Favorable Long-Term Outcomes of Endoscopic Submucosal Dissection for Differentiated-Type-Predominant Early Gastric Cancer with Histological Heterogeneity
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Definitions
2.3. Histopathological Evaluation
2.4. Follow Up after Endoscopic Submucosal Dissection
2.5. Statistical Analysis
3. Results
3.1. Comparison of Clinicopathologic Characteristics of Differentiated-Type-Predominant Early Gastric Cancers with Histological Heterogeneity versus Pure Differentiated-Type Early Gastric Cancer
3.2. Comparison of Short-Term Outcomes of Endoscopic Submucosal Dissection of Differentiated-Type-Predominant Early Gastric Cancers with Histological Heterogeneity versus Pure Differentiated-Type Early Gastric Cancer
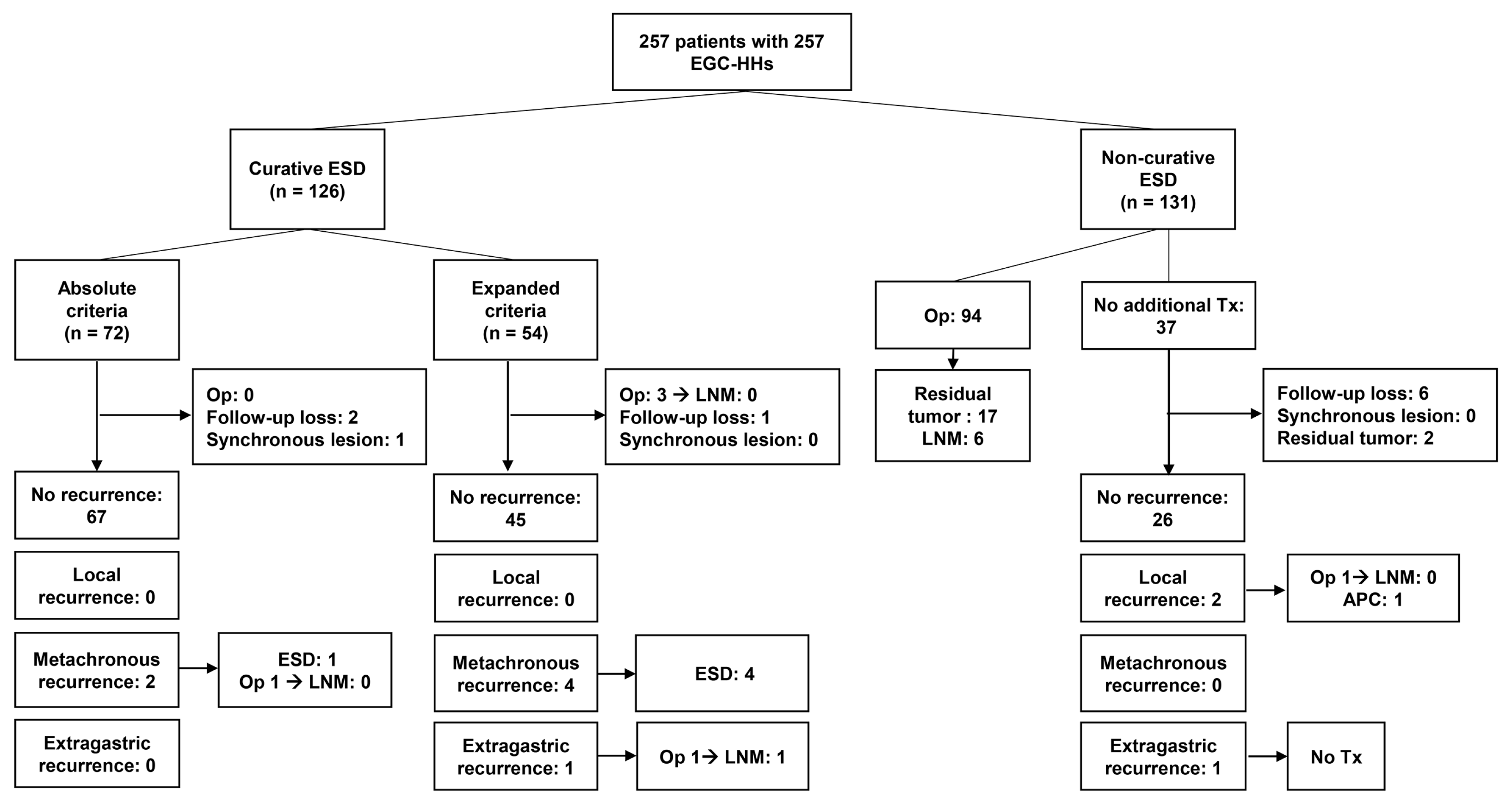
3.3. Recurrence Pattern after Endoscopic Submucosal Dissection for Differentiated-Type-Predominant Early Gastric Cancers with Histological Heterogeneity
3.4. Comparison of Long-Term Outcomes of Endoscopic Submucosal Dissection of Differentiated-Type-Predominant Early Gastric Cancers with Histological Heterogeneity versus Pure Differentiated-Type Early Gastric Cancer
3.5. Pathology Review of Undifferentiated-Type Component in Differentiated-Type-Predominant Early Gastric Cancer with Histological Heterogeneity
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines. 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Park, C.K.; Kim, Y.B.; Kim, Y.W.; Kim, H.G.; Bae, H.I. A standardized pathology report for gastric cancer. Korean J. Pathol. 2005, 39, 106–113. [Google Scholar]
- Hanaoka, N.; Tanabe, S.; Mikami, T.; Okayasu, I.; Saigenji, K. Mixed-Histologic-Type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: Feasibility of endoscopic submucosal dissection. Endoscopy 2009, 41, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Min, B.H.; Kim, K.M.; Park, C.K.; Lee, J.H.; Rhee, P.L.; Rhee, J.C.; Kim, J.J. Outcomes of endoscopic submucosal dissection for differentiated-type early gastric cancer with histological heterogeneity. Gastric Cancer 2015, 18, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Shimoda, T. Risk factors for lymph node metastasis of submucosal invasive differentiated type gastric carcinoma: Clinical significance of histological heterogeneity. J. Gastroenterol. 2001, 36, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, K.; Ono, H.; Kakushima, N.; Tanaka, M.; Hasuike, N.; Matsubayashi, H.; Yamagichi, Y.; Bando, E.; Terashima, M.; Kusafuka, K.; et al. Risk of lymph node metastases from intramucosal gastric cancer in relation to histological types: How to manage the mixed histological type for endoscopic submucosal dissection. Gastric Cancer 2013, 16, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, K.; Ono, H.; Yamamoto, Y.; Katai, H.; Hori, S.; Yano, T.; Umegaki, E.; Sasaki, S.; Iizuka, T.; Kawagoe, K.; et al. Incidence of lymph node metastasis in intramucosal gastric cancer measuring 30 mm or less, with ulceration; mixed, predominantly differentiated-type histology; and no lymphovascular invasion: A multicenter retrospective study. Gastric Cancer 2016, 19, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Min, B.H.; Kim, K.M.; Lee, J.H.; Rhee, P.L.; Kim, J.J. Endoscopic submucosal dissection for papillary adenocarcinoma of the stomach: Low curative resection rate but favorable long-term outcomes after curative resection. Gastric Cancer 2019, 22, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Min, B.H.; Kim, E.R.; Kim, K.M.; Park, C.K.; Lee, J.H.; Rhee, P.L.; Kim, J.J. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy 2015, 47, 784–793. [Google Scholar] [PubMed]
- Pyo, J.H.; Lee, H.; Min, B.H.; Lee, J.H.; Choi, M.G.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Kim, K.M.; Ahn, J.H.; et al. Long-Term Outcome of Endoscopic Resection vs. Surgery for Early Gastric Cancer: A Non-inferiority-Matched Cohort Study. Am. J. Gastroenterol. 2016, 111, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, N.; Tanabe, S.; Higuchi, K.; Sasaki, T.; Nakatani, K.; Ishido, K.; Ae, T.; Koizumi, W.; Saigenji, K.; Mikami, T. A rare case of histologically mixed-type intramucosal gastric cancer accompanied by nodal recurrence and liver metastasis after endoscopic submucosal dissection. Gastrointest. Endosc. 2009, 69, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.H.; Bae, Y.S.; Yoon, S.O.; Lee, Y.C.; Kim, H.; Noh, S.H.; Park, H.; Choi, S.H.; Kim, J.H.; Kim, H. Poorly Differentiated Carcinoma Component in Submucosal Layer Should be Considered as an Additional Criterion for Curative Endoscopic Resection of Early Gastric Cancer. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), 772–777. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, K.; Hatta, W.; Nakagawa, M.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Hirano, M.; Esaki, M.; et al. The Role of an Undifferentiated Component in Submucosal Invasion and Submucosal Invasion Depth after Endoscopic Submucosal Dissection for Early Gastric Cancer. Digestion 2018, 98, 161–168. [Google Scholar] [CrossRef] [PubMed]



| Variables | EGC-HH (n = 257) | PuD-EGC (n = 2386) | p-Value |
|---|---|---|---|
| Age (years) | 0.159 | ||
| Mean ± SD | 61.9 ± 10.6 | 63.1 ± 9.8 | |
| Median (range) | 63 (27–86) | 64 (31–90) | |
| Sex (%) | 0.172 | ||
| Male | 192 (74.7) | 1871 (78.4) | |
| Female | 65 (25.3) | 515 (21.6) | |
| Tumor site (%) | 0.107 | ||
| Antrum/angle | 176 (68.5) | 1778 (74.5) | |
| Body | 75 (29.2) | 558 (23.4) | |
| Fundus/cardia | 6 (10.7) | 50 (2.1) | |
| Tumor shape (%) | 0.288 | ||
| Elevated | 153 (59.5) | 1338 (56.1) | |
| Flat or depressed | 104 (40.5) | 1048 (43.9) | |
| Tumor size on pathology (cm) | <0.001 | ||
| Mean ± SD | 2.3 ± 1.1 | 1.5 ± 1.0 | |
| Median (range) | 2.0 (0.3–6.4) | 1.3 (0.1–11.0) | |
| Tumor depth (%) | <0.001 | ||
| Lamina propria | 39 (15.2) | 979 (41.0) | |
| Muscularis mucosae | 108 (42.0) | 1045 (43.8) | |
| SM1 | 38 (14.8) | 176 (7.4) | |
| SM2 or SM3 | 72 (28.0) | 186 (7.8) | |
| Differentiation (%) | <0.001 | ||
| Well-differentiated | 15 (5.8) | 967 (40.5) | |
| Moderately-differentiated | 230 (89.5) | 1361 (57.0) | |
| Papillary adenocarcinoma | 12 (4.7) | 58 (2.4) | |
| Lymphatic invasion (%) | <0.001 | ||
| Absent | 182 (70.8) | 2259 (94.7) | |
| Present | 75 (29.2) | 127 (5.3) | |
| Vascular invasion (%) | <0.001 | ||
| Absent | 246 (95.7) | 2369 (99.3) | |
| Present | 11 (4.3) | 17 (0.7) | |
| Lateral margin (%) | <0.001 | ||
| Negative | 231 (89.9) | 2321 (97.3) | |
| Positive/undetermined | 26 (10.1) | 65 (2.7) | |
| Vertical margin (%) | 0.002 | ||
| Negative | 242 (94.2) | 2332 (97.7) | |
| Positive/undetermined | 15 (5.8) | 54 (2.3) |
| Variables | EGC-HH (n = 257) | PuD-EGC (n = 2386) | p-Value |
|---|---|---|---|
| En bloc resection (%) | 0.163 | ||
| Yes | 248 (96.5) | 2335 (97.9) | |
| No | 9 (3.5) | 51 (2.1) | |
| R0 resection (%) | <0.001 | ||
| Yes | 220 (85.6) | 2286 (95.8) | |
| No or undetermined | 37 (14.4) | 87 (4.2) | |
| En bloc and R0 resection (%) | <0.001 | ||
| Yes | 216 (84.0) | 2259 (94.7) | |
| No or undetermined | 41 (16.0) | 127 (5.3) | |
| Curative resection (%) | <0.001 | ||
| Yes | 126 (49.0) | 2057 (86.2) | |
| No | 131 (51.0) | 329 (13.8) | |
| Perforation (%) | 0.183 | ||
| Absent | 249 (96.9) | 2341 (98.1) | |
| Present | 8 (3.1) | 45 (1.9) | |
| Bleeding (%) | 0.651 | ||
| Absent | 244 (94.9) | 2280 (95.6) | |
| Present | 13 (5.1) | 106 (4.4) |
| Case | Age | Sex | Tumor Site | Shape | Pathologic Size (mm) | Depth | Pathology | UD Component | UD Component Length (mm) | UD Component in Submucosa | Recurrence | Recurrence-Free Survival (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 53 | M | Body | Elevated | 46 | MM | MD | PD, 30% | 24 | No | No | 63 |
| Case 2 | 45 | F | Antrum | Depressed | 40 | MM | MD | SRC, 20% | 24 | No | No | 84 |
| Case 3 | 40 | F | Antrum | Flat | 32 | LP | MD | SRC, 45% | 24 | No | No | 63 |
| Case 4 | 56 | M | Body | Depressed | 30 | SM1 | MD | PD, 20% | 8 | Yes a | Op, LNM (-) | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-S.; Shin, H.C.; Min, B.-H.; Kim, K.-M.; Min, Y.W.; Lee, H.; Lee, J.H.; Rhee, P.-L.; Kim, J.J. Favorable Long-Term Outcomes of Endoscopic Submucosal Dissection for Differentiated-Type-Predominant Early Gastric Cancer with Histological Heterogeneity. J. Clin. Med. 2020, 9, 1064. https://doi.org/10.3390/jcm9041064
Kim T-S, Shin HC, Min B-H, Kim K-M, Min YW, Lee H, Lee JH, Rhee P-L, Kim JJ. Favorable Long-Term Outcomes of Endoscopic Submucosal Dissection for Differentiated-Type-Predominant Early Gastric Cancer with Histological Heterogeneity. Journal of Clinical Medicine. 2020; 9(4):1064. https://doi.org/10.3390/jcm9041064
Chicago/Turabian StyleKim, Tae-Se, Hyeong Chan Shin, Byung-Hoon Min, Kyoung-Mee Kim, Yang Won Min, Hyuk Lee, Jun Haeng Lee, Poong-Lyul Rhee, and Jae J. Kim. 2020. "Favorable Long-Term Outcomes of Endoscopic Submucosal Dissection for Differentiated-Type-Predominant Early Gastric Cancer with Histological Heterogeneity" Journal of Clinical Medicine 9, no. 4: 1064. https://doi.org/10.3390/jcm9041064
APA StyleKim, T.-S., Shin, H. C., Min, B.-H., Kim, K.-M., Min, Y. W., Lee, H., Lee, J. H., Rhee, P.-L., & Kim, J. J. (2020). Favorable Long-Term Outcomes of Endoscopic Submucosal Dissection for Differentiated-Type-Predominant Early Gastric Cancer with Histological Heterogeneity. Journal of Clinical Medicine, 9(4), 1064. https://doi.org/10.3390/jcm9041064






