Bedside 3D Visualization of Lymphatic Vessels with a Handheld Multispectral Optoacoustic Tomography Device
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
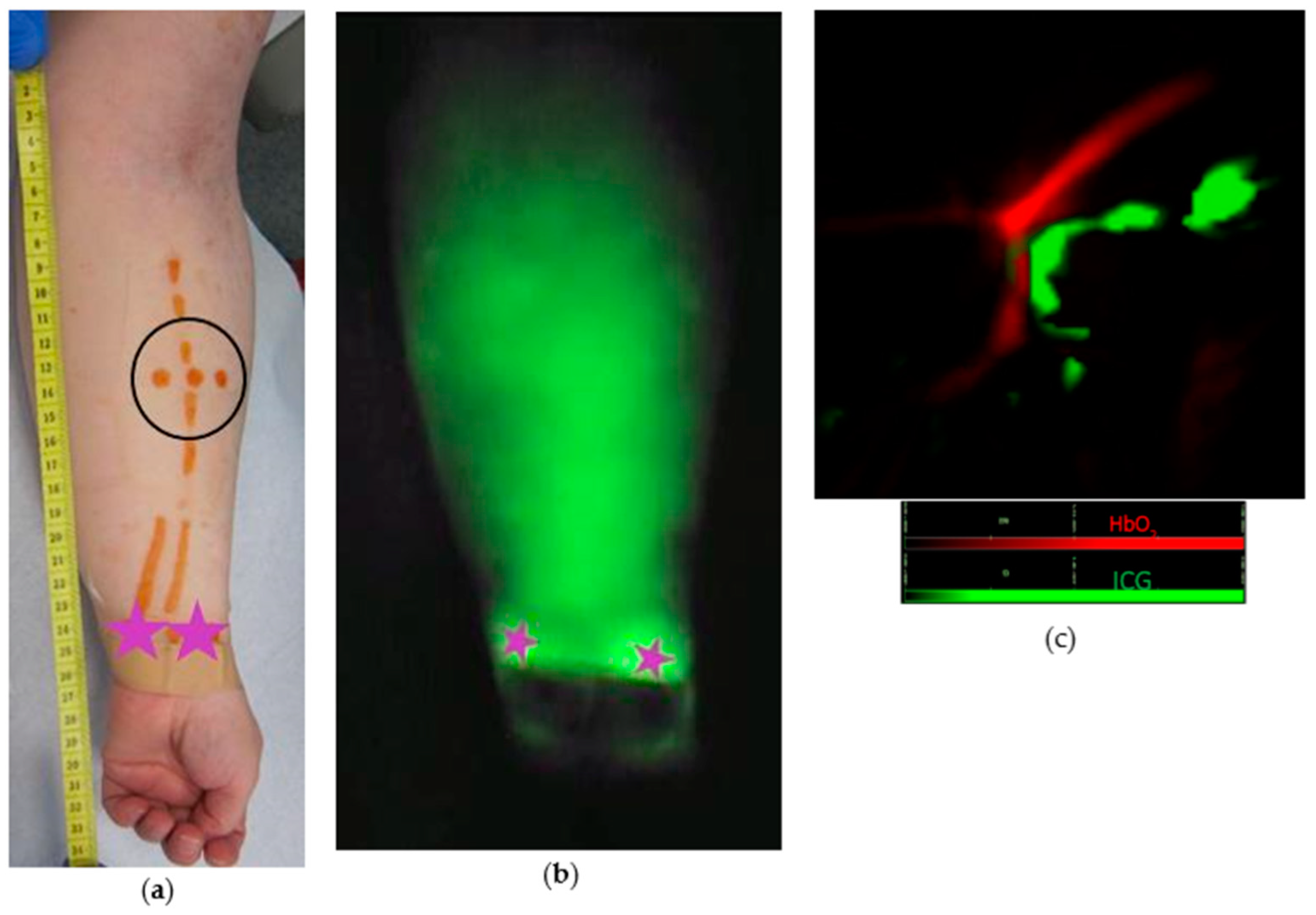
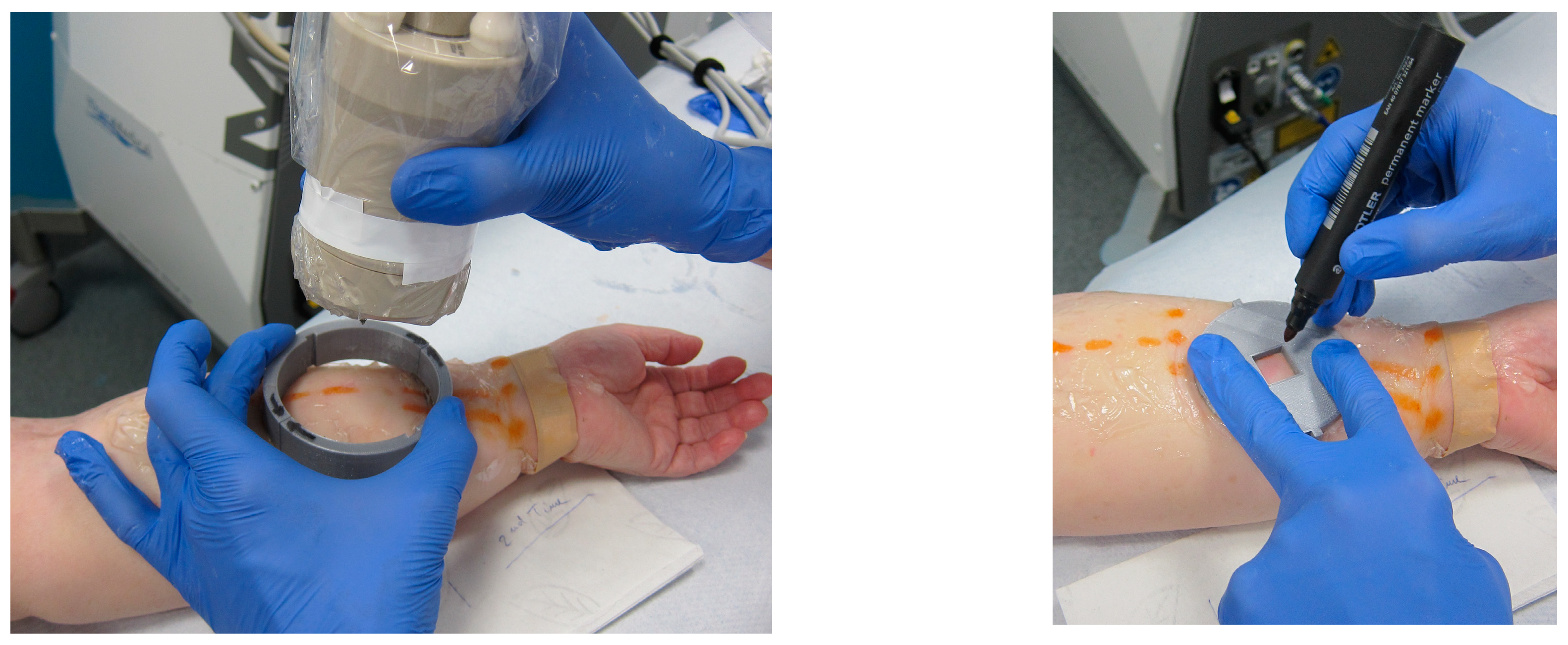
2.2. Study Protocol
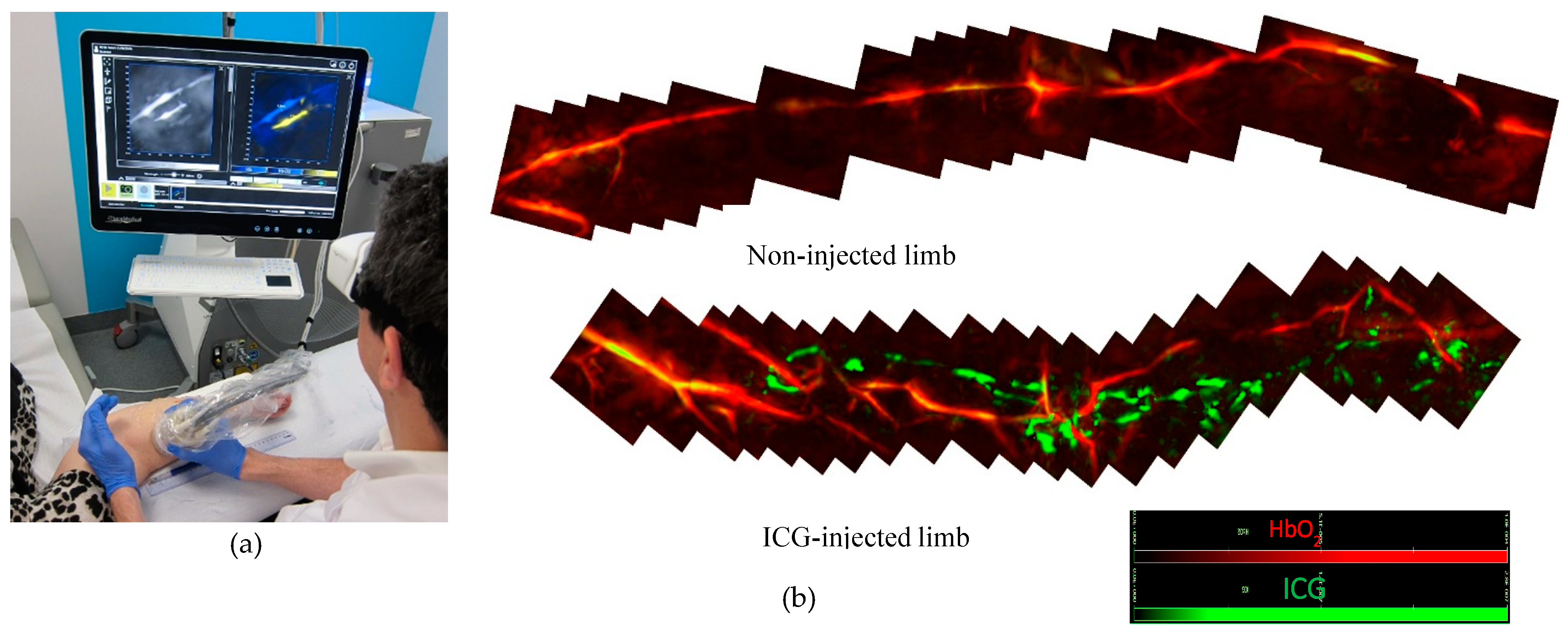
2.3. MSOT Image Acquisition and Data Analysis
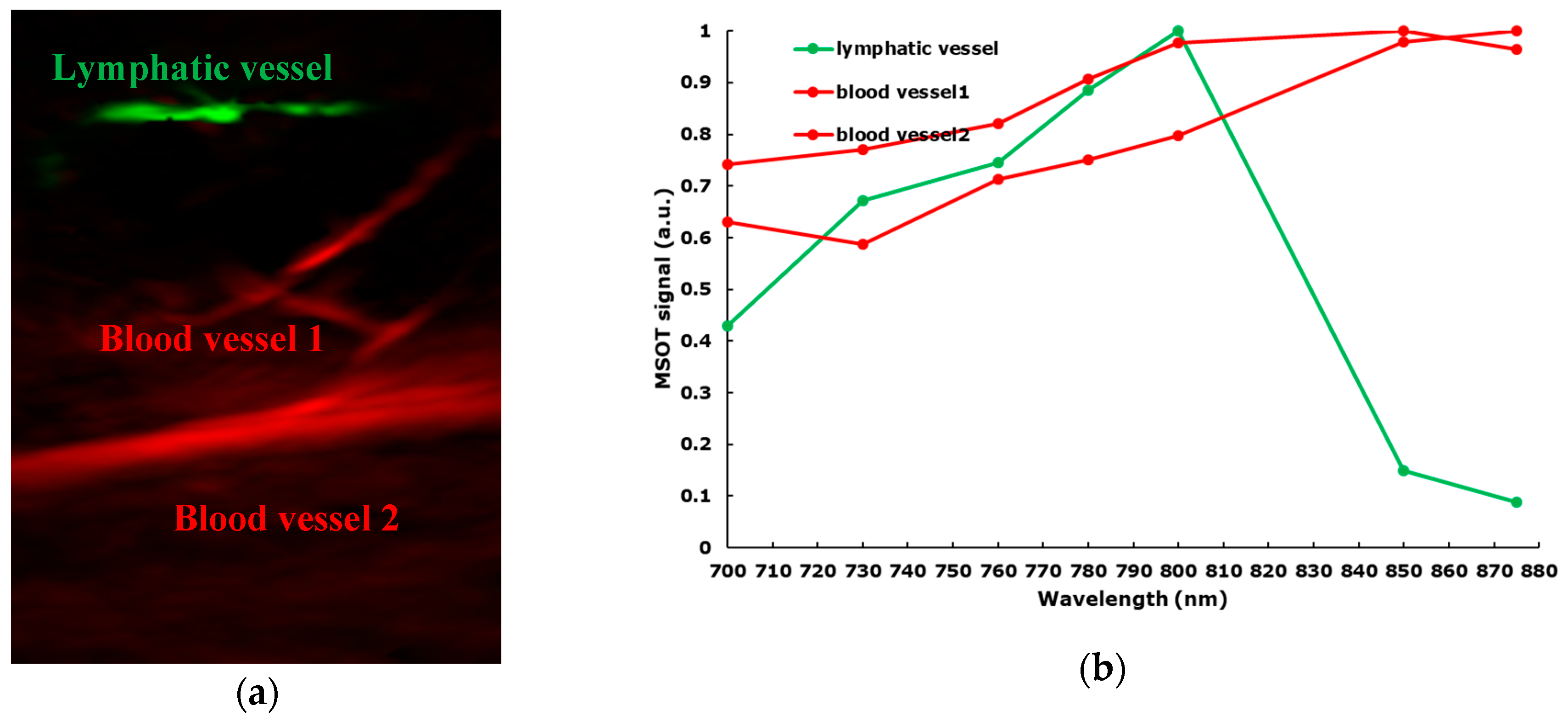
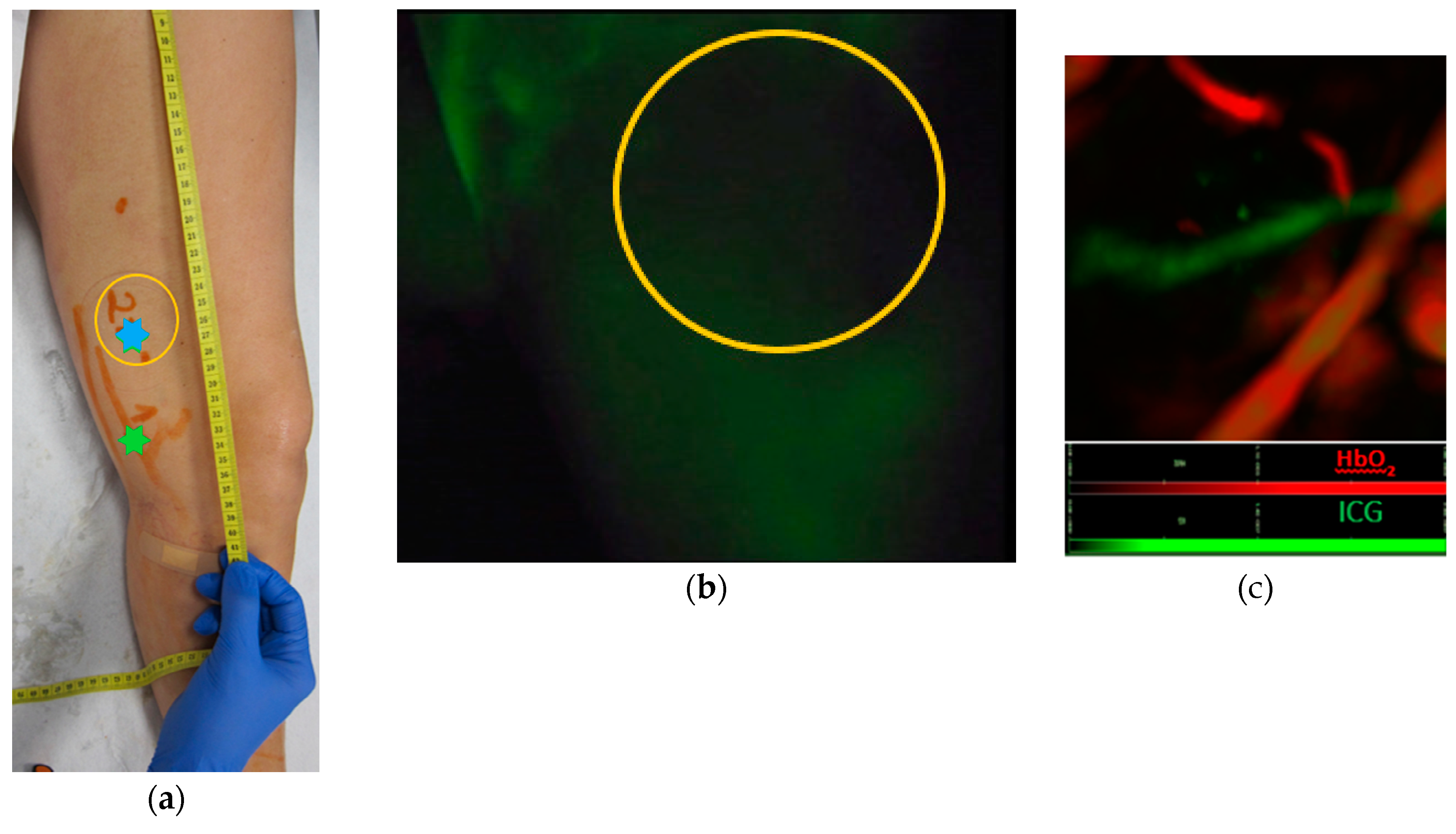
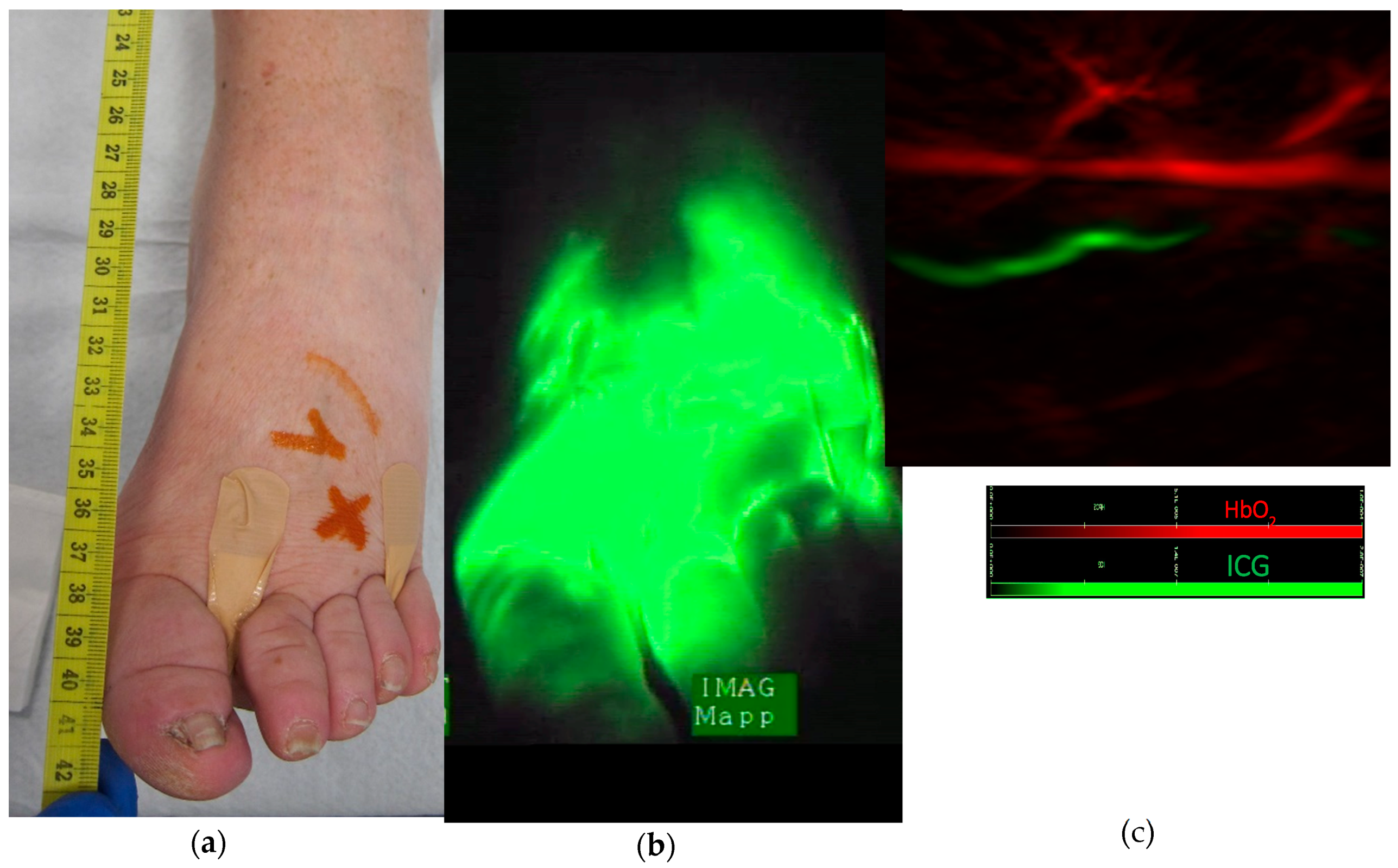
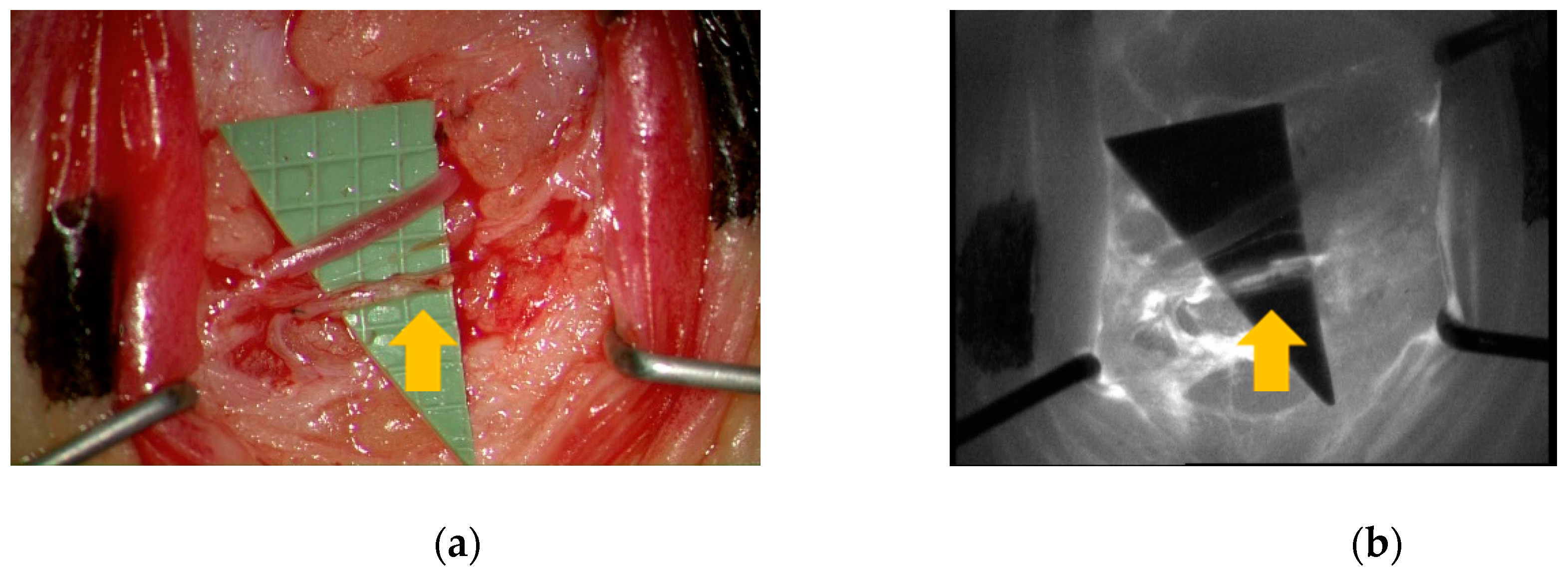
3. Results
4. Discussion
- MSOT differentiates between distinct types of vessels including lymphatics
- MSOT detects lymphatic vessels in areas of dermal backflow
- MSOT provides images in real-time with high spatiotemporal resolution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dori, Y. Novel Lymphatic Imaging Techniques. Tech. Vasc. Interv. Radiol. 2016, 19, 255–261. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Zhang, Y.; Jeon, M.; Liu, C.; Song, L.; Lovell, J.F.; Kim, C. Dual-color photoacoustic lymph node imaging using nanoformulated naphthalocyanines. Biomaterials 2015, 73, 142–148. [Google Scholar] [CrossRef]
- Zackrisson, S.; Van De Ven, S.M.W.Y.; Gambhir, S.S. Light in and sound out: Emerging translational strategies for photoacoustic imaging. Cancer Res. 2014, 74, 979–1004. [Google Scholar] [CrossRef]
- Taruttis, A.; Van Dam, G.M.; Ntziachristos, V. Mesoscopic and Macroscopic Optoacoustic Imaging of Cancer. Cancer Res. 2015, 75, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, I.; Morscher, S.; Helfrich, I.; Hillen, U.; Lehy, J.; Burton, N.C.; Sardella, T.C.P.; Claussen, J.; Poeppel, T.D.; Bachmann, H.S.; et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci. Transl. Med. 2015, 7, 317ra199. [Google Scholar] [CrossRef] [PubMed]
- Regensburger, A.P.; Fonteyne, L.M.; Jüngert, J.; Wagner, A.L.; Gerhalter, T.; Nagel, A.M.; Heiss, R.; Flenkenthaler, F.; Qurashi, M.; Neurath, M.F.; et al. Detection of collagens by multispectral optoacoustic tomography as an imaging biomarker for Duchenne muscular dystrophy. Nat. Med. 2019, 25, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Knieling, F.; Neufert, C.; Hartmann, A.; Claussen, J.; Urich, A.; Egger, C.; Vetter, M.; Fischer, S.; Pfeifer, L.; Hagel, A.; et al. Multispectral Optoacoustic Tomography for Assessment of Crohn’s Disease Activity. N. Engl. J. Med. 2017, 376, 1292–1294. [Google Scholar] [CrossRef]
- Kajita, H.; Kishi, K. High-Resolution Imaging of Lymphatic Vessels with Photoacoustic Lymphangiography. Radiology 2019, 292, 35. [Google Scholar] [CrossRef]
- Nagae, K.; Asao, Y.; Sudo, Y.; Murayama, N.; Tanaka, Y.; Ohira, K.; Ishida, Y.; Otsuka, A.; Matsumoto, Y.; Saito, S.; et al. Real-time 3D Photoacoustic Visualization System with a Wide Field of View for Imaging Human Limbs. F1000Research 2018, 7, 1813. [Google Scholar] [CrossRef]
- Taruttis, A.; Timmermans, A.C.; Wouters, P.C.; Kacprowicz, M.; Van Dam, G.M.; Ntziachristos, V. Optoacoustic Imaging of Human Vasculature: Feasibility by Using a Handheld Probe. Radiology 2016, 281, 256–263. [Google Scholar] [CrossRef]
- Masthoff, M.; Helfen, A.; Claussen, J.; Karlas, A.; Markwardt, N.A.; Ntziachristos, V.; Eisenblätter, M.; Wildgruber, M. Use of Multispectral Optoacoustic Tomography to Diagnose Vascular Malformations. JAMA Dermatol. 2018, 154, 1457. [Google Scholar] [CrossRef] [PubMed]
- Campisi, C.; Bellini, C.; Campisi, C.; Accogli, S.; Bonioli, E.; Boccardo, F. Microsurgery for lymphedema: Clinical research and long-term results. Microsurgery 2010, 30. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Narushima, M.; Doi, K.; Oshima, A.; Ogata, F.; Mihara, M.; Koshima, I.; Mundinger, G.S. Characteristic Indocyanine Green Lymphography Findings in Lower Extremity Lymphedema: The Generation of a Novel Lymphedema Severity Staging System Using Dermal Backflow Patterns. Plast. Reconstr. Surg. 2011, 127, 1979–1986. [Google Scholar] [CrossRef]
- Becker, A.; Masthoff, M.; Claussen, J.; Ford, S.J.; Roll, W.; Burg, M.; Heindel, W.; Eisenblätter, M.; Wildgruber, M.; Barth, P.J.; et al. Multispectral optoacoustic tomography of the human breast: Characterisation of healthy tissue and malignant lesions using a hybrid ultrasound-optoacoustic approach. Eur. Radiol. 2017, 28, 602–609. [Google Scholar] [CrossRef]
- Ford, S.J.; Bigliardi, P.L.; Sardella, T.C.; Urich, A.; Burton, N.C.; Kacprowicz, M.; Bigliardi, M.; Olivo, M.; Razansky, D. Structural and Functional Analysis of Intact Hair Follicles and Pilosebaceous Units by Volumetric Multispectral Optoacoustic Tomography. J. Investig. Dermatol. 2016, 136, 753–761. [Google Scholar] [CrossRef]
- Hayashi, A.; Giacalone, G.; Yamamoto, T.; Belva, F.; Visconti, G.; Hayashi, N.; Handa, M.; Yoshimatsu, H.; Salgarello, M. Ultra High-frequency Ultrasonographic Imaging with 70 MHz Scanner for Visualization of the Lymphatic Vessels. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2086. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, N.; Yoshimatsu, H.; Narushima, M.; Koshima, I. Factors Associated with Lymphosclerosis. Plast. Reconstr. Surg. 2017, 140, 734–741. [Google Scholar] [CrossRef]
- Diot, G.; Liapis, E.; Ntziachristos, V.; Metz, S.; Noske, A.; Schroeder, B.; Ovsepian, S.V.; Meier, R.; Rummeny, E. Multispectral Optoacoustic Tomography (MSOT) of Human Breast Cancer. Clin. Cancer Res. 2017, 23, 6912–6922. [Google Scholar] [CrossRef]
- McNally, L.; Mezera, M.; Morgan, D.E.; Frederick, P.J.; Yang, E.S.; Eltoum, I.-E.; Grizzle, W.E. Current and Emerging Clinical Applications of Multispectral Optoacoustic Tomography (MSOT) in Oncology. Clin. Cancer Res. 2016, 22, 3432–3439. [Google Scholar] [CrossRef]
- Helfen, A.; Masthoff, M.; Claussen, J.; Gerwing, M.; Heindel, W.; Ntziachristos, V.; Eisenblätter, M.; Köhler, M.; Wildgruber, M. Multispectral Optoacoustic Tomography: Intra- and Interobserver Variability Using a Clinical Hybrid Approach. J. Clin. Med. 2019, 8, 63. [Google Scholar] [CrossRef]
- Kajita, H.; Oh, A.; Urano, M.; Takemaru, M.; Imanishi, N.; Otaki, M.; Yagi, T.; Aiso, S.; Kishi, K. Photoacoustic lymphangiography. J. Surg. Oncol. 2019, 121, 48–50. [Google Scholar] [CrossRef] [PubMed]
| Patient Number | Gender | Age | Primary or Secondary Lymphedema | Prior Cancer Treatment | Localization of Lymphedema | Lymphedema Staging | ICG Patterns on Lymphography | MSOT Detection of Lymphatic Vessels | MSOT Detection of Veins |
|---|---|---|---|---|---|---|---|---|---|
| 1 * | F | 63 | Secondary | Breast | Left arm | 4 | L/SD/D | Yes | Yes |
| 2 | F | 72 | Secondary | Breast | Right arm | 4 | D | No | Yes |
| 3 | M | 66 | Secondary | Prostate | Left leg | 3 | L/SD | No (hair) | Yes |
| 4 | F | 49 | Secondary | Cervix | Left leg | 3 | L/SD | Yes | Yes |
| 5 | f | 74 | Secondary | Breast | Right arm | 4 | L/D | Yes | Yes |
| 6 | M | 63 | Secondary | Prostate | Left leg | 3 | L/SD/D | Yes | Yes |
| 7 | M | 73 | Primary | Left leg | 3 | D # | Yes | Yes | |
| 8 | F | 43 | Secondary | Cervix | Right leg | 4 | D | No | Yes |
| 9 | F | 72 | Secondary | Endometrial | Left leg | 4 | D | Yes | Yes |
| 10 | F | 54 | Secondary | Cervix | Left leg | 4 | L/SD/D | Yes | Yes |
| 11 | F | 60 | Primary | Left leg | 4 | D # | Yes | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacalone, G.; Yamamoto, T.; Belva, F.; Hayashi, A. Bedside 3D Visualization of Lymphatic Vessels with a Handheld Multispectral Optoacoustic Tomography Device. J. Clin. Med. 2020, 9, 815. https://doi.org/10.3390/jcm9030815
Giacalone G, Yamamoto T, Belva F, Hayashi A. Bedside 3D Visualization of Lymphatic Vessels with a Handheld Multispectral Optoacoustic Tomography Device. Journal of Clinical Medicine. 2020; 9(3):815. https://doi.org/10.3390/jcm9030815
Chicago/Turabian StyleGiacalone, Guido, Takumi Yamamoto, Florence Belva, and Akitatsu Hayashi. 2020. "Bedside 3D Visualization of Lymphatic Vessels with a Handheld Multispectral Optoacoustic Tomography Device" Journal of Clinical Medicine 9, no. 3: 815. https://doi.org/10.3390/jcm9030815
APA StyleGiacalone, G., Yamamoto, T., Belva, F., & Hayashi, A. (2020). Bedside 3D Visualization of Lymphatic Vessels with a Handheld Multispectral Optoacoustic Tomography Device. Journal of Clinical Medicine, 9(3), 815. https://doi.org/10.3390/jcm9030815






