Male Infertility: Shining a Light on Lipids and Lipid-Modulating Enzymes in the Male Germline
Abstract
1. Introduction
2. Physiological and Pathophysiological Roles of Lipids
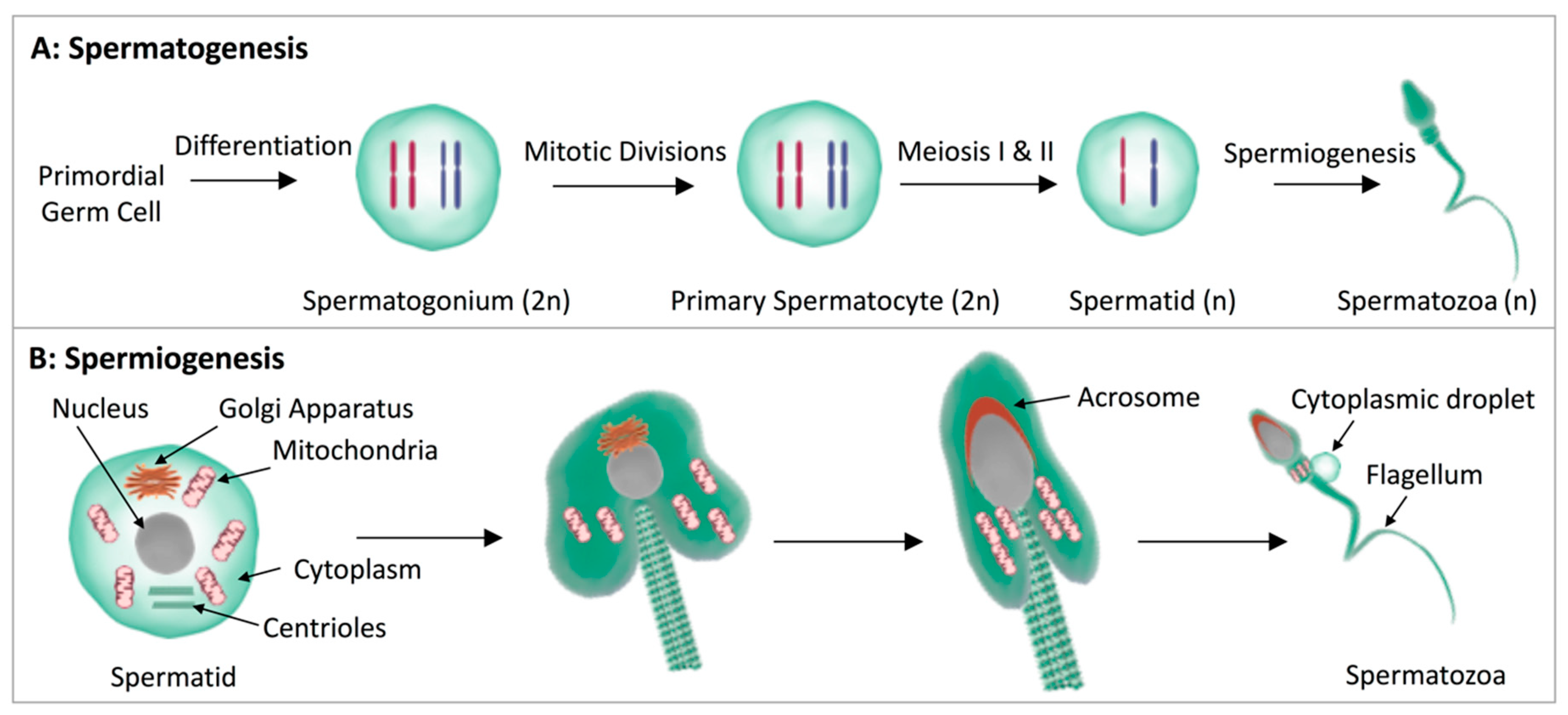
3. The Changing Profile of Lipids during Sperm Maturation
4. The Role of Lipids and Lipid-Associated Proteins in Spermatozoa and Infertility
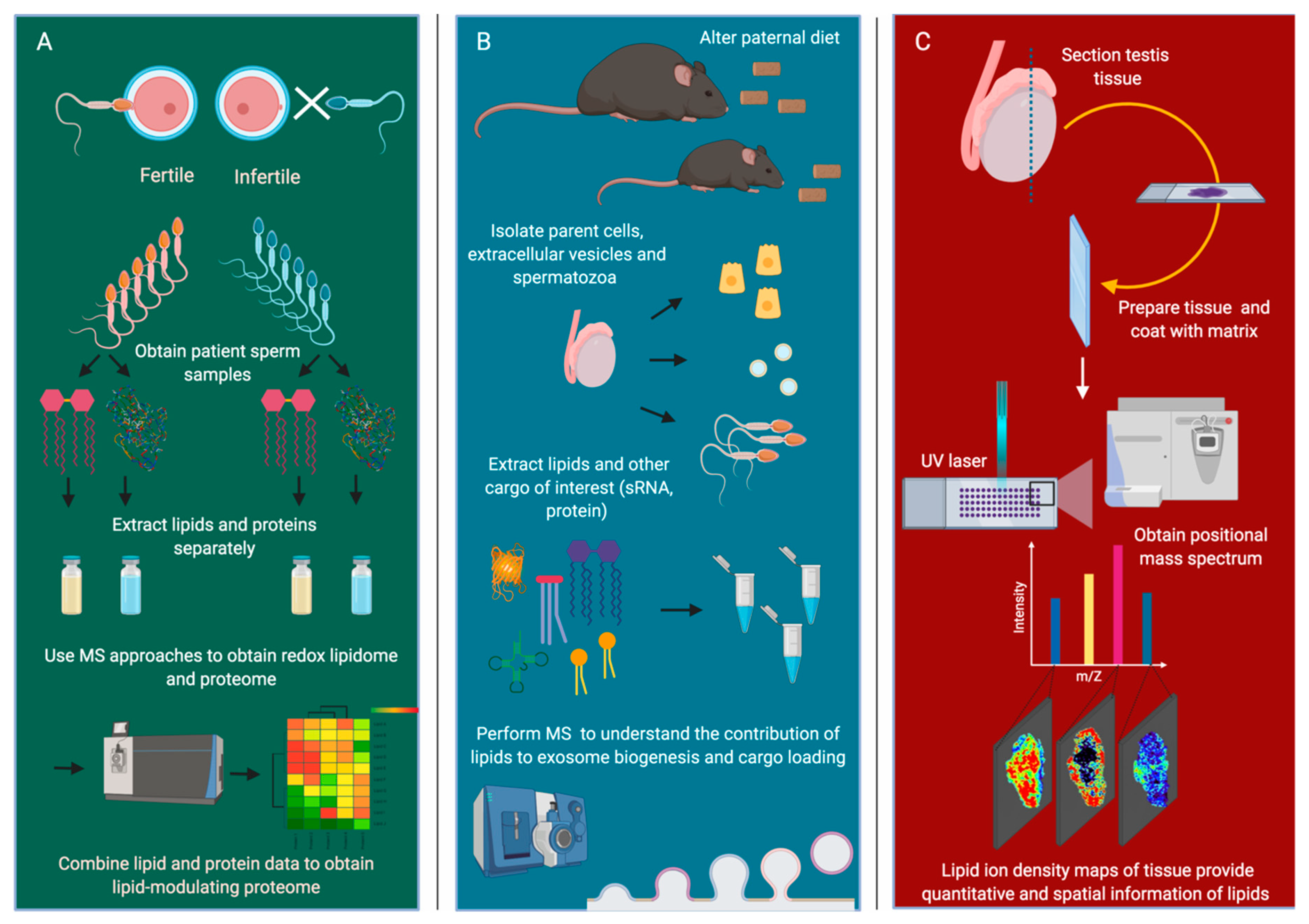
5. Analytical Lipid Technologies and Their Potential Application to Infertility Research
6. Redox Lipidomics, Lipid-Targeted Antioxidants, and Male Infertility
7. The Involvement of Lipids in the Biogenesis and Cargo Loading of Extracellular Vesicles
8. Mass Spectrometry Imaging of Lipids and Potential Applications for Reproductive Tissues
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oliva, A.; Spira, A.; Multigner, L. Contribution of environmental factors to the risk of male infertility. Hum. Reprod. 2001, 16, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369. [Google Scholar] [CrossRef] [PubMed]
- Djaladat, H.; Burner, E.; Parikh, P.M.; Beroukhim Kay, D.; Hays, K. The association between testis cancer and semen abnormalities before orchiectomy: A systematic review. J. Adolesc. Young Adult Oncol. 2014, 3, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef]
- Punab, M.; Poolamets, O.; Paju, P.; Vihljajev, V.; Pomm, K.; Ladva, R.; Korrovits, P.; Laan, M. Causes of male infertility: A 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum. Reprod. 2016, 32, 18–31. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Oxidative stress, sperm survival and fertility control. Mol. Cell. Endocrinol. 2006, 250, 66–69. [Google Scholar] [CrossRef]
- Bromfield, E.G.; Aitken, R.J.; Anderson, A.L.; McLaughlin, E.A.; Nixon, B. The impact of oxidative stress on chaperone-mediated human sperm–egg interaction. Hum. Reprod. 2015, 30, 2597–2613. [Google Scholar] [CrossRef]
- Walters, J.; De Iuliis, G.; Nixon, B.; Bromfield, E. Oxidative stress in the male germline: A review of novel strategies to reduce 4-hydroxynonenal production. Antioxidants 2018, 7, 132. [Google Scholar] [CrossRef]
- Kao, S.-H.; Chao, H.-T.; Chen, H.-W.; Hwang, T.I.; Liao, T.-L.; Wei, Y.-H. Increase of oxidative stress in human sperm with lower motility. Fertil. Steril. 2008, 89, 1183–1190. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A.; Nada, E.A.; El-Tonsy, M.H.; Sharma, R.K.; Meyer, A.; Nelson, D.R.; Thomas, A.J., Jr. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil. Steril. 2003, 79, 1597–1605. [Google Scholar] [CrossRef]
- Gharagozloo, P.; Aitken, R.J. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum. Reprod. 2011, 26, 1628–1640. [Google Scholar] [CrossRef]
- Fernandez, M.C.; O’Flaherty, C. Peroxiredoxin 6 is the primary antioxidant enzyme for the maintenance of viability and DNA integrity in human spermatozoa. Hum. Reprod. 2018, 33, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. Biomed. 2016, 14, 729. [Google Scholar] [CrossRef]
- Ross, C.; Morriss, A.; Khairy, M.; Khalaf, Y.; Braude, P.; Coomarasamy, A.; El-Toukhy, T. A systematic review of the effect of oral antioxidants on male infertility. Reprod. Biomed. Online 2010, 20, 711–723. [Google Scholar] [CrossRef]
- Majzoub, A.; Agarwal, A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef]
- Bernabò, N.; Agostino, R.D.; Ordinelli, A.; Mattioli, M.; Barboni, B. The maturation of murine spermatozoa membranes within the epididymis, a computational biology perspective. Syst. Biol. Reprod. Med. 2016, 62, 299–308. [Google Scholar] [CrossRef][Green Version]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Wymann, M.P.; Schneiter, R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008, 9, 162. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive lipids and chronic inflammation: Managing the fire within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The Role of Lipids in Parkinson’s Disease. Cells 2019, 8, 27. [Google Scholar] [CrossRef]
- Di Paolo, G.; Kim, T.-W. Linking lipids to Alzheimer’s disease: Cholesterol and beyond. Nat. Rev. Neurosci. 2011, 12, 284. [Google Scholar] [CrossRef]
- Singh, M.; Nam, D.T.; Arseneault, M.; Ramassamy, C. Role of by-products of lipid oxidation in Alzheimer’s disease brain: A focus on acrolein. J. Alzheimer’s Dis. 2010, 21, 741–756. [Google Scholar] [CrossRef]
- De Lau, L.; Bornebroek, M.; Witteman, J.; Hofman, A.; Koudstaal, P.J.; Breteler, M. Dietary fatty acids and the risk of Parkinson disease: The Rotterdam study. Neurology 2005, 64, 2040–2045. [Google Scholar] [CrossRef]
- Selley, M.L. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson’s disease. Free Radic. Biol. Med. 1998, 25, 169–174. [Google Scholar] [CrossRef]
- Anand, S.; Barnes, J.M.; Young, S.A.; Garcia, D.M.; Tolley, H.D.; Kauwe, J.S.; Graves, S.W. Discovery and confirmation of diagnostic serum lipid biomarkers for Alzheimer’s disease using direct infusion mass spectrometry. J. Alzheimer’s Dis. 2017, 59, 277–290. [Google Scholar]
- Proitsi, P.; Kim, M.; Whiley, L.; Simmons, A.; Sattlecker, M.; Velayudhan, L.; Lupton, M.K.; Soininen, H.; Kloszewska, I.; Mecocci, P. Association of blood lipids with Alzheimer’s disease: A comprehensive lipidomics analysis. Alzheimer’s Dement. 2017, 13, 140–151. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Vigor, C.; Galano, J.-M.; Oger, C.; Durand, T.; Ferrer, I.; Cuevas, A.; Lopez-Cuevas, R.; Baquero, M.; Lopez-Nogueroles, M. Plasma lipid peroxidation biomarkers for early and non-invasive Alzheimer disease detection. Free Radic. Biol. Med. 2018, 124, 388–394. [Google Scholar] [CrossRef]
- Telles, S.; Pal, S.; Sharma, S.K.; Singh, A.; Kala, N.; Balkrishna, A. The association between the lipid profile and fasting blood glucose with weight related outcomes in healthy obese adults. BMC Res. Notes 2018, 11, 383. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Cuervo, M.; Goni, L.; Martinez, J. Prediction of Blood Lipid Phenotypes Using Obesity-Related Genetic Polymorphisms and Lifestyle Data in Subjects with Excessive Body Weight. Int. J. Genom. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Orozco-Beltran, D.; Gil-Guillen, V.F.; Redon, J.; Martin-Moreno, J.M.; Pallares-Carratala, V.; Navarro-Perez, J.; Valls-Roca, F.; Sanchis-Domenech, C.; Fernandez-Gimenez, A.; Perez-Navarro, A. Lipid profile, cardiovascular disease and mortality in a Mediterranean high-risk population: The ESCARVAL-RISK study. PLoS ONE 2017, 12, e0186196. [Google Scholar] [CrossRef]
- Chait, A.; Eckel, R.H. Lipids, lipoproteins, and cardiovascular disease: Clinical pharmacology now and in the future. J. Clin. Endocrinol. Metab. 2016, 101, 804–814. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Wigger, L.; Cruciani-Guglielmacci, C.; Nicolas, A.; Denom, J.; Fernandez, N.; Fumeron, F.; Marques-Vidal, P.; Ktorza, A.; Kramer, W.; Schulte, A. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep. 2017, 18, 2269–2279. [Google Scholar] [CrossRef]
- Perrotti, F.; Rosa, C.; Cicalini, I.; Sacchetta, P.; Del Boccio, P.; Genovesi, D.; Pieragostino, D. Advances in lipidomics for cancer biomarkers discovery. Int. J. Mol. Sci. 2016, 17, 1992. [Google Scholar] [CrossRef]
- Long, J.; Zhang, C.-J.; Zhu, N.; Du, K.; Yin, Y.-F.; Tan, X.; Liao, D.-F.; Qin, L. Lipid metabolism and carcinogenesis, cancer development. Am. J. Cancer Res. 2018, 8, 778. [Google Scholar]
- Sunshine, H.; Iruela-Arispe, M.L. Membrane lipids and cell signaling. Curr. Opin. Lipidol. 2017, 28, 408. [Google Scholar] [CrossRef]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Fadeel, B.; Xue, D. The ins and outs of phospholipid asymmetry in the plasma membrane: Roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 264–277. [Google Scholar] [CrossRef]
- Futerman, A.H.; Hannun, Y.A. The complex life of simple sphingolipids. EMBO Rep. 2004, 5, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Bernardino de la Serna, J.; Schütz, G.J.; Eggeling, C.; Cebecauer, M. There is no simple model of the plasma membrane organization. Front. Cell Dev. Biol. 2016, 4, 106. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; de Kroon, A.I. Lipid map of the mammalian cell. J. Cell Sci. 2011, 124, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Yang, H.C.; Rosenberger, T.A.; Horrocks, L.A. Phospholipase A2 and its role in brain tissue. J. Neurochem. 1997, 69, 889–901. [Google Scholar] [CrossRef]
- Brown, W.J.; Chambers, K.; Doody, A. Phospholipase A2 (PLA2) enzymes in membrane trafficking: Mediators of membrane shape and function. Traffic 2003, 4, 214–221. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82, 969–974. [Google Scholar] [CrossRef]
- Ingold, K.U.; Pratt, D.A. Advances in radical-trapping antioxidant chemistry in the 21st century: A kinetics and mechanisms perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef]
- Brash, A.R. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Stoyanovsky, D.; Tyurina, Y.; Shrivastava, I.; Bahar, I.; Tyurin, V.; Protchenko, O.; Jadhav, S.; Bolevich, S.; Kozlov, A.; Vladimirov, Y. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free Radic. Biol. Med. 2019, 133, 153–161. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Grocin, A.G.; da Silva, T.N.X.; Panzilius, E.; Scheel, C. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 2019, 1. [Google Scholar] [CrossRef]
- Masaldan, S.; Bush, A.I.; Devos, D.; Rolland, A.S.; Moreau, C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic. Biol. Med. 2019, 133, 221–233. [Google Scholar] [CrossRef]
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017, 12, 8–17. [Google Scholar] [CrossRef]
- Weiland, A.; Wang, Y.; Wu, W.; Lan, X.; Han, X.; Li, Q.; Wang, J. Ferroptosis and its role in diverse brain diseases. Mol. Neurobiol. 2019, 56, 4880–4893. [Google Scholar] [CrossRef]
- Li, Y.; Feng, D.; Wang, Z.; Zhao, Y.; Sun, R.; Tian, D.; Liu, D.; Zhang, F.; Ning, S.; Yao, J. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019, 1, 2284–2299. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180. [Google Scholar] [CrossRef]
- Zille, M.; Karuppagounder, S.S.; Chen, Y.; Gough, P.J.; Bertin, J.; Finger, J.; Milner, T.A.; Jonas, E.A.; Ratan, R.R. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke 2017, 48, 1033–1043. [Google Scholar] [CrossRef]
- Tuo, Q.; Lei, P.; Jackman, K.; Li, X.; Xiong, H.; Liuyang, Z.; Roisman, L.; Zhang, S.; Ayton, S.; Wang, Q. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatry 2017, 22, 1520. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef]
- Chen, L.; Hambright, W.S.; Na, R.; Ran, Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J. Biol. Chem. 2015, 290, 28097–28106. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Baba, Y.; Higa, J.K.; Shimada, B.K.; Horiuchi, K.M.; Suhara, T.; Kobayashi, M.; Woo, J.D.; Aoyagi, H.; Marh, K.S.; Kitaoka, H. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2017, 314, H659–H668. [Google Scholar] [CrossRef]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell. Mol. Med. 2019, 23, 4900–4912. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, L.; Zhang, P.; Luo, M.; Du, J.; Gao, T.; O’Connell, D.; Wang, G.; Wang, H.; Yang, Y. miR-9 regulates ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in melanoma. Mol. Carcinog. 2018, 57, 1566–1576. [Google Scholar] [CrossRef]
- Magtanong, L.; Ko, P.; Dixon, S. Emerging roles for lipids in non-apoptotic cell death. Cell Death Differ. 2016, 23, 1099. [Google Scholar] [CrossRef]
- Parisi, L.R.; Morrow, L.M.; Visser, M.B.; Atilla-Gokcumen, G.E. Turning the spotlight on lipids in non-apoptotic cell death. ACS Chem. Biol. 2018, 13, 506–515. [Google Scholar] [CrossRef]
- Huang, C.; Freter, C. Lipid metabolism, apoptosis and cancer therapy. Int. J. Mol. Sci. 2015, 16, 924–949. [Google Scholar] [CrossRef]
- Schaefer, E.J.; Bongard, V.; Beiser, A.S.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.L.; Kyle, D.J.; Wilson, P.W.; Wolf, P.A. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: The Framingham Heart Study. Arch. Neurol. 2006, 63, 1545–1550. [Google Scholar] [CrossRef]
- Maciejewska, D.; Drozd, A.; Ossowski, P.; Ryterska, K.; Jamioł-Milc, D.; Banaszczak, M.; Raszeja-Wyszomirska, J.; Kaczorowska, M.; Sabinicz, A.; Stachowska, E. Fatty acid changes help to better understand regression of nonalcoholic fatty liver disease. World J. Gastroenterol. WJG 2015, 21, 301. [Google Scholar] [CrossRef]
- Wilson, D.P.; Jacobson, T.A.; Jones, P.H.; Koschinsky, M.L.; McNeal, C.J.; Nordestgaard, B.G.; Orringer, C.E. Use of lipoprotein (a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. Don P. Wilson, MD, on behalf of the Writing group. J. Clin. Lipidol. 2019, 13, 374–392. [Google Scholar] [CrossRef]
- Tsimikas, S. A test in context: Lipoprotein (a): Diagnosis, prognosis, controversies, and emerging therapies. J. Am. Coll. Cardiol. 2017, 69, 692–711. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Langsted, A. Lipoprotein (a) as a cause of cardiovascular disease: Insights from epidemiology, genetics, and biology. J. Lipid Res. 2016, 57, 1953–1975. [Google Scholar] [CrossRef]
- Zarrouk, A.; Debbabi, M.; Bezine, M.; Karym, E.M.; Badreddine, A.; Rouaud, O.; Moreau, T.; Cherkaoui-Malki, M.; El Ayeb, M.; Nasser, B. Lipid biomarkers in Alzheimer’s disease. Curr. Alzheimer Res. 2018, 15, 303–312. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Cheng, X.-l.; Lin, R.-C. Lipidomics applications for discovering biomarkers of diseases in clinical chemistry. In International Review of Cell and Molecular Biology; Academic Press: New York, NY, USA, 2014; pp. 1–26. [Google Scholar]
- Yan, F.; Zhao, H.; Zeng, Y. Lipidomics: A promising cancer biomarker. Clin. Transl. Med. 2018, 7, 21. [Google Scholar] [CrossRef]
- Beaudoin-Chabot, C.; Wang, L.; Smarun, A.V.; Vidović, D.; Shchepinov, M.S.; Thibault, G. Deuterated polyunsaturated fatty acids reduce oxidative stress and extend the lifespan of C. elegans. Front. Physiol. 2019, 10, 641. [Google Scholar] [CrossRef]
- Elharram, A.; Czegledy, N.M.; Golod, M.; Milne, G.L.; Pollock, E.; Bennett, B.M.; Shchepinov, M.S. Deuterium-reinforced polyunsaturated fatty acids improve cognition in a mouse model of sporadic Alzheimer’s disease. FEBS J. 2017, 284, 4083–4095. [Google Scholar] [CrossRef]
- Hatami, A.; Zhu, C.; Relaño-Gines, A.; Elias, C.; Galstyan, A.; Jun, M.; Milne, G.; Cantor, C.R.; Chesselet, M.F.; Shchepinov, M.S. Deuterium-reinforced linoleic acid lowers lipid peroxidation and mitigates cognitive impairment in the Q140 knock in mouse model of Huntington’s disease. FEBS J. 2018, 285, 3002–3012. [Google Scholar] [CrossRef]
- Raefsky, S.M.; Furman, R.; Milne, G.; Pollock, E.; Axelsen, P.; Mattson, M.P.; Shchepinov, M.S. Deuterated polyunsaturated fatty acids reduce brain lipid peroxidation and hippocampal amyloid β-peptide levels, without discernable behavioral effects in an APP/PS1 mutant transgenic mouse model of Alzheimer’s disease. Neurobiol. Aging 2018, 66, 165–176. [Google Scholar] [CrossRef]
- Jensen, T.K.; Andersson, A.-M.; Jørgensen, N.; Andersen, A.-G.; Carlsen, E.; Skakkebæk, N.E. Body mass index in relation to semen quality and reproductive hormonesamong 1,558 Danish men. Fertil. Steril. 2004, 82, 863–870. [Google Scholar] [CrossRef]
- Jensen, T.K.; Jacobsen, R.; Christensen, K.; Nielsen, N.C.; Bostofte, E. Good semen quality and life expectancy: A cohort study of 43,277 men. Am. J. Epidemiol. 2009, 170, 559–565. [Google Scholar] [CrossRef]
- Do Van, B.; Gouel, F.; Jonneaux, A.; Timmerman, K.; Gelé, P.; Pétrault, M.; Bastide, M.; Laloux, C.; Moreau, C.; Bordet, R. Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol. Dis. 2016, 94, 169–178. [Google Scholar] [CrossRef]
- Yoshida, M.; Minagawa, S.; Araya, J.; Sakamoto, T.; Hara, H.; Tsubouchi, K.; Hosaka, Y.; Ichikawa, A.; Saito, N.; Kadota, T. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat. Commun. 2019, 10, 3145. [Google Scholar] [CrossRef]
- Li, X.; Duan, L.; Yuan, S.; Zhuang, X.; Qiao, T.; He, J. Ferroptosis inhibitor alleviates Radiation-induced lung fibrosis (RILF) via down-regulation of TGF-β1. J. Inflamm. 2019, 16, 11. [Google Scholar] [CrossRef]
- Ganna, A.; Salihovic, S.; Sundström, J.; Broeckling, C.D.; Hedman, Å.K.; Magnusson, P.K.; Pedersen, N.L.; Larsson, A.; Siegbahn, A.; Zilmer, M. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS Genet. 2014, 10, e1004801. [Google Scholar] [CrossRef]
- Meikle, P.J.; Wong, G.; Tsorotes, D.; Barlow, C.K.; Weir, J.M.; Christopher, M.J.; MacIntosh, G.L.; Goudey, B.; Stern, L.; Kowalczyk, A. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2723–2732. [Google Scholar] [CrossRef]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef]
- Yang, M.H.; Rampal, S.; Sung, J.; Choi, Y.-H.; Son, H.J.; Lee, J.H.; Kim, Y.H.; Chang, D.K.; Rhee, P.-L.; Kim, J.J. The association of serum lipids with colorectal adenomas. Am. J. Gastroenterol. 2013, 108, 833. [Google Scholar] [CrossRef]
- Lv, J.; Gao, D.; Zhang, Y.; Wu, D.; Shen, L.; Wang, X. Heterogeneity of lipidomic profiles among lung cancer subtypes of patients. J. Cell. Mol. Med. 2018, 22, 5155–5159. [Google Scholar] [CrossRef]
- Shen, S.; Yang, L.; Li, L.; Bai, Y.; Cai, C.; Liu, H. A plasma lipidomics strategy reveals perturbed lipid metabolic pathways and potential lipid biomarkers of human colorectal cancer. J. Chromatogr. B 2017, 1068, 41–48. [Google Scholar] [CrossRef]
- Zhou, X.; Mao, J.; Ai, J.; Deng, Y.; Roth, M.R.; Pound, C.; Henegar, J.; Welti, R.; Bigler, S.A. Identification of plasma lipid biomarkers for prostate cancer by lipidomics and bioinformatics. PLoS ONE 2012, 7, e48889. [Google Scholar] [CrossRef]
- Ma, S.; Henson, E.; Chen, Y.; Gibson, S. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016, 7, e2307. [Google Scholar] [CrossRef]
- Probst, L.; Dächert, J.; Schenk, B.; Fulda, S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem. Pharmacol. 2017, 140, 41–52. [Google Scholar] [CrossRef]
- Nishimura, H.; L’Hernault, S.W. Spermatogenesis. Curr. Biol. 2017, 27, R988–R994. [Google Scholar] [CrossRef]
- Orgebin-Crist, M. Sperm maturation in rabbit epididymis. Nature 1967, 216, 816. [Google Scholar] [CrossRef]
- Cooper, T.G.; Yeung, C.H. Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microsc. Res. Tech. 2003, 61, 28–38. [Google Scholar] [CrossRef]
- Bedford, J.M. The epididymis re-visited: A personal view. Asian J. Androl. 2015, 17, 693. [Google Scholar] [CrossRef]
- Ickowicz, D.; Finkelstein, M.; Breitbart, H. Mechanism of sperm capacitation and the acrosome reaction: Role of protein kinases. Asian J. Androl. 2012, 14, 816. [Google Scholar] [CrossRef]
- Aitken, R.J.; Nixon, B. Sperm capacitation: A distant landscape glimpsed but unexplored. Mol. Hum. Reprod. 2013, 19, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Oresti, G.M.; Reyes, J.G.; Luquez, J.M.; Osses, N.; Furland, N.E.; Aveldaño, M.I. Differentiation-related changes in lipid classes with long-chain and very long-chain polyenoic fatty acids in rat spermatogenic cells. J. Lipid Res. 2010, 51, 2909–2921. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.K.; Gray, M.E.; Coniglio, J.G. The lipid composition of isolated rat spermatids and spermatocytes. Biochim. Biophys. Acta BBA Lipids Lipid Metab. 1978, 530, 367–374. [Google Scholar] [CrossRef]
- Roqueta-Rivera, M.; Stroud, C.K.; Haschek, W.M.; Akare, S.J.; Segre, M.; Brush, R.S.; Agbaga, M.-P.; Anderson, R.E.; Hess, R.A.; Nakamura, M.T. Docosahexaenoic acid supplementation fully restores fertility and spermatogenesis in male delta-6 desaturase-null mice. J. Lipid Res. 2010, 51, 360–367. [Google Scholar] [CrossRef]
- Kim, N.; Nakamura, H.; Masaki, H.; Kumasawa, K.; Hirano, K.-i.; Kimura, T. Effect of lipid metabolism on male fertility. Biochem. Biophys. Res. Commun. 2017, 485, 686–692. [Google Scholar] [CrossRef]
- Wang, B.; Charukeshi Chandrasekera, P.; Pippin, J. Leptin-and leptin receptor-deficient rodent models: Relevance for human type 2 diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef]
- Steger, K. Transcriptional and translational regulation of gene expression in haploid spermatids. Anat. Embryol. 1999, 199, 471–487. [Google Scholar] [CrossRef]
- Evans, R.; Setchell, B. Lipid changes during epididymal maturation in ram spermatozoa collected at different times of the year. Reproduction 1979, 57, 197–203. [Google Scholar] [CrossRef]
- Parks, J.E.; Hammerstedt, R.H. Developmental changes occurring in the lipids of ram epididymal spermatozoa plasma membrane. Biol. Reprod. 1985, 32, 653–668. [Google Scholar] [CrossRef]
- Angrimani, D.S.R.; Nichi, M.; Losano, J.D.A.; Lucio, C.F.; Veiga, G.A.L.; Franco, M.V.J.; Vannucchi, C.I. Fatty acid content in epididymal fluid and spermatozoa during sperm maturation in dogs. J. Oanimal Sci. Biotechnol. 2017, 8, 18. [Google Scholar] [CrossRef]
- Sullivan, R.; Saez, F. Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction 2013, 146, R21–R35. [Google Scholar] [CrossRef]
- Nixon, B.; De Iuliis, G.N.; Hart, H.M.; Zhou, W.; Mathe, A.; Bernstein, I.R.; Anderson, A.L.; Stanger, S.J.; Skerrett-Byrne, D.A.; Jamaluddin, M.F.B. Proteomic profiling of mouse epididymosomes reveals their contributions to post-testicular sperm maturation. Mol. Cell. Proteom. 2019, 18, S91–S108. [Google Scholar] [CrossRef]
- Trigg, N.A.; Eamens, A.L.; Nixon, B. The contribution of epididymosomes to the sperm small RNA profile. Reproduction 2019, 1, R209–R223. [Google Scholar] [CrossRef]
- Zhou, W.; Stanger, S.J.; Anderson, A.L.; Bernstein, I.R.; De Iuliis, G.N.; McCluskey, A.; McLaughlin, E.A.; Dun, M.D.; Nixon, B. Mechanisms of tethering and cargo transfer during epididymosome-sperm interactions. BMC Biol. 2019, 17, 35. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Rejraji, H.; Sion, B.; Prensier, G.; Carreras, M.; Motta, C.; Frenoux, J.-M.; Vericel, E.; Grizard, G.; Vernet, P.; Drevet, J.R. Lipid remodeling of murine epididymosomes and spermatozoa during epididymal maturation. Biol. Reprod. 2006, 74, 1104–1113. [Google Scholar] [CrossRef]
- Bernecic, N.; Zhang, M.; Gadella, B.; Brouwers, J.; Jansen, J.; Arkesteijn, G.; de Graaf, S.; Leahy, T. BODIPY-cholesterol can be reliably used to monitor cholesterol efflux from capacitating mammalian spermatozoa. Sci. Rep. 2019, 9, 9804. [Google Scholar] [CrossRef]
- Osheroff, J.E.; Visconti, P.E.; Valenzuela, J.P.; Travis, A.J.; Alvarez, J.; Kopf, G.S. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol. Hum. Reprod. 1999, 5, 1017–1026. [Google Scholar] [CrossRef]
- Hamdi, S.M.; Vieitez, G.; Jaspard, B.; Barbaras, R.; Perret, B.; Mieusset, R.; Parinaud, J.; Collet, X. Effects of human follicular fluid and high-density lipoproteins on early spermatozoa hyperactivation and cholesterol efflux. J. Lipid Res. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Harrison, R.; Ashworth, P.; Miller, N. Bicarbonate/CO2, an effector of capacitation, induces a rapid and reversible change in the lipid architecture of boar sperm plasma membranes. Mol. Reprod. Dev. Inc. Gamete Res. 1996, 45, 378–391. [Google Scholar] [CrossRef]
- Leahy, T.; Gadella, B.M. New insights into the regulation of cholesterol efflux from the sperm membrane. Asian J. Androl. 2015, 17, 561. [Google Scholar]
- Leemans, B.; Stout, T.A.E.; De Schauwer, C.; Heras, S.; Nelis, H.; Hoogewijs, M.; Van Soom, A.; Gadella, B.M. Update on mammalian sperm capacitation: How much does the horse differ from other species? Reproduction 2019, 1, R181–R197. [Google Scholar] [CrossRef]
- Boerke, A.; Brouwers, J.F.; Olkkonen, V.M.; van de Lest, C.H.; Sostaric, E.; Schoevers, E.J.; Helms, J.B.; Gadella, B.M. Involvement of bicarbonate-induced radical signaling in oxysterol formation and sterol depletion of capacitating mammalian sperm during in vitro fertilization. Biol. Reprod. 2013, 88, 21. [Google Scholar] [CrossRef]
- Zerbinati, C.; Caponecchia, L.; Puca, R.; Ciacciarelli, M.; Salacone, P.; Sebastianelli, A.; Pastore, A.; Palleschi, G.; Petrozza, V.; Porta, N. Mass spectrometry profiling of oxysterols in human sperm identifies 25-hydroxycholesterol as a marker of sperm function. Redox Biol. 2017, 11, 111–117. [Google Scholar] [CrossRef]
- Cross, N.L. Reorganization of lipid rafts during capacitation of human sperm. Biol. Reprod. 2004, 71, 1367–1373. [Google Scholar] [CrossRef]
- Nixon, B.; Bielanowicz, A.; Mclaughlin, E.A.; Tanphaichitr, N.; Ensslin, M.A.; Aitken, R.J. Composition and significance of detergent resistant membranes in mouse spermatozoa. J. Cell. Physiol. 2009, 218, 122–134. [Google Scholar] [CrossRef]
- Nixon, B.; Mitchell, L.A.; Anderson, A.L.; Mclaughlin, E.A.; O’bryan, M.K.; Aitken, R.J. Proteomic and functional analysis of human sperm detergent resistant membranes. J. Cell. Physiol. 2011, 226, 2651–2665. [Google Scholar] [CrossRef]
- Nixon, B.; Aitken, R.J. The biological significance of detergent-resistant membranes in spermatozoa. J. Reprod. Immunol. 2009, 83, 8–13. [Google Scholar] [CrossRef]
- Cardona, C.; Neri, Q.V.; Simpson, A.J.; Moody, M.A.; Ostermeier, G.C.; Seaman, E.K.; Paniza, T.; Rosenwaks, Z.; Palermo, G.D.; Travis, A.J. Localization patterns of the ganglioside GM1 in human sperm are indicative of male fertility and independent of traditional semen measures. Mol. Reprod. Dev. 2017, 84, 423–435. [Google Scholar] [CrossRef]
- Jones, R.; James, P.S.; Howes, L.; Bruckbauer, A.; Klenerman, D. Supramolecular organization of the sperm plasma membrane during maturation and capacitation. Asian J. Androl. 2007, 9, 438–444. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Makker, K.; Agarwal, A.; Sharma, R. Oxidative stress & male infertility. Indian J. Med Res. 2009, 129, 357. [Google Scholar]
- Saleh, R.A.; HCLD, A.A. Oxidative stress and male infertility: From research bench to clinical practice. J. Androl. 2002, 23, 737–752. [Google Scholar]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical relevance of oxidative stress in male factor infertility: An update. Am. J. Reprod. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef]
- Sikka, S.C.; Rajasekaran, M.; Hellstrom, W.J. Role of oxidative stress and antioxidants in male infertility. J. Androl. 1995, 16, 464–468. [Google Scholar]
- Bromfield, E.G.; Mihalas, B.P.; Dun, M.D.; Aitken, R.J.; McLaughlin, E.A.; Walters, J.L.; Nixon, B. Inhibition of arachidonate 15-lipoxygenase prevents 4-hydroxynonenal-induced protein damage in male germ cells. Biol. Reprod. 2017, 96, 598–609. [Google Scholar] [CrossRef]
- Walters, J.L.; De Iuliis, G.N.; Dun, M.D.; Aitken, R.J.; McLaughlin, E.A.; Nixon, B.; Bromfield, E.G. Pharmacological inhibition of arachidonate 15-lipoxygenase protects human spermatozoa against oxidative stress. Biol. Reprod. 2018, 98, 784–794. [Google Scholar] [CrossRef]
- Bromfield, E.G.; Walters, J.L.; Cafe, S.L.; Bernstein, I.R.; Stanger, S.J.; Anderson, A.L.; Aitken, R.J.; McLaughlin, E.A.; Dun, M.D.; Gadella, B.M. Differential cell death decisions in the testis: Evidence for an exclusive window of ferroptosis in round spermatids. MHR Basic Sci. Reprod. Med. 2019, 25, 241–256. [Google Scholar] [CrossRef]
- Ortega, C.; Verheyen, G.; Raick, D.; Camus, M.; Devroey, P.; Tournaye, H. Absolute asthenozoospermia and ICSI: What are the options? Hum. Reprod. Update 2011, 17, 684–692. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Liang, J.; He, D. Comparative proteomic study between human normal motility sperm and idiopathic asthenozoospermia. World J. Urol. 2013, 31, 1395–1401. [Google Scholar] [CrossRef]
- Yu, L.; Yang, X.; Ma, B.; Ying, H.; Shang, X.; He, B.; Zhang, Q. Abnormal arachidonic acid metabolic network may reduce sperm motility via P38 MAPK. Open Biol. 2019, 9, 180091. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E.; Lin, D.S.; Wolf, D.; Alexander, M. Uneven distribution of desmosterol and docosahexaenoic acid in the heads and tails of monkey sperm. J. Lipid Res. 1998, 39, 1404–1411. [Google Scholar] [PubMed]
- Am-In, N.; Kirkwood, R.; Techakumphu, M.; Tantasuparuk, W. Lipid profiles of sperm and seminal plasma from boars having normal or low sperm motility. Theriogenology 2011, 75, 897–903. [Google Scholar] [CrossRef]
- Zalata, A.A.; Christophe, A.B.; Depuydt, C.E.; Schoonjans, F.; Comhaire, F.H. The fatty acid composition of phospholipids of spermatozoa from infertile patients. Mol. Hum. Reprod. 1998, 4, 111–118. [Google Scholar] [CrossRef]
- Gulaya, N.; Margitich, V.; Govseeva, N.; Klimashevsky, V.; Gorpynchenko, I.; Boyko, M. Phospholipid composition of human sperm and seminal plasma in relation to sperm fertility. Arch. Androl. 2001, 46, 169–175. [Google Scholar]
- Connor, W.E.; Weleber, R.G.; DeFrancesco, C.; Lin, D.S.; Wolf, D.P. Sperm abnormalities in retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2619–2628. [Google Scholar]
- Fariello, R.M.; Pariz, J.R.; Spaine, D.M.; Gozzo, F.C.; Pilau, E.J.; Fraietta, R.; Bertolla, R.P.; Andreoni, C.; Cedenho, A.P. Effect of smoking on the functional aspects of sperm and seminal plasma protein profiles in patients with varicocele. Hum. Reprod. 2012, 27, 3140–3149. [Google Scholar] [CrossRef]
- Andersen, J.M.; Rønning, P.O.; Herning, H.; Bekken, S.D.; Haugen, T.B.; Witczak, O. Fatty acid composition of spermatozoa is associated with BMI and with semen quality. Andrology 2016, 4, 857–865. [Google Scholar] [CrossRef]
- Jia, Y.-F.; Feng, Q.; Ge, Z.-Y.; Guo, Y.; Zhou, F.; Zhang, K.-S.; Wang, X.-W.; Lu, W.-H.; Liang, X.-W.; Gu, Y.-Q. Obesity impairs male fertility through long-term effects on spermatogenesis. BMC Urol. 2018, 18, 42. [Google Scholar] [CrossRef]
- Kahn, B.E.; Brannigan, R.E. Obesity and male infertility. Curr. Opin. Urol. 2017, 27, 441–445. [Google Scholar] [CrossRef]
- Craig, J.R.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. Obesity, male infertility, and the sperm epigenome. Fertil. Steril. 2017, 107, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Moscatelli, N.; Di Giacomo, M.; Zara, V. Dietary fatty acids influence sperm quality and function. Andrology 2017, 5, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Attaman, J.A.; Toth, T.L.; Furtado, J.; Campos, H.; Hauser, R.; Chavarro, J.E. Dietary fat and semen quality among men attending a fertility clinic. Hum. Reprod. 2012, 27, 1466–1474. [Google Scholar] [CrossRef]
- Tyurina, Y.Y.; Tyurin, V.A.; Anthonymuthu, T.; Amoscato, A.A.; Sparvero, L.J.; Nesterova, A.M.; Baynard, M.L.; Sun, W.; He, R.; Khaitovich, P. Redox lipidomics technology: Looking for a needle in a haystack. Chem. Phys. Lipids 2019, 93, 221. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Kothapalli, K.S.; Brenna, J.T. Desaturase and elongase limiting endogenous long chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 103. [Google Scholar] [CrossRef]
- Gardner, H.W. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic. Biol. Med. 1989, 7, 65–86. [Google Scholar] [CrossRef]
- Maier, C.S.; Chavez, J.; Wang, J.; Wu, J. Protein adducts of aldehydic lipid peroxidation products: Identification and characterization of protein adducts using an aldehyde/keto-reactive probe in combination with mass spectrometry. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 305–330. [Google Scholar]
- Chen, Y.; Liu, Y.; Lan, T.; Qin, W.; Zhu, Y.; Qin, K.; Gao, J.; Wang, H.; Hou, X.; Chen, N. Quantitative profiling of protein carbonylations in ferroptosis by an aniline-derived probe. J. Am. Chem. Soc. 2018, 140, 4712–4720. [Google Scholar] [CrossRef]
- Aitken, R.J.; Whiting, S.; De Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060. [Google Scholar] [CrossRef]
- Baker, M.A.; Weinberg, A.; Hetherington, L.; Villaverde, A.-I.; Velkov, T.; Baell, J.; Gordon, C.P. Defining the mechanisms by which the reactive oxygen species by-product, 4-hydroxynonenal, affects human sperm cell function. Biol. Reprod. 2015, 92, 108–110. [Google Scholar] [CrossRef]
- Lord, T.; Martin, J.H.; Aitken, R.J. Accumulation of electrophilic aldehydes during postovulatory aging of mouse oocytes causes reduced fertility, oxidative stress, and apoptosis. Biol. Reprod. 2015, 92, 33. [Google Scholar] [CrossRef]
- Mihalas, B.P.; Bromfield, E.G.; Sutherland, J.M.; De Iuliis, G.N.; McLaughlin, E.A.; Aitken, R.J.; Nixon, B. Oxidative damage in naturally aged mouse oocytes is exacerbated by dysregulation of proteasomal activity. J. Biol. Chem. 2018, 293, 18944–18964. [Google Scholar] [CrossRef]
- Ni, Z.; Goracci, L.; Cruciani, G.; Fedorova, M. Computational solutions in redox lipidomics–Current strategies and future perspectives. Free Radic. Biol. Med. 2019, 144, 110–123. [Google Scholar] [CrossRef]
- Levy, B.D.; Vachier, I.; Serhan, C.N. Resolution of inflammation in asthma. Clin. Chest Med. 2012, 33, 559–570. [Google Scholar] [CrossRef]
- Lewis, R.A.; Austen, K.F.; Soberman, R.J. Leukotrienes and other products of the 5-lipoxygenase pathway: Biochemistry and relation to pathobiology in human diseases. N. Engl. J. Med. 1990, 323, 645–655. [Google Scholar]
- Tyurina, Y.Y.; St. Croix, C.M.; Watkins, S.C.; Watson, A.M.; Epperly, M.W.; Anthonymuthu, T.S.; Kisin, E.R.; Vlasova, I.I.; Krysko, O.; Krysko, D.V. Redox (phospho) lipidomics of signaling in inflammation and programmed cell death. J. Leukoc. Biol. 2019, 106, 57–81. [Google Scholar]
- O’Donnell, V.B.; Ekroos, K.; Liebisch, G.; Wakelam, M. Lipidomics: Current state of the art in a fast moving field. Wiley Interdiscip. Rev. Syst. Biol. Med. 2019, 12, e1466. [Google Scholar] [CrossRef]
- Spickett, C.M.; Pitt, A.R. Oxidative lipidomics coming of age: Advances in analysis of oxidized phospholipids in physiology and pathology. Antioxid. Redox Signal. 2015, 22, 1646–1666. [Google Scholar] [CrossRef]
- Koelmel, J.P.; Kroeger, N.M.; Ulmer, C.Z.; Bowden, J.A.; Patterson, R.E.; Cochran, J.A.; Beecher, C.W.; Garrett, T.J.; Yost, R.A. LipidMatch: An automated workflow for rule-based lipid identification using untargeted high-resolution tandem mass spectrometry data. BMC Bioinform. 2017, 18, 331. [Google Scholar] [CrossRef]
- Ulmer, C.Z.; Koelmel, J.P.; Ragland, J.M.; Garrett, T.J.; Bowden, J.A. LipidPioneer: A comprehensive user-generated exact mass template for lipidomics. J. Am. Soc. Mass Spectrom. 2017, 28, 562–565. [Google Scholar] [CrossRef]
- Parker, B.L.; Calkin, A.C.; Seldin, M.M.; Keating, M.F.; Tarling, E.J.; Yang, P.; Moody, S.C.; Liu, Y.; Zerenturk, E.J.; Needham, E.J. An integrative systems genetic analysis of mammalian lipid metabolism. Nature 2019, 567, 187. [Google Scholar] [CrossRef]
- Shah, R.; Margison, K.; Pratt, D.A. The potency of diarylamine radical-trapping antioxidants as inhibitors of ferroptosis underscores the role of autoxidation in the mechanism of cell death. ACS Chem. Biol. 2017, 12, 2538–2545. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, P.; Mao, Y.; Wang, C.; Zheng, M.; Zhao, Z. The nitroxide Tempo inhibits hydroxyl radical production from the Fenton-like reaction of iron (II)-citrate with hydrogen peroxide. Biochem. Biophys. Res. Commun. 2017, 483, 159–164. [Google Scholar] [CrossRef]
- Kagan, V.E.; Tyurin, V.A.; Jiang, J.; Tyurina, Y.Y.; Ritov, V.B.; Amoscato, A.A.; Osipov, A.N.; Belikova, N.A.; Kapralov, A.A.; Kini, V. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005, 1, 223. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.F.; Doll, S.; St Croix, C.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017, 13, 81. [Google Scholar] [CrossRef]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef]
- Pollet, H.; Conrard, L.; Cloos, A.-S.; Tyteca, D. Plasma membrane lipid domains as platforms for vesicle biogenesis and shedding? Biomolecules 2018, 8, 94. [Google Scholar] [CrossRef]
- Egea-Jimenez, A.L.; Zimmermann, P. Lipids in Exosome Biology. Handb. Exp. Pharmacol. 2019, 2019, 1–28. [Google Scholar]
- Egea-Jimenez, A.L.; Zimmermann, P. Phospholipase D and phosphatidic acid in the biogenesis and cargo loading of extracellular vesicles. J. Lipid Res. 2018, 59, 1554–1560. [Google Scholar] [CrossRef]
- Brouwers, J.F.; Aalberts, M.; Jansen, J.W.; van Niel, G.; Wauben, M.H.; Stout, T.A.; Helms, J.B.; Stoorvogel, W. Distinct lipid compositions of two types of human prostasomes. Proteomics 2013, 13, 1660–1666. [Google Scholar] [CrossRef]
- Brzozowski, J.S.; Jankowski, H.; Bond, D.R.; McCague, S.B.; Munro, B.R.; Predebon, M.J.; Scarlett, C.J.; Skelding, K.A.; Weidenhofer, J. Lipidomic profiling of extracellular vesicles derived from prostate and prostate cancer cell lines. Lipids Health Dis. 2018, 17, 211. [Google Scholar] [CrossRef]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Haraszti, R.A.; Didiot, M.-C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Kim, D.K.; Kim, Y.K.; Gho, Y.S. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 2013, 13, 1554–1571. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.L.; Caprioli, R.M. Imaging mass spectrometry: A new tool for pathology in a molecular age. Proteom. Clin. Appl. 2013, 7, 733–738. [Google Scholar] [CrossRef]
- Spengler, B. Mass spectrometry imaging of biomolecular information. Anal. Chem. 2014, 87, 64–82. [Google Scholar] [CrossRef]
- Bodzon-Kulakowska, A.; Suder, P. Imaging mass spectrometry: Instrumentation, applications, and combination with other visualization techniques. Mass Spectrom. Rev. 2016, 35, 147–169. [Google Scholar] [CrossRef]
- Ucal, Y.; Durer, Z.A.; Atak, H.; Kadioglu, E.; Sahin, B.; Coskun, A.; Baykal, A.T.; Ozpinar, A. Clinical applications of MALDI imaging technologies in cancer and neurodegenerative diseases. Biochim. Biophys. Acta BBA Proteins Proteom. 2017, 1865, 795–816. [Google Scholar] [CrossRef]
- Mallah, K.; Quanico, J.; Raffo-Romero, A.; Cardon, T.; Aboulouard, S.; Devos, D.; Kobeissy, F.; Zibara, K.; Salzet, M.; Fournier, I. Matrix-Assisted Laser Desorption/Ionization-Mass Spectrometry Imaging of Lipids in Experimental Model of Traumatic Brain Injury Detecting Acylcarnitines as Injury Related Markers. Anal. Chem. 2019, 91, 11879–11887. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.E.; Essner, J.J.; Lee, Y.J. 3D MALDI mass spectrometry imaging of a single cell: Spatial mapping of lipids in the embryonic development of zebrafish. Sci. Rep. 2017, 7, 14946. [Google Scholar] [CrossRef] [PubMed]
- Cobice, D.F.; Livingstone, D.E.; Mackay, C.L.; Goodwin, R.J.; Smith, L.B.; Walker, B.R.; Andrew, R. Spatial localization and quantitation of androgens in mouse testis by mass spectrometry imaging. Anal. Chem. 2016, 88, 10362–10367. [Google Scholar] [CrossRef] [PubMed]

| Disease | Technology | Evidence 1 |
|---|---|---|
| Neurological disorders | Manipulation of ferroptosis | • Deletion of GPX4 in an AD mouse model led to memory and learning deficits [54]. |
| • Significant improvements to locomotive activity in mice and decreases in ferroptotic cell death were observed following use of ferrostatin-1 in a mouse model for Parkinson’s disease [85]. | ||
| Deuteration | • Initial studies of C. elegans supplemented with D-PUFAs show reduced cellular stress as measured by ROS and lipid peroxidation levels. This treatment subsequently improved the lifespan, highlighting the promise for the prevention of age-related disorders [79]. | |
| • A diet supplemented with D-PUFAs significantly improved memory performance in an AD mouse model [80]. | ||
| • A D-PUFA diet in a Huntington’s disease mouse model resulted in improvements to memory recognition and reduction in lipid peroxidation markers [81]. | ||
| • A mouse model for AD confirmed D-PUFA supplementation as a promising strategy to lower amyloid β-peptide production but did not improve learning deficits [82]. | ||
| Lipidomics and novel biomarkers | • An extensive lipidomic approach has identified 35 potential lipid biomarkers that varied between healthy controls and AD blood samples [27]. | |
| • Blood lipidomics between aged healthy individuals and those with AD has identified 24 biomarkers that could be used to confirm AD with >70% accuracy [28]. | ||
| • The levels of six lipid peroxidation markers were monitored between healthy and AD blood samples to provide a promising model for AD diagnosis [29]. | ||
| Cardiovascular and lung diseases | Manipulation of ferroptosis | • A COPD mouse model induced via cigarette exposure demonstrated that GPX4 gene deletion resulted in an exacerbation of hallmark features of COPD and increased lipid peroxidation and ferroptotic cell death [86]. |
| • Using a radiation-induced lung fibrosis (RILF) mouse model, GPX4 levels were shown to be significantly reduced compared to healthy controls. Further, the addition of the ferroptosis inhibitor liproxstatin-1 lowered levels of cellular stress and improved the GPX4 concentration [87]. | ||
| Lipidomics and novel biomarkers | • A lipidomic study was completed on 1028 subjects to identify lipid metabolites indicative of risk for coronary heart disease. Metabolites identified included lysophosphatidylcholine 18:1, lysophosphatidylcholine 18:2, monoglyceride 18:2, and sphingomyelin 28:1 [88]. | |
| • A study of 220 individuals highlighted unique differences in the lipid profiles between unstable and stable coronary heart disease [89]. | ||
| • A lipidomic study completed on 685 blood samples highlighted that the relative risk of cardiovascular disease was associated with increased levels of cholesterol esters and triacylglycerols [90]. | ||
| • The identification of lipoprotein(a) as a risk factor for ASCVD has led to a clinical trial set to begin in 2020, which will examine the possibility of targeting lipoprotein(a) production to protect against the disease [73]. | ||
| Cancer | Lipidomics and novel biomarkers | • Screening of almost 20,000 individuals found that colorectal adenomas (advanced and non-advanced) were associated with increased levels of triglycerides while ApoA-1 and HDL cholesterol were linked to non-advanced adenomas [91]. |
| • A positive relationship has been observed between phosphatidylserine and lyso-phosphatidylserine and lung cancer prevalence and a negative correlation with lyso-phosphatidylethanolamine and phosphatidylethanolamine and lung cancer. Furthermore, this study identified that the lipidomic profile varied between different subtypes of lung cancer [92]. | ||
| • A lipidomic analysis identified 64 potential lipid biomarkers that were either up or downregulated in the presence of colorectal cancer [93]. | ||
| • A lipidomic analysis comparing prostate cancer patients with healthy controls identified 35 potential lipid biomarkers for diagnostic use [94]. | ||
| Manipulation of ferroptosis | • A recent study confirmed SKBr3 breast cancer cells as sensitive to ferroptosis using the ferroptosis inhibitors deferoxamine and ferrostatin-1 [95]. | |
| • A study confirmed the sensitivity of acute lymphoblastic leukemia cells to ferroptosis induced through RSL3 treatment. Furthermore, ferroptosis and lipid peroxidation were prevented through Ferrostatin-1 treatment and lipoxygenase inhibition [96]. |
| PUFA | Temporal Accumulation and Consequences of Dysregulation 1 |
|---|---|
| Docosahexanoic acid (22:6) | ↑ Increased levels of DHA in ram spermatozoa collected from the epididymis compared to the testes [109]. |
| ↑ Increased levels of DHA in dog spermatozoa isolated from the distal versus proximal epididymis [111]. | |
| ↑ Extremely high levels of DHA found in the monkey sperm tail compared to the sperm head [143]. | |
| ↓ Reduced levels of DHA correlated with low motility in boar spermatozoa compared to normal motility controls [144]. | |
| ↓ Significantly lower levels of DHA present in patients with asthenozoospermia and oligozoospermia compared to normozoospermic controls [145]. | |
| ↓ Significantly lower levels of DHA reported in infertile human semen samples compared to healthy controls [146]. | |
| ↓ Patients presenting with retinitis pigmentosa had significantly lower levels of DHA within their sperm [147]. | |
| ↓ A negative correlation was reported between human sperm DHA levels, DNA damage, and BMI; DHA content was correlated positively with normal semen parameters such as sperm count, vitality and motility [149]. | |
| Docosapentanoic acid (22:5) | ↑ Increased levels of DPA accompanies transition of rat germ cells from spermatocytes to post-meiotic spermatids [104]. |
| ↓ Reduced levels of DPA correlated with boar spermatozoa presenting with low motility compared to normal motility controls [144]. | |
| Eicosapentaenoic acid (20:5) | ↓ Significantly lower levels reported in human semen samples from infertile individuals compared to healthy controls [146] |
| Arachidonic acid (20:4) | ↑ Increased levels of AA were observed in the seminal plasma of human patient samples with asthenozoospermia compared to healthy controls [142]. |
| ↑ Increased levels of AA found in the tails compared to the heads of monkey spermatozoa [143]. | |
| ↓ Reduced AA levels were observed in ram spermatozoa from the epididymis compared to the testes [109]. | |
| ↓ Patients presenting with retinitis pigmentosa had significantly lower levels of AA within their sperm [147]. | |
| Dihomo-γ-linolenic acid (20:3) | ↑ Higher levels of DGLA found in the tails compared to the heads of monkey spermatozoa [143]. |
| ↑ Significantly higher levels of DGLA were reported in infertile human semen samples compared to healthy controls [146]. | |
| ↓ Patients presenting with retinitis pigmentosa had significantly lower levels of DGLA within their spermatozoa [147]. | |
| γ-Linolenic acid (18:3) | ↑ Higher levels of LA found in the tails compared to the heads of monkey spermatozoa [143]. |
| ↑ Significantly higher levels of LA present in the spermatozoa of patients suffering from asthenozoospermia and oligozoospermia compared to normozoospermic controls [145]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walters, J.L.H.; Gadella, B.M.; Sutherland, J.M.; Nixon, B.; Bromfield, E.G. Male Infertility: Shining a Light on Lipids and Lipid-Modulating Enzymes in the Male Germline. J. Clin. Med. 2020, 9, 327. https://doi.org/10.3390/jcm9020327
Walters JLH, Gadella BM, Sutherland JM, Nixon B, Bromfield EG. Male Infertility: Shining a Light on Lipids and Lipid-Modulating Enzymes in the Male Germline. Journal of Clinical Medicine. 2020; 9(2):327. https://doi.org/10.3390/jcm9020327
Chicago/Turabian StyleWalters, Jessica L.H., Bart M. Gadella, Jessie M. Sutherland, Brett Nixon, and Elizabeth G. Bromfield. 2020. "Male Infertility: Shining a Light on Lipids and Lipid-Modulating Enzymes in the Male Germline" Journal of Clinical Medicine 9, no. 2: 327. https://doi.org/10.3390/jcm9020327
APA StyleWalters, J. L. H., Gadella, B. M., Sutherland, J. M., Nixon, B., & Bromfield, E. G. (2020). Male Infertility: Shining a Light on Lipids and Lipid-Modulating Enzymes in the Male Germline. Journal of Clinical Medicine, 9(2), 327. https://doi.org/10.3390/jcm9020327





