Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
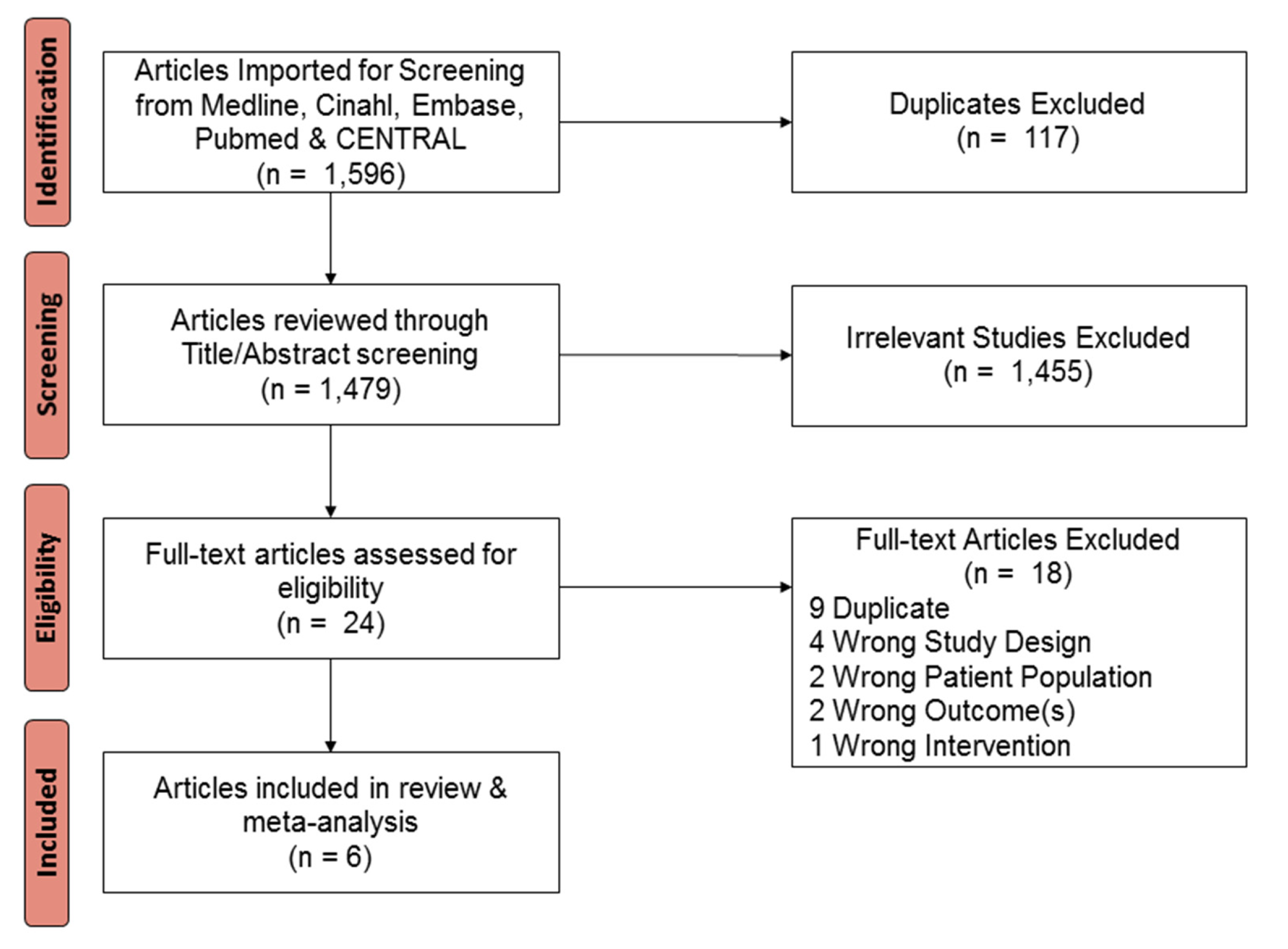
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Statistical Analysis
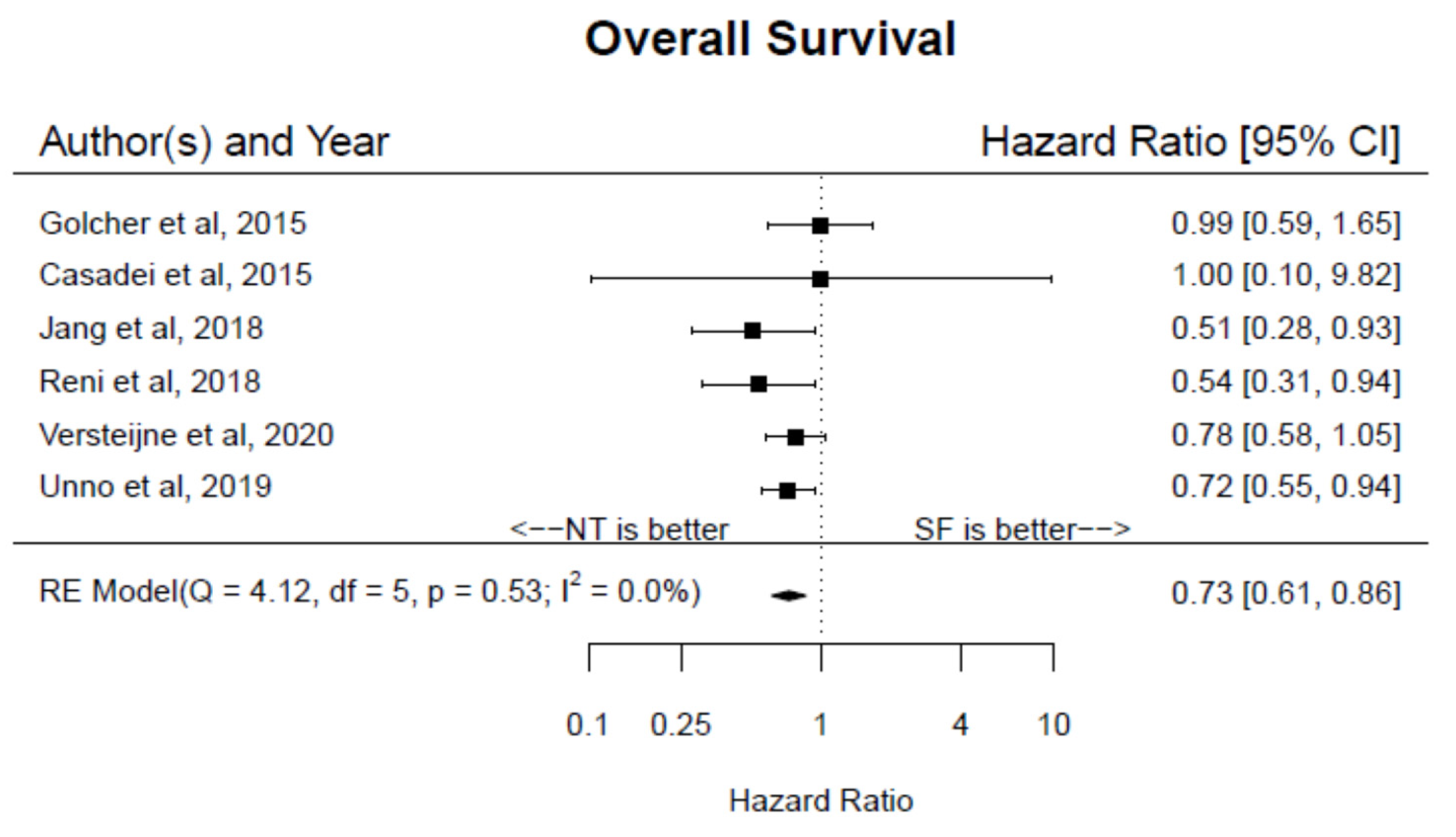
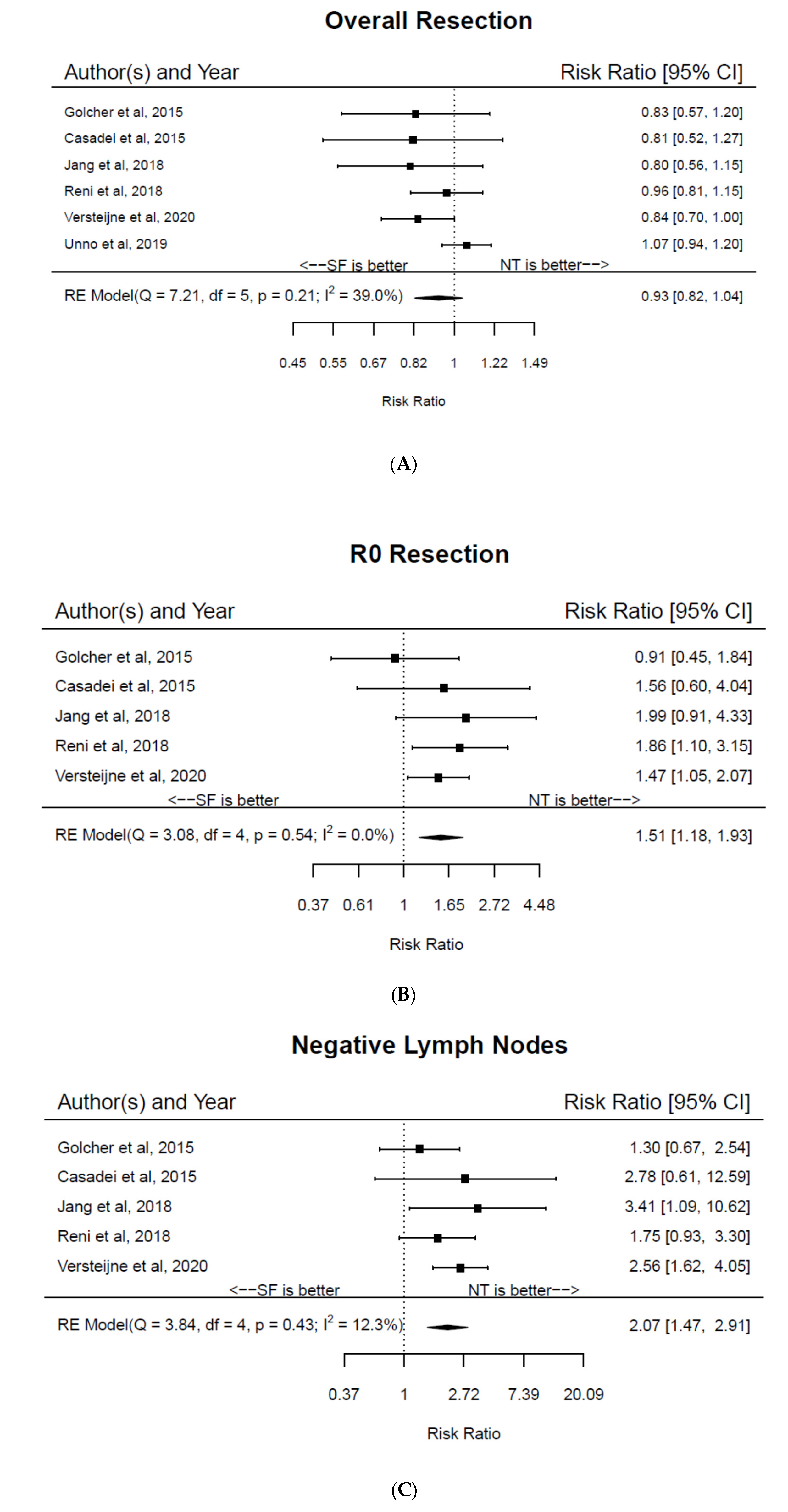
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Groot, V.P.; Rezaee, N.; Wu, W.; Cameron, J.L.; Fishman, E.K.; Hruban, R.H.; Weiss, M.J.; Zheng, L.; Wolfgang, C.L.; He, J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2017, 267, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Post, S.; Neuhaus, P.; Gellert, K.; Langrehr, J.; Ridwelski, K.; Schramm, H.; Fahlke, J.; Zuelke, C.; Burkart, C.; et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 2007, 297, 267–277. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Bentrem, D.J.; Ko, C.Y.; Tomlinson, J.S.; Stewart, A.K.; Winchester, D.P.; Talamonti, M.S. Multimodality therapy for pancreatic cancer in the U.S.: Utilization, outcomes, and the effect of hospital volume. Cancer 2007, 110, 1227–1234. [Google Scholar] [CrossRef]
- Altman, A.M.; Wirth, K.; Marmor, S.; Lou, E.; Chang, K.; Hui, J.Y.C.; Tuttle, T.M.; Jensen, E.H.; Denbo, J.W. Completion of Adjuvant Chemotherapy After Upfront Surgical Resection for Pancreatic Cancer Is Uncommon Yet Associated With Improved Survival. Ann. Surg. Oncol. 2019, 26, 4108–4116. [Google Scholar] [CrossRef]
- He, J.; Ahuja, N.; Makary, M.A.; Cameron, J.L.; Eckhauser, F.E.; Choti, M.A.; Hruban, R.H.; Pawlik, T.M.; Wolfgang, C.L. 2564 resected periampullary adenocarcinomas at a single institution: Trends over three decades. HPB 2014, 16, 83–90. [Google Scholar] [CrossRef]
- Winter, J.M.; Brennan, M.F.; Tang, L.H.; D’Angelica, M.I.; Dematteo, R.P.; Fong, Y.; Klimstra, D.S.; Jarnagin, W.R.; Allen, P.J. Survival after resection of pancreatic adenocarcinoma: Results from a single institution over three decades. Ann. Surg. Oncol. 2012, 19, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.A.; Minter, R.M.; Zhu, H.; Augustine, M.M.; Porembka, M.R.; Wang, S.C.; Yopp, A.C.; Mansour, J.C.; Choti, M.A.; Polanco, P.M. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J. Clin. Oncol. 2016, 35, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Takahashi, N.; Farnell, M.B.; Smyrk, T.C.; Truty, M.J.; Nagorney, D.M.; Smoot, R.L.; Chari, S.T.; Carter, R.E.; Kendrick, M.L. Survival benefit of neoadjuvant therapy in patients with non-metastatic pancreatic ductal adenocarcinoma: A propensity matching and intention-to-treat analysis. J. Surg. Oncol. 2019, 120, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Katz, M.H.G.; Prakash, L.; Varadhachary, G.R.; Wolff, R.A.; Shroff, R.T.; Javle, M.; Fogelman, D.; Overman, M.; Crane, C.H.; et al. Preoperative Therapy and Pancreatoduodenectomy for Pancreatic Ductal Adenocarcinoma: A 25-Year Single-Institution Experience. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2017, 21, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Wang, H.; Egger, M.E.; Tzeng, C.-W.D.; Prakash, L.R.; Maitra, A.; Varadhachary, G.R.; Shroff, R.; Javle, M.; Fogelman, D.; et al. Association of Clinical Factors With a Major Pathologic Response Following Preoperative Therapy for Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2017, 152, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Vogel, J.A.; Besselink, M.G.; Busch, O.R.C.; Wilmink, J.W.; Daams, J.G.; van Eijck, C.H.J.; Groot Koerkamp, B.; Rasch, C.R.N.; van Tienhoven, G.; et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br. J. Surg. 2018, 105, 946–958. [Google Scholar] [CrossRef] [PubMed]
- De Geus, S.W.L.; Evans, D.B.; Bliss, L.A.; Eskander, M.F.; Smith, J.K.; Wolff, R.A.; Miksad, R.A.; Weinstein, M.C.; Tseng, J.F. Neoadjuvant therapy versus upfront surgical strategies in resectable pancreatic cancer: A Markov decision analysis. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2016, 42, 1552–1560. [Google Scholar] [CrossRef]
- Khorana, A.A.; Mangu, P.B.; Berlin, J.; Engebretson, A.; Hong, T.S.; Maitra, A.; Mohile, S.G.; Mumber, M.; Schulick, R.; Shapiro, M.; et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2541–2556. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Chiorean, E.G.; Czito, B.; Scaife, C.; Narang, A.K.; Fountzilas, C.; Wolpin, B.M.; Al-Hawary, M.; Asbun, H.; et al. Pancreatic Adenocarcinoma, Version 1.2019. J. Natl. Compr. Cancer Netw. JNCCN 2019, 17, 202–210. [Google Scholar] [CrossRef]
- Abrams, R.A.; Lowy, A.M.; O’Reilly, E.M.; Wolff, R.A.; Picozzi, V.J.; Pisters, P.W.T. Combined modality treatment of resectable and borderline resectable pancreas cancer: Expert consensus statement. Ann. Surg. Oncol. 2009, 16, 1751–1756. [Google Scholar] [CrossRef]
- Youngwirth, L.M.; Nussbaum, D.P.; Thomas, S.; Adam, M.A.; Blazer, D.G.; Roman, S.A.; Sosa, J.A. Nationwide trends and outcomes associated with neoadjuvant therapy in pancreatic cancer: An analysis of 18,243 patients. J. Surg. Oncol. 2017, 116, 127–132. [Google Scholar] [CrossRef]
- Hashmi, A.; Kozick, Z.; Fluck, M.; Hunsinger, M.A.; Wild, J.; Arora, T.K.; Shabahang, M.M.; Blansfield, J.A. Neoadjuvant versus Adjuvant Chemotherapy for Resectable Pancreatic Adenocarcinoma: A National Cancer Database Analysis. Am. Surg. 2018, 84, 1439–1445. [Google Scholar]
- Casadei, R.; Di Marco, M.; Ricci, C.; Santini, D.; Serra, C.; Calculli, L.; D’Ambra, M.; Guido, A.; Morselli-Labate, A.M.; Minni, F. Neoadjuvant Chemoradiotherapy and Surgery Versus Surgery Alone in Resectable Pancreatic Cancer: A Single-Center Prospective, Randomized, Controlled Trial Which Failed to Achieve Accrual Targets. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2015, 19, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Golcher, H.; Brunner, T.B.; Witzigmann, H.; Marti, L.; Bechstein, W.-O.; Bruns, C.; Jungnickel, H.; Schreiber, S.; Grabenbauer, G.G.; Meyer, T.; et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: Results of the first prospective randomized phase II trial. Strahlenther. Onkol. Organ Dtsch. Röntgenges. Al. 2015, 191, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.; Van Der Meer, R. Upfront Surgery versus Neoadjuvant Therapy for Resectable Pancreatic Cancer: Systematic Review and Bayesian Network Meta-analysis. Sci. Rep. 2019, 9, 4354. [Google Scholar] [CrossRef] [PubMed]
- Unno, M.; Hata, T.; Motoi, F. Long-term outcome following neoadjuvant therapy for resectable and borderline resectable pancreatic cancer compared to upfront surgery: A meta-analysis of comparative studies by intention-to-treat analysis. Surg. Today 2019, 49, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, Q.; Chen, Y.; Fu, Q.; Li, X.; Bai, X.; Liang, T. Neoadjuvant chemotherapy for primary resectable pancreatic cancer: A systematic review and meta-analysis. HPB 2020. [Google Scholar] [CrossRef]
- Pan, L.; Fang, J.; Tong, C.; Chen, M.; Zhang, B.; Juengpanich, S.; Wang, Y.; Cai, X. Survival benefits of neoadjuvant chemo(radio)therapy versus surgery first in patients with resectable or borderline resectable pancreatic cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2019, 18, 1. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Beller, E.M.; Glasziou, P.P.; Altman, D.G.; Hopewell, S.; Bastian, H.; Chalmers, I.; Gøtzsche, P.C.; Lasserson, T.; Tovey, D. PRISMA for Abstracts Group PRISMA for Abstracts: Reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013, 10, e1001419. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36. [Google Scholar] [CrossRef]
- Zang, J.; Xu, J.; Xiang, C.; Zou, S.; He, J. Statistical modeling and verification for the synthesis of median survival time in multilevel meta-analysis of survival data. J. Epidemiol. Res. 2015, 1, 25. [Google Scholar] [CrossRef]
- Reni, M.; Balzano, G.; Zanon, S.; Zerbi, A.; Rimassa, L.; Castoldi, R.; Pinelli, D.; Mosconi, S.; Doglioni, C.; Chiaravalli, M.; et al. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): A randomised, open-label, phase 2–3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 413–423. [Google Scholar] [CrossRef]
- Unno, M.; Motoi, F.; Matsuyama, Y.; Satoi, S.; Matsumoto, I.; Aosasa, S.; Shirakawa, H.; Wada, K.; Fujii, T.; Yoshitomi, H.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). J. Clin. Oncol. 2019, 37, 189. [Google Scholar] [CrossRef]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Jang, J.-Y.; Han, Y.; Lee, H.; Kim, S.-W.; Kwon, W.; Lee, K.-H.; Oh, D.-Y.; Chie, E.K.; Lee, J.M.; Heo, J.S.; et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann. Surg. 2018, 268, 215–222. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Groot, V.P.; Gemenetzis, G.; Blair, A.B.; Rivero-Soto, R.J.; Yu, J.; Javed, A.A.; Burkhart, R.A.; Rinkes, I.H.M.B.; Molenaar, I.Q.; Cameron, J.L.; et al. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019, 269, 1154–1162. [Google Scholar] [CrossRef]
- Evans, D.B.; Rich, T.A.; Byrd, D.R.; Cleary, K.R.; Connelly, J.H.; Levin, B.; Charnsangavej, C.; Fenoglio, C.J.; Ames, F.C. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch. Surg. Chic. III 1960 1992, 127, 1335–1339. [Google Scholar] [CrossRef]
- Evans, D.B.; Varadhachary, G.R.; Crane, C.H.; Sun, C.C.; Lee, J.E.; Pisters, P.W.T.; Vauthey, J.-N.; Wang, H.; Cleary, K.R.; Staerkel, G.A.; et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 3496–3502. [Google Scholar] [CrossRef] [PubMed]
- Pisters, P.W.T.; Wolff, R.A.; Janjan, N.A.; Cleary, K.R.; Charnsangavej, C.; Crane, C.N.; Lenzi, R.; Vauthey, J.N.; Lee, J.E.; Abbruzzese, J.L.; et al. Preoperative paclitaxel and concurrent rapid-fractionation radiation for resectable pancreatic adenocarcinoma: Toxicities, histologic response rates, and event-free outcome. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 2537–2544. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.; Shen, C.; Santry, H.; Bridges, J.; Dillhoff, M.; Ejaz, A.; Pawlik, T.; Tsung, A. Disparities in the Use of Neoadjuvant Therapy for Resectable Pancreatic Ductal Adenocarcinoma. J. Natl. Compr. Canc. Netw. 2020, 18, 1–8. [Google Scholar]
- Ettrich, T.J.; Berger, A.W.; Perkhofer, L.; Daum, S.; König, A.; Dickhut, A.; Wittel, U.; Wille, K.; Geissler, M.; Algül, H.; et al. Neoadjuvant plus adjuvant or only adjuvant nab-paclitaxel plus gemcitabine for resectable pancreatic cancer—The NEONAX trial (AIO-PAK-0313), a prospective, randomized, controlled, phase II study of the AIO pancreatic cancer group. BMC Cancer 2018, 18, 1298. [Google Scholar] [CrossRef]
- Hozaeel, W.; Pauligk, C.; Homann, N.; Luley, K.; Kraus, T.W.; Trojan, J.; Bechstein, W.O.; Grimm, K.; Heise, B.; Schmiegel, W.; et al. Randomized multicenter phase II/III study with adjuvant gemcitabine versus neoadjuvant/adjuvant FOLFIRINOX in resectable pancreatic cancer: The NEPAFOX trial. J. Clin. Oncol. 2015, 33, TPS4152. [Google Scholar] [CrossRef]
- Labori, K.J.; Lassen, K.; Hoem, D.; Grønbech, J.E.; Søreide, J.A.; Mortensen, K.; Smaaland, R.; Sorbye, H.; Verbeke, C.; Dueland, S. Neoadjuvant chemotherapy versus surgery first for resectable pancreatic cancer (Norwegian Pancreatic Cancer Trial—1 (NorPACT-1))—Study protocol for a national multicentre randomized controlled trial. BMC Surg. 2017, 17, 94. [Google Scholar] [CrossRef]
- Schwarz, L.; Vernerey, D.; Bachet, J.-B.; Tuech, J.-J.; Portales, F.; Michel, P.; Cunha, A.S. Resectable pancreatic adenocarcinoma neo-adjuvant FOLF(IRIN)OX-based chemotherapy—A multicenter, non-comparative, randomized, phase II trial (PANACHE01-PRODIGE48 study). BMC Cancer 2018, 18, 762. [Google Scholar] [CrossRef]
- Dutch Pancreatic Cancer Group PREOPANC-2—DPCG. Available online: http://www.dpcg.nl/projecten/preopanc-2.html (accessed on 18 February 2020).
- European Study Group for Pancreatic Cancer ESPAC-5F: European Study Group for Pancreatic Cancer—Trial 5F. Available online: http://www.isrctn.com/ISRCTN89500674 (accessed on 18 February 2020).
- Sohal, D.; McDonough, S.; Ahmad, S.A.; Gandhi, N.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L.; Guthrie, K.A.; Lowy, A.M.; Philip, P.A.; et al. SWOG S1505: Initial findings on eligibility and neoadjuvant chemotherapy experience with mfolfirinox versus gemcitabine/nab-paclitaxel for resectable pancreatic adenocarcinoma. J. Clin. Oncol. 2019, 37, 4137. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Chen, H.-C.; Wang, X.; Tzeng, C.-W.D.; Kim, M.P.; Aloia, T.A.; Vauthey, J.-N.; Lee, J.E.; Katz, M.H.G. Chemotherapy Versus Chemoradiation as Preoperative Therapy for Resectable Pancreatic Ductal Adenocarcinoma: A Propensity Score Adjusted Analysis. Pancreas 2019, 48, 216–222. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Crane, C.H.; Koay, E.J.; Das, P.; Krishnan, S.; Prakash, L.; Snyder, R.A.; Varadhachary, G.R.; Wolff, R.A.; Javle, M.; et al. Impact of hypofractionated and standard fractionated chemoradiation before pancreatoduodenectomy for pancreatic ductal adenocarcinoma. Cancer 2016, 122, 2671–2679. [Google Scholar] [CrossRef]
- Katz, M.H.G.; Shi, Q.; Ahmad, S.A.; Herman, J.M.; Marsh Rde, W.; Collisson, E.; Schwartz, L.; Frankel, W.; Martin, R.; Conway, W.; et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 2016, 151, e161137. [Google Scholar] [PubMed]
- Katz, M.H.G.; Ou, F.-S.; Herman, J.M.; Ahmad, S.A.; Wolpin, B.; Marsh, R.; Behr, S.; Shi, Q.; Chuong, M.; Schwartz, L.H.; et al. Alliance for clinical trials in oncology (ALLIANCE) trial A021501: Preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer 2017, 17, 505. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; Hill, C. Meta-analyses of randomised clinical trials in oncology. Lancet Oncol. 2001, 2, 475–482. [Google Scholar] [CrossRef]
- Kontopantelis, E.; Springate, D.A.; Reeves, D. A re-analysis of the Cochrane Library data: The dangers of unobserved heterogeneity in meta-analyses. PLoS ONE 2013, 8, e69930. [Google Scholar] [CrossRef]
| Author | Institution | Origin | Setting | PR/BR Definition | Sample Size | Neoadjuvant Therapy | Regimen | Adjuvant Therapy |
|---|---|---|---|---|---|---|---|---|
| Golcher | Multi- | Germany | PR | ≤180 “peripancreatic vessels” | 66 | CRT | Gemcitabine/Cisplatin; 56Gy | Gemcitabine |
| Casadei | Single- | Italy | PR | <180 SMV/PV; No contact to CA/HA/SMA | 38 | CRT | Gemcitabine; 54Gy | Gemcitabine |
| Jang | Multi- | Korea | BR | 2012 NCCN criteria | 50 | CRT | Gemcitabine; 54Gy | CRT, Gemcitabine |
| Reni | Multi- | Italy | PR | No invasion of SMA/SMV/PV/CA/HA | 88 | Chemo | Cisplatin, Epirubicin, Gemcitabine, Capecitabine | Cisplatin, Epirubicin, Gemcitabine, Capecitabine or Gemcitabine |
| Versteijne | Multi- | Netherlands | PR/BR | PR: <90 SMV/PV; no CA/HA/SMA contact BR: <90 CA/HA/SMA; 90–270 PV/SMV without occlusion | 246 | CRT | Gemcitabine; 36Gy | Gemcitabine |
| Unno | Multi- | Japan | PR | No CA/HA/SMA abutment | 362 | Chemo | Gemcitabine, S-1 | S1 |
| Author | SAE Rate (%) | Resected Rate (%) | R0 Resection Rate (%) | pN0 Rate (%) | Grade ≥3 Postoperative Morbidity (%) | Overall Survival (mo) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NT | NT | SF | NT | SF | NT | SF | NT | SF | NT | SF | |
| Golcher | 45.5 | 57.6 | 69.7 | 52.6 | 47.8 | 68.4 | 43.5 | 31.56 | 65.2 | 17.4 | 14.4 |
| Casadei | 22.2 | 61.1 | 75.0 | 63.6 | 33.3 | 45.5 | 13.3 | N/A | N/A | 22.4 | 19.5 |
| Jang | 11.1 | 63.0 | 78.3 | 82.4 | 33.3 | 70.6 | 16.7 | 23.5 | 16.7 | 21.0 | 12.0 |
| Reni | 34.4 | 84.4 | 87.5 | 63.0 | 32.7 | 48.1 | 26.5 | 11.1 | 20.4 | 38.2 | 20.4–26.4 |
| Versteijne | N/A | 60.5 | 72.4 | 70.8 | 40.2 | 66.7 | 21.7 | 68.1 * | 50.0 * | 16.0 | 14.3 |
| Unno | 72.0 | 76.9 | 72.2 | N/A | N/A | N/A | N/A | N/A | N/A | 36.7 | 26.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cloyd, J.M.; Heh, V.; Pawlik, T.M.; Ejaz, A.; Dillhoff, M.; Tsung, A.; Williams, T.; Abushahin, L.; Bridges, J.F.P.; Santry, H. Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 1129. https://doi.org/10.3390/jcm9041129
Cloyd JM, Heh V, Pawlik TM, Ejaz A, Dillhoff M, Tsung A, Williams T, Abushahin L, Bridges JFP, Santry H. Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2020; 9(4):1129. https://doi.org/10.3390/jcm9041129
Chicago/Turabian StyleCloyd, Jordan M., Victor Heh, Timothy M. Pawlik, Aslam Ejaz, Mary Dillhoff, Allan Tsung, Terence Williams, Laith Abushahin, John F. P. Bridges, and Heena Santry. 2020. "Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 9, no. 4: 1129. https://doi.org/10.3390/jcm9041129
APA StyleCloyd, J. M., Heh, V., Pawlik, T. M., Ejaz, A., Dillhoff, M., Tsung, A., Williams, T., Abushahin, L., Bridges, J. F. P., & Santry, H. (2020). Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 9(4), 1129. https://doi.org/10.3390/jcm9041129






