Pattern of Recurrence and Patient Survival after Perioperative Chemotherapy with 5-FU, Leucovorin, Oxaliplatin and Docetaxel (FLOT) for Locally Advanced Esophagogastric Adenocarcinoma in Patients Treated Outside Clinical Trials
Abstract
1. Introduction
2. Experimental Section
2.1. Pretherapeutic Work-Up
2.2. Perioperative Chemotherapy (FLOT)
2.3. Surgery
2.4. Assessment of Histopathological Response to FLOT
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Patients and Tumor Characteristics
3.2. Treatment Characteristics
3.3. Histopathological Analysis
3.4. Pattern of Recurrence
3.5. Overall and Recurrence-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hiripi, E.; Jansen, L.; Gondos, A.; Emrich, K.; Holleczek, B.; Katalinic, A.; Luttmann, S.; Nennecke, A.; Brenner, H. The GEKID Cancer Survival Working Group Survival of stomach and esophagus cancer patients in Germany in the early 21st century. Acta Oncol. 2012, 51, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Makowiec, F.; Baier, P.; Kulemann, B.; Marjanovic, G.; Bronsert, P.; Zirlik, K.; Henke, M.; Hopt, U.T.; Hoeppner, J. Improved Long-Term Survival After Esophagectomy for Esophageal Cancer: Influence of Epidemiologic Shift and Neoadjuvant Therapy. J. Gastrointest. Surg. 2013, 17, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Law, S.; Kwong, D.L.; Kwok, K.-F.; Chu, K.-M.; Sham, J.S.T.; Wong, J.; Wong, K.-H. Improvement in Treatment Results and Long-Term Survival of Patients with Esophageal Cancer: Impact of chemoradiation and change in treatment strategy. Ann. Surg. 2003, 238, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Ronellenfitsch, U.; Schwarzbach, M.; Hofheinz, R.; Kienle, P.; Kieser, M.; E Slanger, T.; Jensen, K. GE adenocarcinoma meta-analysis group Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst. Rev. 2013, 5. [Google Scholar] [CrossRef]
- Xiong, B.-H.; Cheng, Y.; Ma, L.; Zhang, C.-Q. An Updated Meta-Analysis of Randomized Controlled Trial Assessing the Effect of Neoadjuvant Chemotherapy in Advanced Gastric Cancer. Cancer Investig. 2014, 32, 272–284. [Google Scholar] [CrossRef]
- Moorcraft, S.Y.; Smyth, E.C.; Cunningham, D. Adjuvant or neoadjuvant therapy for operable esophagogastric cancer? Gastric Cancer 2014, 18, 1–10. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van De Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Ychou, M.; Boige, V.; Pignon, J.-P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.-M.; Saint-Aubert, B.; et al. Perioperative Chemotherapy Compared with Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An FNCLCC and FFCD Multicenter Phase III Trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef]
- Thuss-Patience, P.; Hofheinz, R.; Arnold, D.; Florschütz, A.; Daum, S.; Kretzschmar, A.; Mantovani-Löffler, L.; Bichev, D.; Breithaupt, K.; Kneba, M.; et al. Perioperative chemotherapy with docetaxel, cisplatin and capecitabine (DCX) in gastro-oesophageal adenocarcinoma: A phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Ann. Oncol. 2012, 23, 2827–2834. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.-G.; Haag, G.M.; Luley, K.B.; Meiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; O Goetze, T.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Mueller, J.; Schulmacher, C.; Ott, K.; Fink, U.; Busch, R.; Böttcher, K.; Siewert, J.R.; Höfler, H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003, 98, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, M.; Alcindor, T.; Ades, S.; Aloraini, A.; Van Huyse, M.; Asselah, J.; David, M.; Frechette, D.; Brisson, S.; Thirlwell, M.; et al. Survival and Recurrence Patterns after Neoadjuvant Docetaxel, Cisplatin, and 5-Fluorouracil (DCF) for Locally Advanced Esophagogastric Adenocarcinoma. Ann. Surg. Oncol. 2014, 22, 324–330. [Google Scholar] [CrossRef]
- Glatz, T.; Bronsert, P.; Schäfer, M.; Kulemann, B.; Marjanovic, G.; Sick, O.; Hopt, U.T.; Zirlik, K.; Makowiec, F.; Hoeppner, J. Perioperative platin-based chemotherapy for locally advanced esophagogastric adenocarcinoma: Postoperative chemotherapy has a substantial impact on outcome. Eur. J. Surg. Oncol. (EJSO) 2015, 41, 1300–1307. [Google Scholar] [CrossRef]
- Sisic, L.; Blank, S.; Nienhüser, H.; Haag, G.M.; Jäger, D.; Bruckner, T.; Ott, K.; Schmidt, T.; Ulrich, A. The postoperative part of perioperative chemotherapy fails to provide a survival benefit in completely resected esophagogastric adenocarcinoma. Surg. Oncol. 2020, 33, 177–188. [Google Scholar] [CrossRef]
- Van Hagen, P.; Van Lanschot, J.; Steyerberg, E.; Nieuwenhuijzen, G.A.; Hospers, G.; Bonenkamp, J.; Cuesta, M.; Blaisse, R.; Busch, O.; Creemers, G.; et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Hoeppner, J.; Zirlik, K.; Brunner, T.; Bronsert, P.; Kulemann, B.; Sick, O.; Marjanovic, G.; Hopt, U.T.; Makowiec, F. Multimodal treatment of locally advanced esophageal adenocarcinoma: Which regimen should we choose? Outcome analysis of perioperative chemotherapy versus neoadjuvant chemoradiation in 105 patients. J. Surg. Oncol. 2013, 109, 287–293. [Google Scholar] [CrossRef]
- Hoeppner, J.; Lordick, F.; Brunner, T.B.; Glatz, T.; Bronsert, P.; Röthling, N.; Schmoor, C.; Lorenz, D.; Ell, C.; Hopt, U.T.; et al. ESOPEC: Prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016, 16, 503. [Google Scholar] [CrossRef]
- Fitzgerald, T.L.; Efird, J.T.; Bellamy, N.; Russo, S.; Jindal, C.; Mosquera, C.; Holliday, E.G.; Biswas, T. Perioperative chemotherapy versus postoperative chemoradiotherapy in patients with resectable gastric/gastroesophageal junction adenocarcinomas: A survival analysis of 5058 patients. Cancer 2017, 123, 2909–2917. [Google Scholar] [CrossRef]
- Gamboa, A.C.; Winer, J.H. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer. Cancers 2019, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Brenkman, H.J.F.; Päeva, M.; Van Hillegersberg, R.; Ruurda, J.P.; Mohammad, N.H. Prophylactic Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Gastric Cancer-A Systematic Review. J. Clin. Med. 2019, 8, 1685. [Google Scholar] [CrossRef] [PubMed]
- Rawicz-Pruszyński, K.; Mielko, J.; Pudło, K.; Lisiecki, R.; Skoczylas, T.; Murawa, D.; Polkowski, W.P. Yield of staging laparoscopy in gastric cancer is influenced by Laurén histologic subtype. J. Surg. Oncol. 2019, 120, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
| Gastric Carcinoma (N = 97) | Esophageal Carcinoma (N = 131) | Total (N = 228) | |
|---|---|---|---|
| Sex | |||
| Female | 34 (35.1%) | 17 (13.0%) | 51 (22.4%) |
| Male | 63 (64.9%) | 114 (87.0%) | 177 (77.6%) |
| Age in years * | 64 (28-86) | 63.4 (30–84) | 64.0 (28–86) |
| ASA classification | |||
| ASA 1–2 | 53 (54.6%) | 68 (51.9%) | 121 (53.1%) |
| ASA 3–4 | 44 (45.4%) | 63 (48.1%) | 107 (46.9%) |
| BMI in kg/m2 * | 25.1 (16.7–37.7) | 26.9 (19.0–48.7) | 26.0 (16.7–48.7) |
| Localization | |||
| AEG I | 0 (0.0%) | 69 (52.7%) | 69 (30.3%) |
| AEG II | 0 (0.0%) | 62 (47.3%) | 62 (27.2%) |
| AEG III | 28 (28.9%) | 0 (0.0%) | 28 (12.3%) |
| Corpus | 43 (44.3%) | 0 (0.0%) | 43 (18.9%) |
| Antrum | 26 (26.8%) | 0 (0.0%) | 26 (11.4%) |
| Preoperative T stage ** | |||
| T1 | 1 (1.1%) | 0 (0.0%) | 1 (0.5%) |
| T2 | 11 (12.5%) | 19 (15.3%) | 30 (14.2%) |
| T3 | 72 (81.8%) | 100 (80.6%) | 172 (81.1%) |
| T4 | 4 (4.5%) | 5 (4.0%) | 9 (4.2%) |
| Preoperative n stage | |||
| N0 | 26 (31.0%) | 28 (22.8%) | 54 (26.1%) |
| N+ | 58 (69.0%) | 95 (77.2%) | 153 (73.9%) |
| Lauren classification | |||
| Intestinal | 49 (50.5%) | 122 (93.1%) | 171 (75.0%) |
| Diffuse | 39 (40.2%) | 6 (4.6%) | 45 (19.7%) |
| Mixed | 9 (9.3%) | 3 (2.3%) | 12 (5.3%) |
| Histology | |||
| No tumor detectable | 11 (11.3%) | 20 (15.3%) | 31 (13.6%) |
| Adenocarcinoma | 67 (69.1%) | 107 (81.7%) | 174 (76.3%) |
| Signet ring cell carcinoma | 19 (19.6%) | 2 (1.5%) | 21 (9.2%) |
| Undifferentiated | 0 (0.0%) | 2 (1.5%) | 2 (0.9%) |
| Resection margin | |||
| R0 | 89 (91.8%) | 126 (96.2%) | 215 (94.3%) |
| R+ | 8 (8.2%) | 5 (3.8%) | 13 (5.7%) |
| Tumor regression grading | |||
| 1a | 11 (11.3%) | 21 (16.0%) | 32 (14.0%) |
| 1b | 27 (27.8%) | 26 (19.8%) | 53 (23.2%) |
| 2 | 30 (30.9%) | 40 (30.5%) | 70 (30.7%) |
| 3 | 25 (25.8%) | 37 (28.2%) | 62 (27.2%) |
| 4 | 4 (4.1%) | 7 (5.3%) | 11 (4.8%) |
| Postop. pathologic T stage | |||
| T0 | 11 (11.3%) | 21 (16.0%) | 32 (14.0%) |
| T1 | 18 (18.6%) | 19 (14.5%) | 37 (16.2%) |
| T2 | 19 (19.6%) | 29 (22.1%) | 48 (21.1%) |
| T3 | 42 (43.3%) | 59 (45.0%) | 101 (44.3%) |
| T4 | 7 (7.2%) | 3 (2.3%) | 10 (4.4%) |
| Postop. pathologic n stage | |||
| N0 | 60 (61.9%) | 73 (55.7%) | 133 (58.3%) |
| N+ | 37 (38.1%) | 58 (44.3%) | 95 (41.7%) |
| Number of removed lymph nodes * | 23 (3–64) | 26 (8–58) | 25 (3–64) |
| Number of positive lymph nodes * | 0 (0–34) | 0 (0–29) | 0 (0–34) |
| Postop. pathologic M stage | |||
| M0 | 94 (96.9%) | 126 (96.2%) | 220 (96.5%) |
| M1 | 3 (3.1%) | 5 (3.8%) | 8 (3.5%) |
| Type of surgery | |||
| Esophagectomy | 0 (0.0%) | 121 (92.4%) | 121 (53.1%) |
| Gastrectomy | 97 (100.0%) | 10 (7.6%) | 107 (46.9%) |
| Laparoscopic surgery | 22 (22.7%) | 66 (50.4%) | 88 (38.6%) |
| Conversion rate | 9 (40.9%) | 3 (4.5%) | 12 (13.6%) |
| Hospital stay in days * | 11 (6–98) | 15 (7–96) | 13.5 (6–98) |
| Perioperative complications ** | 41 (42.3%) | 79 (60.3%) | 120 (52.6%) |
| I | 7 (7.2%) | 11 (8.4%) | 18 (7.9%) |
| II | 13 (13.4%) | 21 (16.0%) | 34 (14.9%) |
| IIIa | 8 (8.2%) | 28 (21.4%) | 36 (15.8%) |
| IIIb | 7 (7.2%) | 10 (7.6%) | 17 (7.5%) |
| IVa | 2 (2.1%) | 5 (3.8%) | 7 (3.1%) |
| IVb | 2 (2.1%) | 1 (0.8%) | 3 (1.3%) |
| V | 2 (2.1%) | 3 (2.3%) | 5 (2.2%) |
| Neoadjuvant chemotherapy | |||
| Complete (≥4 cycles) | 75 (77.3%) | 116 (88.5%) | 191 (83.8%) |
| Incomplete (≥1 cycle–<4 cycles) | 22 (22.7%) | 15 (11.5%) | 37 (16.2%) |
| Cycles of neoadjuvant chemotherapy * | 4 (1–8) | 4 (2–9) | 4 (1–9) |
| Type of adjuvant chemotherapy | |||
| None | 23 (23.7%) | 35 (26.7%) | 58 (25.4%) |
| FLOT | 65 (67.0%) | 84 (64.1%) | 149 (65.4%) |
| Other | 6 (6.2%) | 5 (3.8%) | 11 (4.8%) |
| Not specified | 3 (3.1%) | 7 (5.3%) | 10 (4.4%) |
| Adjuvant chemotherapy | |||
| Complete (≥4 cycles) | 36 (37.1%) | 50 (38.2%) | 86 (37.7%) |
| Incomplete (≥1 cycle–<4 cycles) | 53 (54.6%) | 69 (52.7%) | 122 (53.5%) |
| Not specified | 8 (8.2%) | 12 (9.2%) | 20 (8.8%) |
| Cycles of adjuvant chemotherapy * | 4 (1–4) | 4 (1–5) | 4 (1–5) |
| Gastric Carcinoma (N = 97) | Esophageal Carcinoma (N = 131) | Total (N = 228) | |
|---|---|---|---|
| Recurrence | 36 (37%) | 46 (35%) | 82 (36%) |
| Time of recurrence after surgery (m) | 9 (2–46) | 9.5 (1–42) | 9 (1–46) |
| Type of recurrence | |||
| Local | 3 (8%) | 3 (7%) | 6 (7%) |
| Local and distant metastasis | 2 (6%) | 4 (9%) | 6 (7%) |
| Peritoneal carcinomatosis | 20 (56%) | 3 (7%) | 23 (28%) |
| Hepatic metastasis | 1 (3%) | 11 (24%) | 12 (14%) |
| Pulmonary metastasis | 3 (8%) | 7 (15%) | 10 (12%) |
| Other location of metastasis | 4 (11%) | 11 (24%) | 15 (18%) |
| Multiple distant metastasis | 3 (8%) | 7 (15%) | 10 (12%) |
| Therapy of recurrence | |||
| None | 13 (36%) | 7 (15%) | 20 (24%) |
| Curative surgery | 2 (6%) | 4 (9%) | 6 (7%) |
| Radiotherapy | 0 (0%) | 4 (9%) | 4 (5%) |
| Chemotherapy | 19 (53%) | 23 (50%) | 42 (51%) |
| Chemo- and radiotherapy | 2 (6%) | 8 (17%) | 10 (12%) |
| Parameter | N | Peritoneal Carcinomatosis | P |
|---|---|---|---|
| Total * | 94 | 26% (n = 24) | |
| Sex | 0.294 | ||
| Female | 33 | 30% | |
| Male | 61 | 23% | |
| Age | 0.320 | ||
| <65 | 49 | 29% | |
| ≥65 | 45 | 22% | |
| Localization | 0.680 | ||
| AEG III | 27 | 22% | |
| Corpus | 42 | 24% | |
| Antrum | 25 | 32% | |
| Preoperative T Stage ** | 0.128 | ||
| T1–T2 | 12 | 8% | |
| T3–T4 | 74 | 28% | |
| Preoperative N Stage ** | 0.549 | ||
| N0 | 26 | 27% | |
| N+ | 56 | 29% | |
| Resection Margin | <0.001 | ||
| R0 | 88 | 21% | |
| R+ | 6 | 100% | |
| Lauren Classification | <0.001 | ||
| Intestinal | 57 | 12% | |
| Diffuse | 37 | 46% | |
| Postop. Pathologic T Stage | 0.002 | ||
| yT0 | 11 | 0% | |
| yT1 | 18 | 0% | |
| yT2 | 18 | 22% | |
| yT3 | 41 | 42% | |
| yT4 | 6 | 50% | |
| Postop. Pathologic N Stage | 0.031 | ||
| yN0 | 60 | 18% | |
| yN+ | 34 | 38% | |
| Histopathological Regression | 0.221 | ||
| 1a | 11 | 0% | |
| 1b | 26 | 23% | |
| 2 | 30 | 30% | |
| 3 | 23 | 30% | |
| 4 | 4 | 50% | |
| Adjuvant Chemotherapy ** | 0.530 | ||
| Yes | 62 | 24% | |
| No | 23 | 22% |
| Univariate Analysis | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | n | Overall Survival | p | Recurrence-Free Survival | p | ||
| Total | 228 | 51% | 46% | ||||
| Sex | 0.965 | 0.778 | |||||
| Female | 51 | 49% | 45% | ||||
| Male | 177 | 51% | 46% | ||||
| AGE | 0.423 | 0.761 | |||||
| <65 | 124 | 54% | 47% | ||||
| ≥65 | 104 | 47% | 45% | ||||
| ASA Classification | 0.043 | 0.067 | |||||
| ASA 1–2 | 121 | 55% | 53% | ||||
| ASA 3–4 | 107 | 46% | 37% | ||||
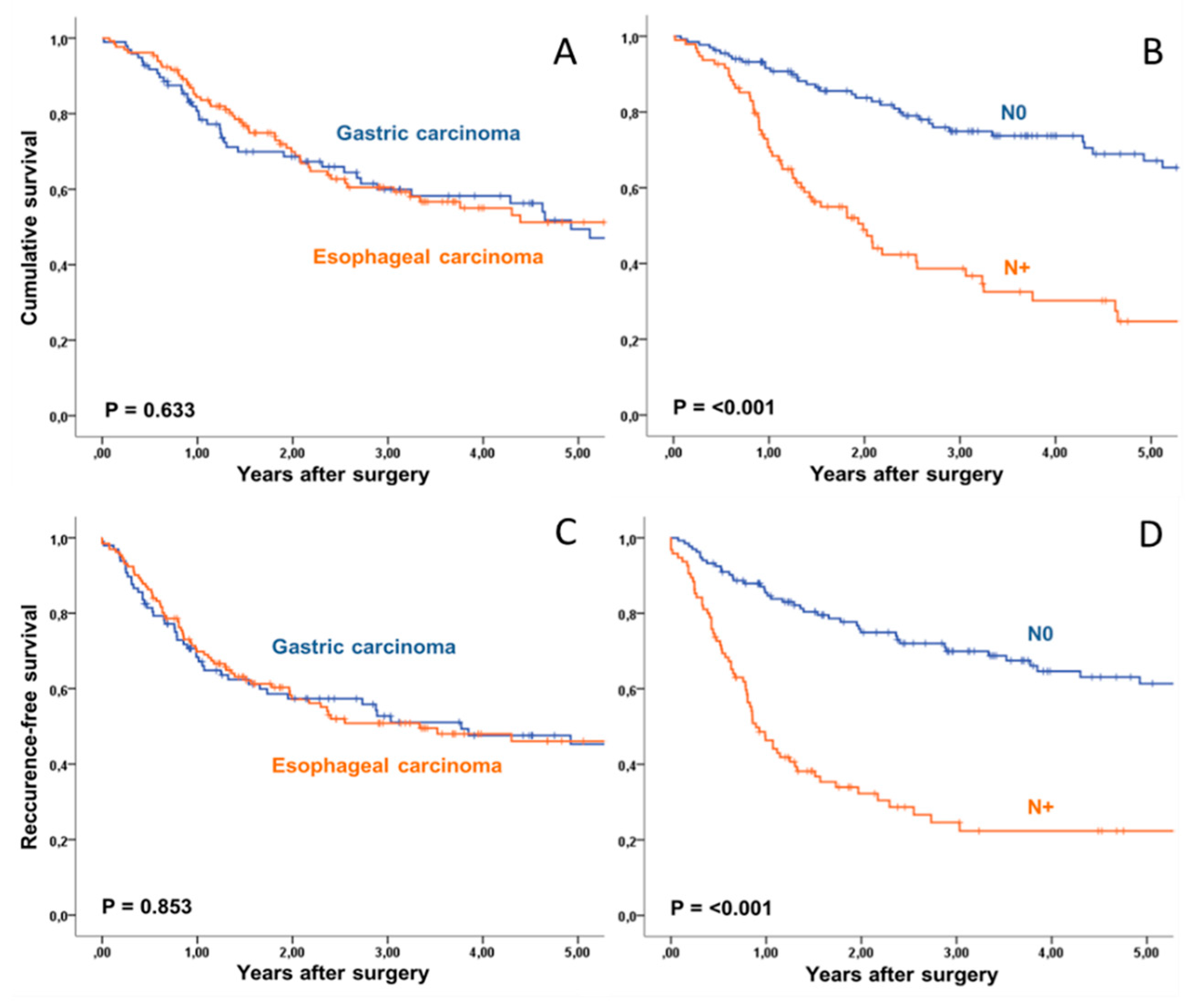
| Type of Carcinoma | 0.633 | 0.853 | |||||
| Esophageal Carcinoma | 131 | 51% | 46% | ||||
| Gastric Carcinoma | 97 | 49% | 45% | ||||
| Localization | 0.988 | 0.727 | |||||
| AEG I | 69 | 53% | 44% | ||||
| AEG II | 62 | 49% | 49% | ||||
| AEG III | 28 | 48% | 40% | ||||
| Corpus | 43 | 49% | 47% | ||||
| Antrum | 26 | 55% | 49% | ||||
| Preoperative T Stage * | 0.069 | 0.069 | |||||
| T1–T2 | 31 | 61% | 60% | ||||
| T3–T4 | 181 | 49% | 44% | ||||
| Preoperative N Stage * | 0.742 | 0.972 | |||||
| N0 | 54 | 46% | 45% | ||||
| N+ | 153 | 53% | 47% | ||||
| Type of Surgery | 0.705 | 0.928 | |||||
| Esophagectomy | 121 | 51% | 45% | ||||
| Gastrectomy | 107 | 50% | 46% | ||||
| Resection Margin | <0.001 | <0.001 | |||||
| R0 | 214 | 53% | 48% | ||||
| R+ | 14 | 16% | 13% | ||||
| Histopathological Regression | <0.001 | <0.001 | |||||
| 1a | 32 | 68% | 69% | ||||
| 1b | 53 | 68% | 62% | ||||
| 2 | 70 | 51% | 44% | ||||
| 3 | 62 | 36% | 30% | ||||
| 4 | 11 | 9% | 9% | ||||
| Postop. Pathologic T Stage | <0.001 | <0.001 | |||||
| yT0 | 32 | 68% | 69% | ||||
| yT1 | 37 | 74% | 72% | ||||
| yT2 | 48 | 62% | 48% | ||||
| yT3 | 101 | 36% | 33% | ||||
| yT4 | 10 | 0% | 0% | ||||
| Postop. Pathologic N Stage | <0.001 | <0.001 | |||||
| yN0 | 133 | 67% | 61% | ||||
| yN+ | 95 | 25% | 22% | ||||
| Postop. Pathologic M Stage | 0.023 | 0.002 | |||||
| M0 | 220 | 51% | 47% | ||||
| M1 | 8 | 22% | 16% | ||||
| Adjuvant Chemotherapy * | 0.024 | 0.025 | |||||
| Yes | 149 | 54% | 49% | ||||
| No | 58 | 42% | 38% | ||||
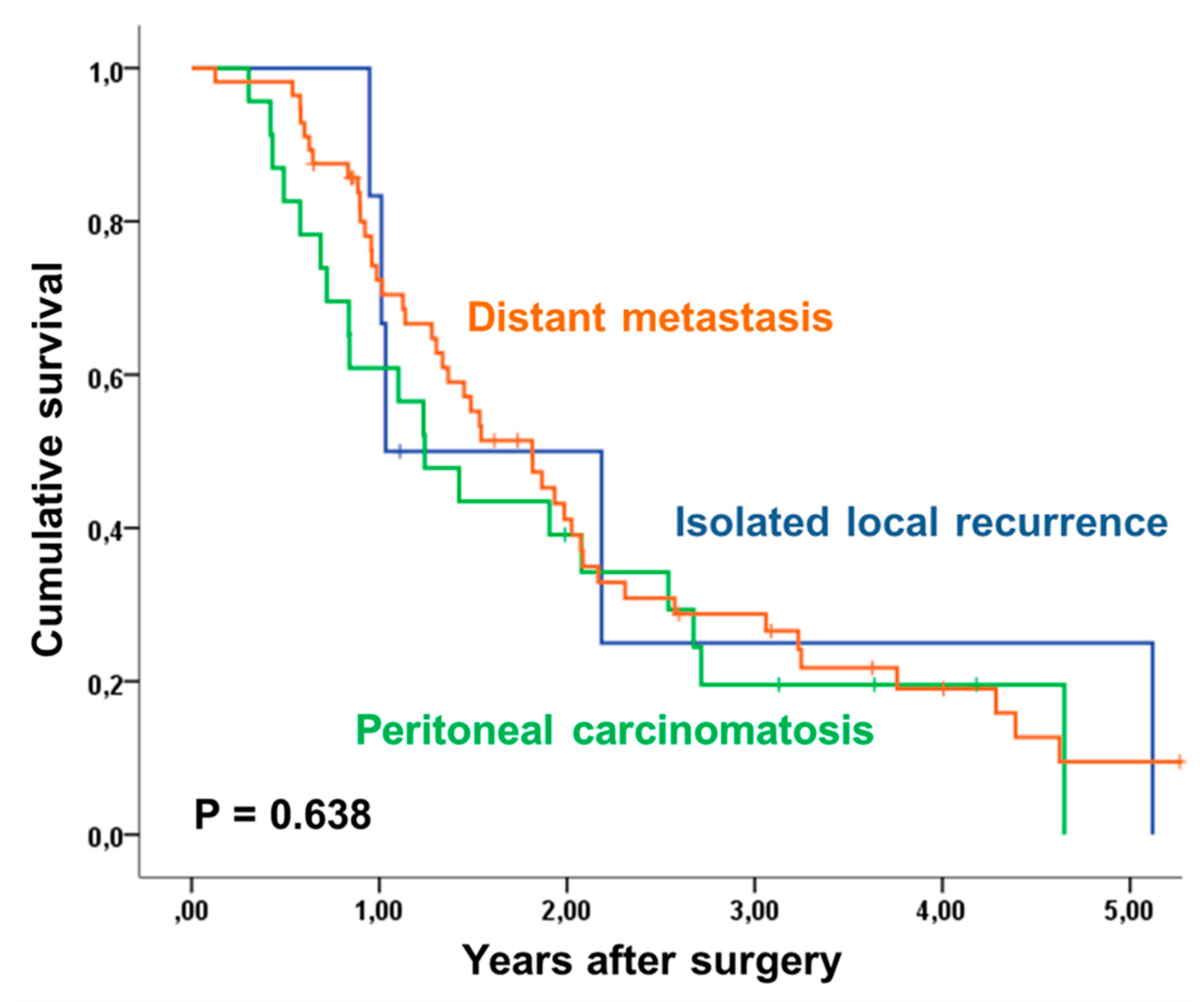
| Recurrence | 0.638 | ||||||
| Isolated Local Recurrence | 6 | 25% | |||||
| Peritoneal Carcinomatosis | 23 | 0% | |||||
| Distant Metastasis | 53 | 9% | |||||
| MULTIVARIATE ANALYSIS | |||||||
| Overall Survival | Recurrence-Free Survival | ||||||
| Parameter | RR | 95%-CI | p | RR | 95%-CI | p | |
| Postop. N Stage (YPN0/YPN+) | 2.53 | 1.54–4.17 | <0.001 | 2.87 | 1.80–4.57 | <0.001 | |
| Postop. T Stage (YPT0-2/YPT3–4) | 2.06 | 1.22–3.46 | 0.006 | 1.77 | 1.10–2.84 | 0.018 | |
| Postop. Chemotherapy (Y/N) | --- | --- | 0.057 | 1.52 | 1.00–2.32 | 0.05 | |
| Histopath. Regr. (<10/10–50 > 50%) | --- | --- | 0.110 | --- | --- | 0.106 | |
| Postop. M Stage (YM0/YM+) | --- | --- | 0.204 | --- | --- | 0.231 | |
| ASA (1–2/3–4) | --- | --- | 0.126 | --- | --- | 0.273 | |
| Resection Margin (R0/R+) | --- | --- | 0.400 | --- | --- | 0.685 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glatz, T.; Verst, R.; Kuvendjiska, J.; Bronsert, P.; Becker, H.; Hoeppner, J.; Kulemann, B. Pattern of Recurrence and Patient Survival after Perioperative Chemotherapy with 5-FU, Leucovorin, Oxaliplatin and Docetaxel (FLOT) for Locally Advanced Esophagogastric Adenocarcinoma in Patients Treated Outside Clinical Trials. J. Clin. Med. 2020, 9, 2654. https://doi.org/10.3390/jcm9082654
Glatz T, Verst R, Kuvendjiska J, Bronsert P, Becker H, Hoeppner J, Kulemann B. Pattern of Recurrence and Patient Survival after Perioperative Chemotherapy with 5-FU, Leucovorin, Oxaliplatin and Docetaxel (FLOT) for Locally Advanced Esophagogastric Adenocarcinoma in Patients Treated Outside Clinical Trials. Journal of Clinical Medicine. 2020; 9(8):2654. https://doi.org/10.3390/jcm9082654
Chicago/Turabian StyleGlatz, Torben, Rasmus Verst, Jasmina Kuvendjiska, Peter Bronsert, Heiko Becker, Jens Hoeppner, and Birte Kulemann. 2020. "Pattern of Recurrence and Patient Survival after Perioperative Chemotherapy with 5-FU, Leucovorin, Oxaliplatin and Docetaxel (FLOT) for Locally Advanced Esophagogastric Adenocarcinoma in Patients Treated Outside Clinical Trials" Journal of Clinical Medicine 9, no. 8: 2654. https://doi.org/10.3390/jcm9082654
APA StyleGlatz, T., Verst, R., Kuvendjiska, J., Bronsert, P., Becker, H., Hoeppner, J., & Kulemann, B. (2020). Pattern of Recurrence and Patient Survival after Perioperative Chemotherapy with 5-FU, Leucovorin, Oxaliplatin and Docetaxel (FLOT) for Locally Advanced Esophagogastric Adenocarcinoma in Patients Treated Outside Clinical Trials. Journal of Clinical Medicine, 9(8), 2654. https://doi.org/10.3390/jcm9082654






