Expression of the Novel Cardiac Biomarkers sST2, GDF-15, suPAR, and H-FABP in HFpEF Patients Compared to ICM, DCM, and Controls
Abstract
1. Introduction
2. Experimental Section
2.1. Laboratory Analysis
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
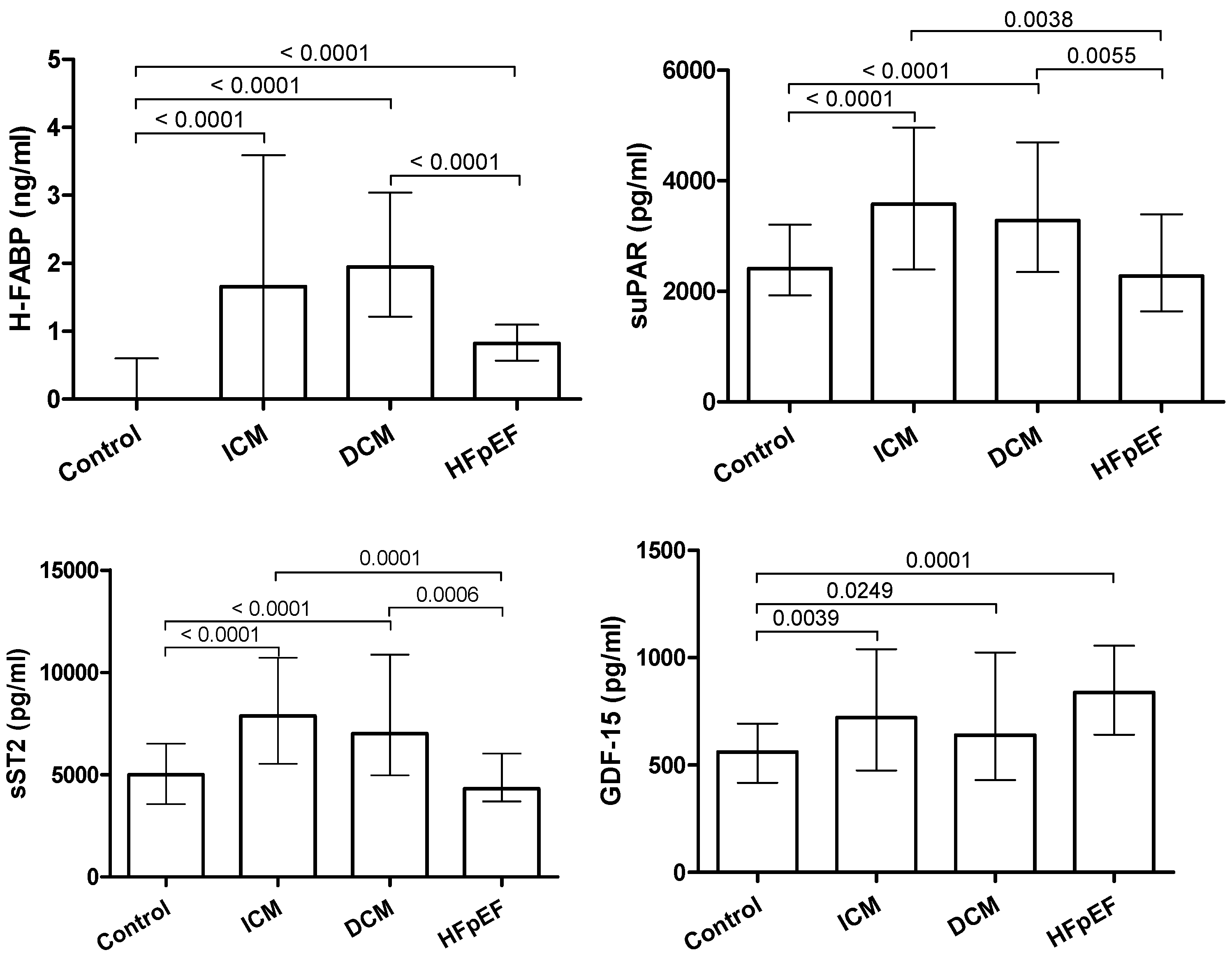
3.2. Biomarkers
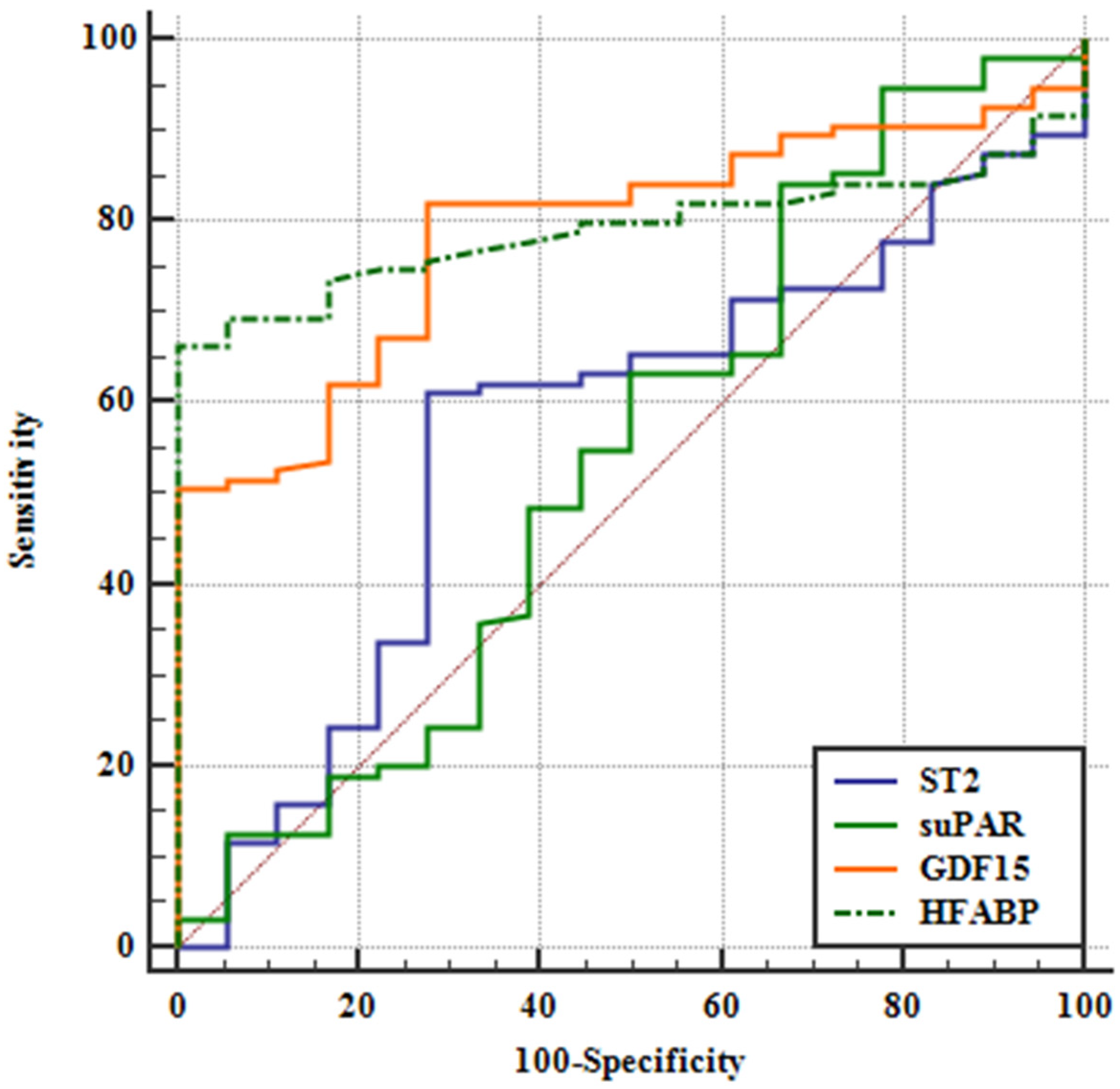
3.3. AUC-Analysis
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| BMI | body mass index |
| BNP | brain natriuretic peptide |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| CRP | C-reactive protein |
| DCM | dilative cardiomyopathy |
| ESC | European society of cardiology |
| ELISA | enzyme-linked immunosorbent assay |
| GDF-15 | growth differentiation factor-15 |
| GFR | glomerular filtration rate |
| Hb | haemoglobin |
| H-FABP | heart-type fatty acid binding protein |
| HFpEF | heart failure with preserved ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| HDL | high density lipoprotein |
| ICM | ischemic cardiomyopathy |
| IL-33 | interleukin 33 |
| LDL | low density lipoprotein |
| LVEF | left ventricular ejection fraction |
| MRA | mineralocorticoid receptor antagonist |
| NYHA | New York heart association |
| PKG | protein kinase G |
| ROC | receiver operating curve |
| sST2 | soluble suppression of tumorigenicity 2 |
| suPAR | soluble urokinase-type plasminogen activator receptor |
References
- Bleumink, G.S.; Knetsch, A.M.; Sturkenboom, M.C.J.M.; Straus, S.M.J.M.; Hofman, A.; Deckers, J.W.; Witteman, J.C.M.; Stricker, B.H.C. Quantifying the heart failure epidemic: Prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur. Heart J. 2004, 25, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Redfield, M.M. Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2017, 376, 897. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [PubMed]
- Borlaug, B.A. The pathophysiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2014, 11, 507–515. [Google Scholar] [CrossRef]
- Gevaert, A.B.; Boen, J.R.A.; Segers, V.F.; van Craenenbroeck, E.M. Heart Failure With Preserved Ejection Fraction: A Review of Cardiac and Noncardiac Pathophysiology. Front. Physiol. 2019, 10, 638. [Google Scholar] [CrossRef]
- Packer, M. Leptin-Aldosterone-Neprilysin Axis: Identification of Its Distinctive Role in the Pathogenesis of the Three Phenotypes of Heart Failure in People with Obesity. Circulation 2018, 137, 1614–1631. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef]
- Lekavich, C.L.; Barksdale, D.J.; Neelon, V.; Wu, J. Heart failure preserved ejection fraction (HFpEF): An integrated and strategic review. Heart Fail. Rev. 2015, 20, 643–653. [Google Scholar] [CrossRef]
- Lichtenauer, M.; Jirak, P.; Wernly, B.; Paar, V.; Rohm, I.; Jung, C.; Schernthaner, C.; Kraus, J.; Motloch, L.J.; Yilmaz, A.; et al. A comparative analysis of novel cardiovascular biomarkers in patients with chronic heart failure. Eur. J. Intern. Med. 2017. [Google Scholar] [CrossRef]
- Schernthaner, C.; Lichtenauer, M.; Wernly, B.; Paar, V.; Pistulli, R.; Rohm, I.; Jung, C.; Figulla, H.R.; Yilmaz, A.; Cadamuro, J.; et al. Multibiomarker analysis in patients with acute myocardial infarction. Eur. J. Clin. Investig. 2017, 47, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Jirak, P.; Fejzic, D.; Paar, V.; Wernly, B.; Pistulli, R.; Rohm, I.; Jung, C.; Hoppe, U.C.; Schulze, P.C.; Lichtenauer, M.; et al. Influences of Ivabradine treatment on serum levels of cardiac biomarkers sST2, GDF-15, suPAR and H-FABP in patients with chronic heart failure. Acta Pharmacol. Sin. 2018, 39, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Otaki, Y.; Watanabe, T.; Kubota, I. Heart-type fatty acid-binding protein in cardiovascular disease: A systemic review. Clin. Chim. Acta 2017, 474, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Figal, D.A.; Lax, A.; Perez-Martinez, M.T.; Asensio-Lopez, M.D.; Sanchez-Mas, J. Clinical relevance of sST2 in cardiac diseases. Clin. Chem. Lab. Med. 2016, 54, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Kempf, T.; Wallentin, L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clin. Chem. 2017, 63, 140–151. [Google Scholar] [CrossRef]
- Eugen-Olsen, J.; Giamarellos-Bourboulis, E.J. suPAR: The unspecific marker for disease presence, severity and prognosis. Int. J. Antimicrob. Agents 2015, 46, 31. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Gao, W. Growth differentiation factor 15 in cardiovascular diseases: From bench to bedside. Biomarkers 2011, 16, 466–475. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Li, M.J.; Zhou, Y.L.; Ma, L.; Yi, X. Growth differentiation factor-15 (GDF-15), novel biomarker for assessing atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease. Int. J. Clin. Exp. Med. 2015, 8, 21201–21207. [Google Scholar]
- Farhan, S.; Freynhofer, M.K.; Brozovic, I.; Bruno, V.; Vogel, B.; Tentzeris, I.; Baumgartner-Parzer, S.; Huber, K.; Kautzky-Willer, A. Determinants of growth differentiation factor 15 in patients with stable and acute coronary artery disease. A prospective observational study. Cardiovasc. Diabetol. 2016, 15, 016–0375. [Google Scholar] [CrossRef]
- Adela, R.; Banerjee, S.K. GDF-15 as a Target and Biomarker for Diabetes and Cardiovascular Diseases: A Translational Prospective. J. Diabetes Res. 2015, 490842, 27. [Google Scholar] [CrossRef]
- Niizeki, T.; Takeishi, Y.; Arimoto, T.; Takabatake, N.; Nozaki, N.; Hirono, O.; Watanabe, T.; Nitobe, J.; Harada, M.; Suzuki, S. Heart-type fatty acid-binding protein is more sensitive than troponin T to detect the ongoing myocardial damage in chronic heart failure patients. J. Card. Fail. 2007, 13, 120–127. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. Heart failure in cardiomyopathies: A position paper from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 553–576. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.J.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Griesenauer, B.; Paczesny, S. The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Najjar, E.; Faxén, U.L.; Hage, C.; Donal, E. ST2 in heart failure with preserved and reduced ejection fraction. Scand. Cardiovasc. J. 2019, 53, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Schelbert, E.B.; Fridman, Y.; Wong, T.C.; Abu Daya, H.; Piehler, K.M.; Kadakkal, A.; et al. Temporal Relation Between Myocardial Fibrosis and Heart Failure With Preserved Ejection Fraction: Association With Baseline Disease Severity and Subsequent Outcome. JAMA Cardiol. 2017, 2, 995–1006. [Google Scholar] [CrossRef]
- Thuno, M.; Macho, B.; Eugen-Olsen, J. suPAR: The molecular crystal ball. Dis. Markers 2009, 27, 157–172. [Google Scholar] [CrossRef]
- Hamie, L.; Daoud, G.; Nemer, G.; Nammour, T.; el Chediak, A.; Uthman, I.W.; Kibbi, A.G.; Eid, A.; Kurban, M. SuPAR, an emerging biomarker in kidney and inflammatory diseases. Postgrad. Med. J. 2018, 94, 517–524. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Curcio, A.; Indolfi, C. Emerging role of microRNAs in cardiovascular diseases. Circ. J. 2014, 78, 567–575. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Eposito, F.; Carella, C.; Strangio, A.; Ammirati, G.; Sabatino, J.; Abbate, F.G.; Iaconetti, C.; Liguori, V.; Pergola, V.; et al. Transcoronary concentration gradients of circulating microRNAs in heart failure. Eur. J. Heart Fail. 2018, 20, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Iaconetti, C.; Sorrentino, S.; De Rosa, S.; Indolfi, C. Exosomal miRNAs in Heart Disease. Physiology 2016, 31, 16–24. [Google Scholar] [CrossRef]
| Controls | HFpEF | ICM | DCM | Total | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (y) | 63.56 | 9.25 | 70.94 | 6.49 | 65.12 | 11.16 | 57.10 | 10.73 | 62.65 | 10.73 | <0.0001 |
| Height (m) | 1.68 | 0.09 | 1.69 | 0.09 | 1.74 | 0.09 | 1.75 | 0.09 | 1.72 | 0.09 | <0.0001 |
| Weight (kg) | 77.68 | 17.22 | 81.86 | 12.82 | 76.66 | 25.58 | 89.09 | 18.74 | 81.06 | 20.27 | 0.001 |
| BMI | 27.22 | 5.71 | 28.68 | 4.63 | 28.08 | 4.37 | 29.02 | 5.24 | 28.30 | 5.08 | 0.334 |
| LVEF (%) | 65.93 | 8.63 | 59.75 | 9.85 | 37.42 | 12.93 | 35.32 | 11.87 | 48.04 | 17.92 | <0.0001 |
| BNP (pg/mL) | 73.74 | 86.08 | 165.22 | 162.54 | 435.75 | 488.22 | 684.64 | 866.83 | 430,10 | 646.27 | <0.0001 |
| Creatinine (μmol/L) | 74.29 | 15.67 | 85.06 | 25.96 | 108.19 | 39.05 | 98.35 | 31.15 | 89.14 | 30.44 | <0.0001 |
| GFR (mL/min) | 83.62 | 13.33 | 71.08 | 13.78 | 66.97 | 17.61 | 74.69 | 24.96 | 75.75 | 17.68 | 0.084 |
| CRP (mg/L) | 2.28 | 2.98 | 5.58 | 8.87 | 4.33 | 4.20 | 7.55 | 14.29 | 4.55 | 9.19 | 0.005 |
| Hb (mmol/L) | 8.79 | 0.56 | 8.16 | 0.87 | 8.51 | 0.94 | 8.92 | 0.91 | 8.67 | 0.91 | 0.005 |
| LDL (mmol/L) | 3.47 | 0.94 | 2.76 | 1.40 | 2.23 | 0.89 | 2.88 | 1.07 | 3.10 | 1.10 | <0.0001 |
| HDL (mmol/L) | 1.49 | 0.41 | 1.29 | 0.35 | 0.99 | 0.22 | 1.15 | 0.31 | 1.32 | 0.40 | <0.0001 |
| Controls | HFpEF | ICM | DCM | Total | p-Value | |
|---|---|---|---|---|---|---|
| Sex (male) | 36% | 44% | 86% | 77% | 61% | <0.0001 |
| Diabetes | 15% | 39% | 36% | 38% | 29% | 0.003 |
| Hypertension | 77% | 89% | 78% | 50% | 70% | <0.001 |
| Atrial Fibrillation | 5% | 50% | 3% | 18% | 15% | <0.0001 |
| Beta Blockers | 39% | 72% | 100% | 99% | 76% | <0.0001 |
| ACE-Inhibitors | 59% | 72% | 96% | 96% | 82% | <0.0001 |
| Loop-Diuretics | 30% | 56% | 79% | 91% | 64% | <0.0001 |
| MRA | 2% | 19% | 61% | 68% | 43% | <0.0001 |
| Controls | HFpEF | ICM | DCM | |||||
|---|---|---|---|---|---|---|---|---|
| Median | Interquartile Range | Median | Interquartile Range | Median | Interquartile Range | Median | Interquartile Range | |
| sST2 (pg/mL) | 4999.00 | 2970.00 | 4318.00 | 2332.00 | 7869.00 | 5191.00 | 7010.00 | 5892.00 |
| GDF-15 (pg/mL) | 561.20 | 276.60 | 838.00 | 415.90 | 720.50 | 565.60 | 639.10 | 595.10 |
| H-FABP (ng/mL) | 0.00 | 0.60 | 0.82 | 0.53 | 1.66 | 3.59 | 1.94 | 1.83 |
| suPAR (pg/mL) | 2414.00 | 1280.00 | 2279.00 | 1753.00 | 3576.00 | 2567.00 | 3280.00 | 2349.00 |
| Variable | AUC | SE a | 95% CI b |
|---|---|---|---|
| ST2 | 0.567 | 0.0725 | 0.470 to 0.660 |
| suPAR | 0.543 | 0.0829 | 0.447 to 0.637 |
| GDF15 | 0.787 | 0.0469 | 0.700 to 0.859 |
| HFABP | 0.792 | 0.0401 | 0.705 to 0.862 |
| ST2 ~ suPAR | |
| Difference between areas | 0.0240 |
| Standard Error a | 0.112 |
| 95% Confidence Interval | −0.196 to 0.244 |
| Z statistic | 0.214 |
| Significance level | p = 0.8307 |
| ST2 ~ GDF15 | |
| Difference between areas | 0.220 |
| Standard Error a | 0.0999 |
| 95% Confidence Interval | 0.0247 to 0.416 |
| Z statistic | 2.207 |
| Significance level | p = 0.0273 |
| ST2 ~ HFABP | |
| Difference between areas | 0.225 |
| Standard Error a | 0.0830 |
| 95% Confidence Interval | 0.0621 to 0.388 |
| Z statistic | 2.708 |
| Significance level | p = 0.0068 |
| suPAR ~ GDF15 | |
| Difference between areas | 0.244 |
| Standard Error a | 0.0996 |
| 95% Confidence Interval | 0.0492 to 0.440 |
| Z statistic | 2.453 |
| Significance level | p = 0.0141 |
| suPAR ~ HFABP | |
| Difference between areas | 0.249 |
| Standard Error a | 0.0983 |
| 95% Confidence Interval | 0.0562 to 0.442 |
| Z statistic | 2.531 |
| Significance level | p = 0.0114 |
| GDF15 ~ HFABP | |
| Difference between areas | 0.00439 |
| Standard Error a | 0.0563 |
| 95% Confidence Interval | −0.106 to 0.115 |
| Z statistic | 0.0779 |
| Significance level | p = 0.9379 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jirak, P.; Pistulli, R.; Lichtenauer, M.; Wernly, B.; Paar, V.; Motloch, L.J.; Rezar, R.; Jung, C.; Hoppe, U.C.; Schulze, P.C.; et al. Expression of the Novel Cardiac Biomarkers sST2, GDF-15, suPAR, and H-FABP in HFpEF Patients Compared to ICM, DCM, and Controls. J. Clin. Med. 2020, 9, 1130. https://doi.org/10.3390/jcm9041130
Jirak P, Pistulli R, Lichtenauer M, Wernly B, Paar V, Motloch LJ, Rezar R, Jung C, Hoppe UC, Schulze PC, et al. Expression of the Novel Cardiac Biomarkers sST2, GDF-15, suPAR, and H-FABP in HFpEF Patients Compared to ICM, DCM, and Controls. Journal of Clinical Medicine. 2020; 9(4):1130. https://doi.org/10.3390/jcm9041130
Chicago/Turabian StyleJirak, Peter, Rudin Pistulli, Michael Lichtenauer, Bernhard Wernly, Vera Paar, Lukas J. Motloch, Richard Rezar, Christian Jung, Uta C. Hoppe, P. Christian Schulze, and et al. 2020. "Expression of the Novel Cardiac Biomarkers sST2, GDF-15, suPAR, and H-FABP in HFpEF Patients Compared to ICM, DCM, and Controls" Journal of Clinical Medicine 9, no. 4: 1130. https://doi.org/10.3390/jcm9041130
APA StyleJirak, P., Pistulli, R., Lichtenauer, M., Wernly, B., Paar, V., Motloch, L. J., Rezar, R., Jung, C., Hoppe, U. C., Schulze, P. C., Kretzschmar, D., Braun-Dullaeus, R. C., & Bekfani, T. (2020). Expression of the Novel Cardiac Biomarkers sST2, GDF-15, suPAR, and H-FABP in HFpEF Patients Compared to ICM, DCM, and Controls. Journal of Clinical Medicine, 9(4), 1130. https://doi.org/10.3390/jcm9041130








