Diagnosis of Non-Alcoholic Fatty Liver Disease (NAFLD) Is Independently Associated with Cardiovascular Risk in a Large Austrian Screening Cohort
Abstract
1. Introduction
2. Methods
2.1. Study Subjects
2.2. Assessment
2.3. Definitions
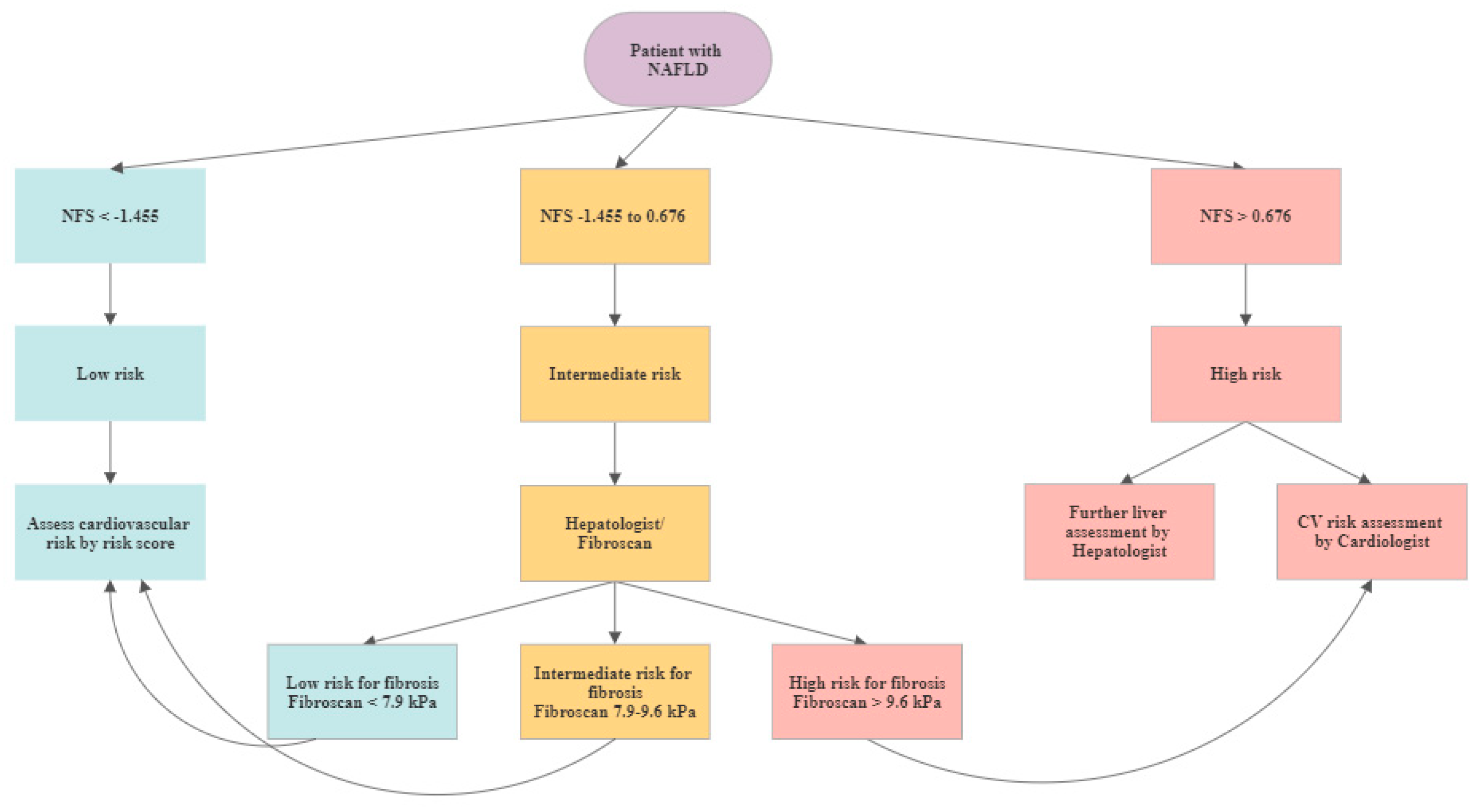
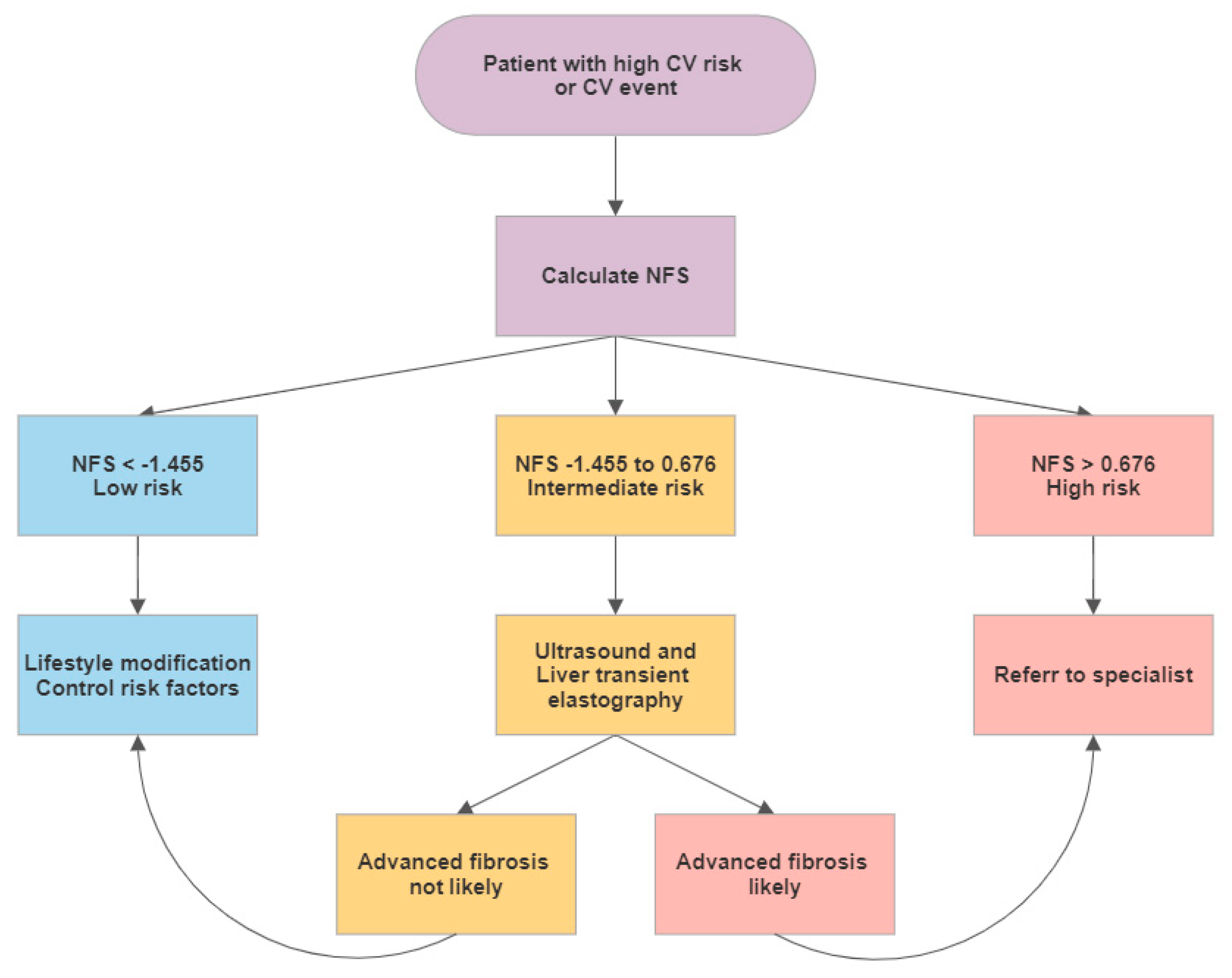
2.4. Cardiovascular Risk Assessment
2.5. Statistical Analysis
3. Results
3.1. Analysis of the Total Study Cohort, NAFLD versus Non-NAFLD Patients
3.2. Analysis of Patients with NAFLD
4. Discussion
4.1. CV Risk Assessment for NALFD Patients
4.2. Screening for NAFLD in CV Patients
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation. Treatment of High Blood Cholesterol in, A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Rafiq, N.; Bai, C.; Fang, Y.; Srishord, M.; McCullough, A.; Gramlich, T.; Younossi, Z.M. Long-term follow-up of patients with nonalcoholic fatty liver. Clin. Gastroenterol. Hepatol. 2009, 7, 234–238. [Google Scholar] [CrossRef]
- Ong, J.P.; Pitts, A.; Younossi, Z.M. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J. Hepatol. 2008, 49, 608–612. [Google Scholar] [CrossRef]
- Oni, E.T.; Agatston, A.S.; Blaha, M.J.; Fialkow, J.; Cury, R.; Sposito, A.; Erbel, R.; Blankstein, R.; Feldman, T.; Al-Mallah, M.H.; et al. A systematic review: Burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis 2013, 230, 258–267. [Google Scholar] [CrossRef]
- Mantovani, A.; Pernigo, M.; Bergamini, C.; Bonapace, S.; Lipari, P.; Pichiri, I.; Bertolini, L.; Valbusa, F.; Barbieri, E.; Zoppini, G.; et al. Nonalcoholic Fatty Liver Disease Is Independently Associated with Early Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. PLoS ONE 2015, 10, e0135329. [Google Scholar] [CrossRef]
- Targher, G.; Day, C.P.; Bonora, E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010, 363, 1341–1350. [Google Scholar] [CrossRef]
- Wu, S.; Wu, F.; Ding, Y.; Hou, J.; Bi, J.; Zhang, Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 33386. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Wieckowska, A.; Papouchado, B.G.; Li, Z.; Lopez, R.; Zein, N.N.; Feldstein, A.E. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2008, 103, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Zoppini, G.; Zenari, L.; Falezza, G. Increased plasma markers of inflammation and endothelial dysfunction and their association with microvascular complications in Type 1 diabetic patients without clinically manifest macroangiopathy. Diabet. Med. 2005, 22, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.; O’Leary, D.H.; Zaccaro, D.; Haffner, S.; Rewers, M.; Hamman, R.; Selby, J.V.; Saad, M.F.; Savage, P.; Bergman, R. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation 1996, 93, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Hagstrom, H.; Nasr, P.; Fredrikson, M.; Stal, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Despotovic, D.; Niederseer, D.; Brunckhorst, C. CME-EKG 60: Akut auftretende Thoraxschmerzen und Dyspnoe: Das EKG als Schlussel zur Diagnose. Praxis 2018, 107, 223–224. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit. Pathw. Cardiol. 2005, 4, 198–203. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Simon, T.G.; Corey, K.E.; Cannon, C.P.; Blazing, M.; Park, J.G.; O’Donoghue, M.L.; Chung, R.T.; Giugliano, R.P. The nonalcoholic fatty liver disease (NAFLD) fibrosis score, cardiovascular risk stratification and a strategy for secondary prevention with ezetimibe. Int. J. Cardiol. 2018, 270, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Loomis, A.K.; Van der Lei, J.; Duarte-Salles, T.; Prieto-Alhambra, D.; Ansell, D.; Pasqua, A.; Lapi, F.; Rijnbeek, P.; Mosseveld, M.; et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: Findings from matched cohort study of 18 million European adults. BMJ 2019, 367, l5367. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Choudhary, N.S.; Duseja, A. Screening of Cardiovascular Disease in Nonalcoholic Fatty Liver Disease: Whom and How? J. Clin. Exp. Hepatol. 2019, 9, 506–514. [Google Scholar] [CrossRef]
- Wernly, B.; Wernly, S.; Niederseer, D.; Datz, C. Hepatitis C virus (HCV) infection and cardiovascular disease: Hepatologists and cardiologists need to talk! Eur. J. Intern. Med. 2020, 71, 87–88. [Google Scholar] [CrossRef]
| No NAFLD | NAFLD | Total Cohort | p-Value | |
|---|---|---|---|---|
| n = 990 | n = 975 | n = 1965 | ||
| Female | 61% | 43% | 52% | <0.001 |
| Age (years) | 58 (10) | 60 (9) | 59 (10) | <0.001 |
| Systolic RR (mmHg) | 128 (18) | 135 (19) | 131 (18) | <0.001 |
| Diastolic RR (mmhg) | 79 (10) | 83 (11) | 81 (10) | <0.001 |
| BMI (kg/m2) | 25 (4) | 26 (5) | 27 (4) | <0.001 |
| Waist circumference (cm) | 90 (11) | 105 (12) | 97 (11) | <0.001 |
| Waist to hip ratio | 1 (0.1) | 1 (0.1) | 1 (0.1) | <0.001 |
| Bilirubine (mg/dL) | 0.72 (0.4) | 0.73 (0.4) | 0.72 (0.4) | 0.4 |
| GGT (U/L) | 31 (46) | 48 (71) | 40 (46) | <0.001 |
| AST (U/L) | 22 (12) | 26 (18) | 24 (12) | <0.001 |
| INR | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.1) | 0.24 |
| Total cholesterol (mg/dL) | 219 (40) | 217 (44) | 218 (40) | 0.25 |
| HDL (mg/dL) | 67 (18) | 56 (16) | 62 (18) | <0.001 |
| LDL (mg/dL) | 137 (36) | 142 (39) | 139 (36) | 0.02 |
| Triglycerices (mg/dL) | 101 (51) | 145 (85) | 123 (51) | <0.001 |
| Thrombocytes (G/L) | 236 (66) | 227 (65) | 232 (66) | 0.001 |
| Fasting glucose (mg/dL) | 97 (15) | 109 (30) | 103 (15) | <0.001 |
| HbA1c (%) | 5.6 (0.5) | 5.9 (0.8) | 5.8 (0.5) | <0.001 |
| Metabolic syndrome | 7% | 33% | 20% | <0.001 |
| T2DM | 9% | 24% | 16% | <0.001 |
| Current smoker | 19% | 17% | 20% | 0.48 |
| Medication | ||||
| ASS | 11% | 17% | 14% | 0.001 |
| Statin | 15% | 23% | 19% | <0.001 |
| ACE-I/ARB | 13% | 27% | 20% | <0.001 |
| Metformin | 2% | 8% | 5% | <0.001 |
| CV risk score | ||||
| FRS | 5.41 (5.20) | 8.71 (6.38) | 7.05 (5.20) | <0.001 |
| FRS 0-2% | 41% | 19% | 30% | <0.001 |
| FRS >2–5% | 21% | 19% | 20% | |
| FRS >5–10% | 22% | 30% | 25% | |
| FRS >10% | 16% | 33% | 24% | |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| Age | 1.03 | 1.02–1.04 | <0.001 | 1.010 | 0.998–1.023 | 0.11 |
| Female gender | 0.48 | 0.40–0.58 | <0.001 | 0.68 | 0.54–0.86 | 0.001 |
| Metabolic syndrome | 6.08 | 4.63–7.99 | <0.001 | 5.02 | 3.77–6.70 | <0.001 |
| FRS | 1.11 | 1.09–1.13 | <0.001 | 1.06 | 1.04–1.08 | <0.001 |
| F0-F2 | Intermediate | F3-F4 | |||||
|---|---|---|---|---|---|---|---|
| n = 604 | n = 138 | n = 10 | |||||
| Mean | SD | Mean | SD | Mean | SD | p-Value | |
| Female | 36% | 43% | 50% | 0.80 | |||
| Age (years) | 59 | 9 | 66 | 8 | 67 | 9 | <0.001 |
| Systolic RR (mmHg) | 134 | 18 | 139 | 19 | 148 | 26 | <0.001 |
| Diastolic RR (mmhg) | 82 | 11 | 85 | 12 | 85 | 12 | 0.07 |
| BMI (kg/m2) | 29 | 4 | 33 | 6 | 35 | 4 | <0.001 |
| Waist circumference (cm) | 103 | 11 | 111 | 12 | 115 | 14 | <0.001 |
| Waist to hip ratio | 0.96 | 0 | 0.97 | 0 | 0.97 | 0 | 0.23 |
| Bilirubine (mg/dL) | 0.70 | 0 | 0.80 | 1 | 1.57 | 1 | <0.001 |
| GGT (U/L) | 48 | 76 | 53 | 70 | 115 | 145 | 0.02 |
| AST (U/L) | 25 | 15 | 30 | 24 | 55 | 61 | <0.001 |
| INR | 0.99 | 0 | 1.02 | 0 | 1.17 | 0 | <0.001 |
| Total cholesterol (mg/dL) | 221 | 44 | 202 | 42 | 221 | 52 | <0.001 |
| HDL (mg/dL | 57 | 16 | 53 | 13 | 57 | 13 | 0.03 |
| LDL (mg/dL) | 145 | 40 | 130 | 37 | 142 | 41 | <0.001 |
| Triglycerices (mg/dL) | 145 | 84 | 147 | 101 | 142 | 68 | 0.97 |
| Thrombocytes (G/L) | 243 | 62 | 176 | 52 | 128 | 88 | <0.001 |
| Fasting glucose (mg/dL) | 107 | 28 | 115 | 28 | 97 | 16 | 0.01 |
| HbA1c (%) | 5.9 | 1 | 6.0 | 1 | 5.6 | 0 | 0.08 |
| Metabolic syndrome | 30% | 43% | 40% | 0.01 | |||
| T2DM | 20% | 44% | 20% | <0.001 | |||
| Current Smoker | 19% | 6% | 0% | 0.02 | |||
| Medication | |||||||
| ASS | 24% | 31% | 13% | 0.21 | |||
| Statin | 24% | 31% | 13% | 0.21 | |||
| ACE-I/ARB | 22% | 38% | 20% | 0.02 | |||
| Metformin | 8% | 10% | 0% | 0.42 | |||
| FRS | 7.83 | 5.92 | 10.87 | 6.29 | 11.70 | 5.44 | <0.001 |
| F0-F2 | Intermediate or F3-F4 | ||
|---|---|---|---|
| n = 604 | n = 148 | p-Value | |
| Female | 41% | 43% | 0.64 |
| Age (years) | 59 (9) | 66 (9) | <0.001 |
| Systolic RR (mmHg) | 134 (18) | 140 (18) | <0.001 |
| Diastolic RR (mmhg) | 82 (11) | 85 (11) | 0.02 |
| BMI (kg/m2) | 29 (4) | 33 (4) | <0.001 |
| Waist circumference (cm) | 103 (11) | 111 (11) | <0.001 |
| Waist to hip ratio | 1 (0) | 1 (0) | 0.09 |
| Bilirubine (mg/dL) | 1 (0) | 1 (0) | <0.001 |
| GGT (U/L) | 48 (76) | 57 (76) | 0.16 |
| AST (U/L) | 25 (15) | 32 (15) | <0.001 |
| INR | 0.99 (0.07) | 1.03 (0.07) | <0.001 |
| Total cholesterol (mg/dL) | 221 (44) | 203 (44) | <0.001 |
| HDL (mg/dL | 57 (16) | 53 (16) | 0.01 |
| LDL (mg/dL) | 145 (40) | 131 (40) | <0.001 |
| Triglycerices (mg/dL) | 145 (84) | 147 (84) | 0.87 |
| Thrombocytes (G/L) | 243 (62) | 173 (62) | <0.001 |
| Fasting glucose (mg/dL) | 107 (28) | 113 (28) | 0.01 |
| HbA1c (%) | 5.9 (0.7) | 6.0 (0.7) | 0.12 |
| Metabolic syndrome | 30% | 43% | 0.003 |
| T2DM | 20% | 40% | <0.001 |
| Current Smoker | 19% | 6% | 0.003 |
| Medication | |||
| ASS | 16% | 21% | 0.11 |
| Statin | 24% | 30% | 0.20 |
| ACE-I/ARB | 22% | 37% | 0.01 |
| Metformin | 8% | 10% | 0.39 |
| FRS | 7.83 (5.92) | 10.92 (5.92) | <0.001 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| Age | 1.11 | 1.09–1.13 | <0.001 | 1.17 | 1.14–1.21 | <0.001 |
| Female gender | 0.15 | 0.10–0.21 | <0.001 | 0.02 | 0.01–0.04 | <0.001 |
| Metabolic syndrome | 2.46 | 1.86–3.26 | <0.001 | 4.15 | 2.64–6.55 | <0.001 |
| FRS | 1.60 | 1.41–1.83 | <0.001 | 1.30 | 1.09–1.54 | 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niederseer, D.; Wernly, S.; Bachmayer, S.; Wernly, B.; Bakula, A.; Huber-Schönauer, U.; Semmler, G.; Schmied, C.; Aigner, E.; Datz, C. Diagnosis of Non-Alcoholic Fatty Liver Disease (NAFLD) Is Independently Associated with Cardiovascular Risk in a Large Austrian Screening Cohort. J. Clin. Med. 2020, 9, 1065. https://doi.org/10.3390/jcm9041065
Niederseer D, Wernly S, Bachmayer S, Wernly B, Bakula A, Huber-Schönauer U, Semmler G, Schmied C, Aigner E, Datz C. Diagnosis of Non-Alcoholic Fatty Liver Disease (NAFLD) Is Independently Associated with Cardiovascular Risk in a Large Austrian Screening Cohort. Journal of Clinical Medicine. 2020; 9(4):1065. https://doi.org/10.3390/jcm9041065
Chicago/Turabian StyleNiederseer, David, Sarah Wernly, Sebastian Bachmayer, Bernhard Wernly, Adam Bakula, Ursula Huber-Schönauer, Georg Semmler, Christian Schmied, Elmar Aigner, and Christian Datz. 2020. "Diagnosis of Non-Alcoholic Fatty Liver Disease (NAFLD) Is Independently Associated with Cardiovascular Risk in a Large Austrian Screening Cohort" Journal of Clinical Medicine 9, no. 4: 1065. https://doi.org/10.3390/jcm9041065
APA StyleNiederseer, D., Wernly, S., Bachmayer, S., Wernly, B., Bakula, A., Huber-Schönauer, U., Semmler, G., Schmied, C., Aigner, E., & Datz, C. (2020). Diagnosis of Non-Alcoholic Fatty Liver Disease (NAFLD) Is Independently Associated with Cardiovascular Risk in a Large Austrian Screening Cohort. Journal of Clinical Medicine, 9(4), 1065. https://doi.org/10.3390/jcm9041065







