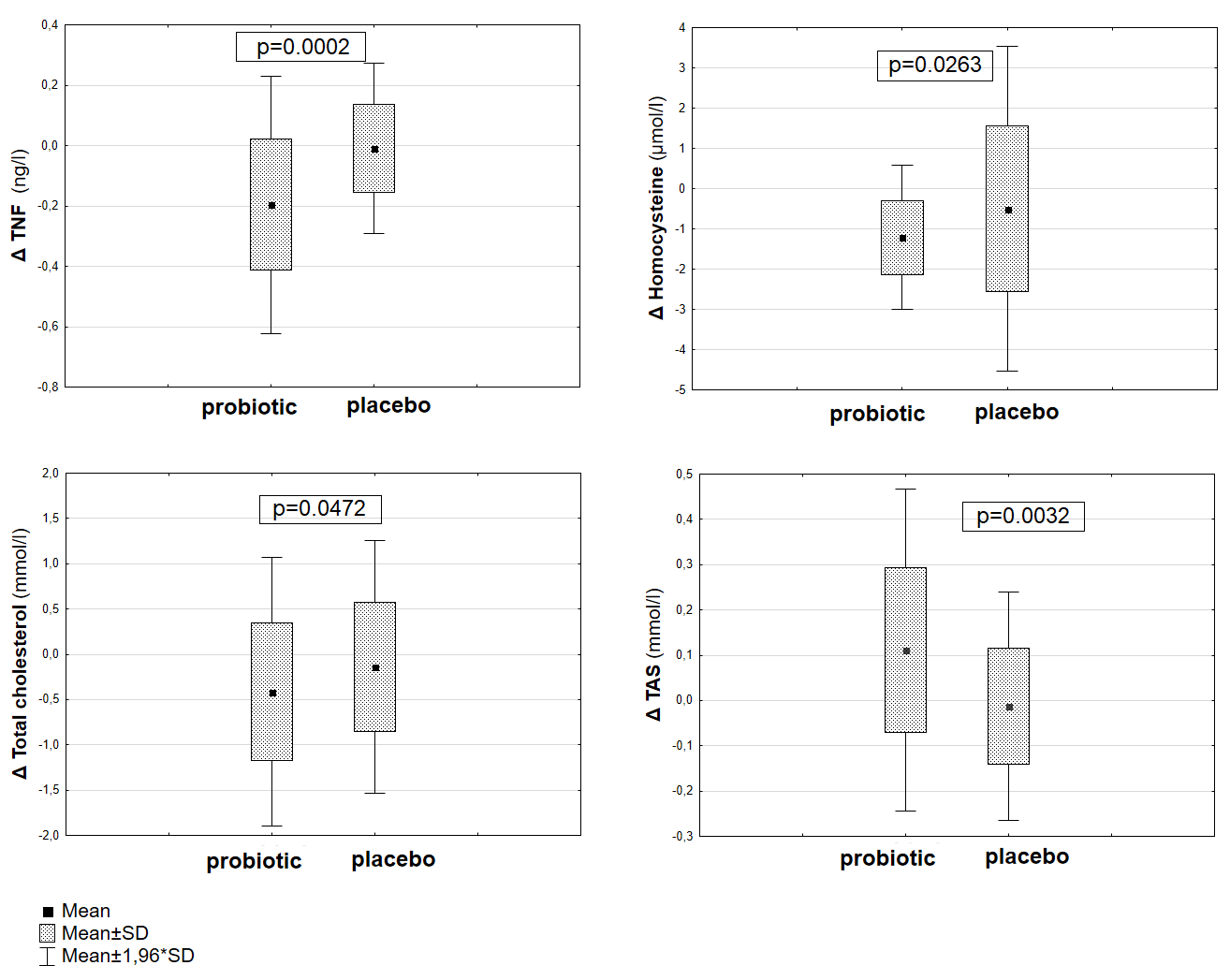
| Hcy(µmol/L) | NS | NS * | 0.0263 |
| TNF-α(ng/L) | NS | 0.0070 | 0.0002 |
| TAS (mmol/L) | NS | 0.0017 * | 0.0032 |
| TC (mmol/L) | NS | NS | 0.0472 |
| LDL (mmol/L) | NS * | NS | NS |
| HDL (mmol/L) | NS * | NS * | NS * |
| TG (mmol/L) | NS | NS | NS |
| Δ Hcy (µmol/L) | RP-Value | |
|---|---|---|
| Δ TNF-α (ng/L) | 0.36 | NS |
| Δ TAS (mmol/L) | 0.12 | NS |
| Δ TC (mmol/L) | 0.15 | NS |
| Δ LDL (mmol/L) | 0.29 | NS |
| Δ HDL (mmol/L) | 0.02 | NS |
| Δ TG (mmol/L) | −0.17 | NS |
| Multiple Linear Regression, Dependent Variable Hcy (µmol/L) | ||||||
|---|---|---|---|---|---|---|
| Before Probiotic Suplementation | After Probiotic Supplementation | |||||
| β | Standard Error of β | p-Value | β | Standard Error of β | p-Value | |
| Intercept | 12.20 | 7.60 | NS | 13.70 | 6.76 | NS |
| TNF-α (ng/L) | 0.44 | 1.68 | NS | 1.09 | 1.87 | NS |
| TAS (mmol/L) | 1.45 | 3.12 | NS | −1.82 | 2.78 | NS |
| TC (mmol/L) | −0.01 | 0.02 | NS | 0.00 | 0.02 | NS |
| LDL (mmol/L) | 0.00 | 0.02 | NS | 0.00 | 0.01 | NS |
| HDL (mmol/L) | −0.02 | 0.07 | NS | −0.02 | 0.07 | NS |
| TG (mmol/L) | 0.00 | 0.01 | NS | 0.00 | 0.01 | NS |
| R | 0.23 | 0.27 | ||||
| R2 | 0.06 | 0.07 | ||||
| F(6,18) | 0.18 | 0.24 | ||||
| Author | Study Group | Probiotics | Study Duration | Dosage | Results |
|---|---|---|---|---|---|
| Valentini et al. [33] | Healthy adults (65–85 years) | B. infantis DSM24737, B. longum DSM24736, B. breve DSM24732, L. acidophilus DSM24735, L. delbrueckii ssp. bulgaricus DSM24734, L. paracasei DSM24733, L. plantarum DSM24730, S. thermophilus DSM24731 | 56 days | 112 × 109 CFU/caps 2 caps/daily | Hcy↓ vitamin B12, folic acid↑ No significant effect on inflammatory biomarkers, lipid profile |
| Rajkumar et al. [34] | Overweight, healthy adults (40–60 years) | B. longum, B. infantis, B. breve, L. acidophilus, L. paracasei, L. delbrueckii spp. bulgaricus, L. plantarum, S. salivarius spp. Thermophilus | 6 weeks | 112.5 × 109 CFU/caps | HDL ↑ TC, TG, LDL, VLDL↓ hs-CRP↓ |
| Vagher-Mehrabany et al. [35] | Women with rheumatoid arthritis (20–80 years) | Lactobacillus casei 01 | 8 weeks | 108 CFU/caps | Il-6, Il-12, TNF-α,↓ Il-10 ↑ No significant effect on Il-1β |
| Asemi et al. [36] | Diabetic patients (35–70 years) | L. acidophilus, L.casei, L. rhamnosus, L. bulgaricus, B. breve, B. longum, S. thermophilus, Fructo-oligosaccharide. | 8 weeks | 2 × 109 CFU 7 × 109 CFU 1.5 × 109 CFU 2 × 108 CFU 2 × 1010 CFU 7 × 109 CFU 1.5 × 109 CFU 100 mg/caps | GSH, TAC, LDL↑ hs-CRP, HDL ↓ No significant effect on TC, TG, |
| Barreto et al. [18] | Postmenopau-sal women with metabolic syndrome | L. plantarum | 90 days | 1.25 × 107 CFU/g (fermented milk–80 mL/d) | Hcy, TC, Il-6↓ No significant effect on HDL, LDL, TG, CRP, TNF-α |
| Present study | Obese women (45–65 years) | Probiotic Ecologic® BARRIER | 12 weeks | 2.5 × 109 CFU/g | Hcy, TC, TNF-α↓ TAS↑ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewska, K.; Kręgielska-Narożna, M.; Jakubowski, H.; Szulińska, M.; Bogdański, P. The Multispecies Probiotic Effectively Reduces Homocysteine Concentration in Obese Women: A Randomized Double-Blind Placebo-Controlled Study. J. Clin. Med. 2020, 9, 998. https://doi.org/10.3390/jcm9040998
Majewska K, Kręgielska-Narożna M, Jakubowski H, Szulińska M, Bogdański P. The Multispecies Probiotic Effectively Reduces Homocysteine Concentration in Obese Women: A Randomized Double-Blind Placebo-Controlled Study. Journal of Clinical Medicine. 2020; 9(4):998. https://doi.org/10.3390/jcm9040998
Chicago/Turabian StyleMajewska, Karolina, Matylda Kręgielska-Narożna, Hieronim Jakubowski, Monika Szulińska, and Paweł Bogdański. 2020. "The Multispecies Probiotic Effectively Reduces Homocysteine Concentration in Obese Women: A Randomized Double-Blind Placebo-Controlled Study" Journal of Clinical Medicine 9, no. 4: 998. https://doi.org/10.3390/jcm9040998
APA StyleMajewska, K., Kręgielska-Narożna, M., Jakubowski, H., Szulińska, M., & Bogdański, P. (2020). The Multispecies Probiotic Effectively Reduces Homocysteine Concentration in Obese Women: A Randomized Double-Blind Placebo-Controlled Study. Journal of Clinical Medicine, 9(4), 998. https://doi.org/10.3390/jcm9040998







