Cannabinoids in Glaucoma Patients: The Never-Ending Story
Abstract
1. Introduction
1.1. Phytocannabinoids and Endocannabinoids: Mechanism of Action
1.2. Cannabinoids Effects on Intraocular Pressure
1.3. Cannabinoids’ Neuroprotective Effects
2. Results
2.1. Oral Cannabinoids in Glaucoma and Their IOP Lowering Effects
2.2. Inhaled Cannabinoids in Glaucoma and Their IOP Lowering Effects
2.3. Topical Cannabinoids in Glaucoma and Their IOP Lowering Effects
2.4. Intravenous Cannabinoids in Glaucoma and Their IOP Lowering Effects
3. Discussion
Future Directions
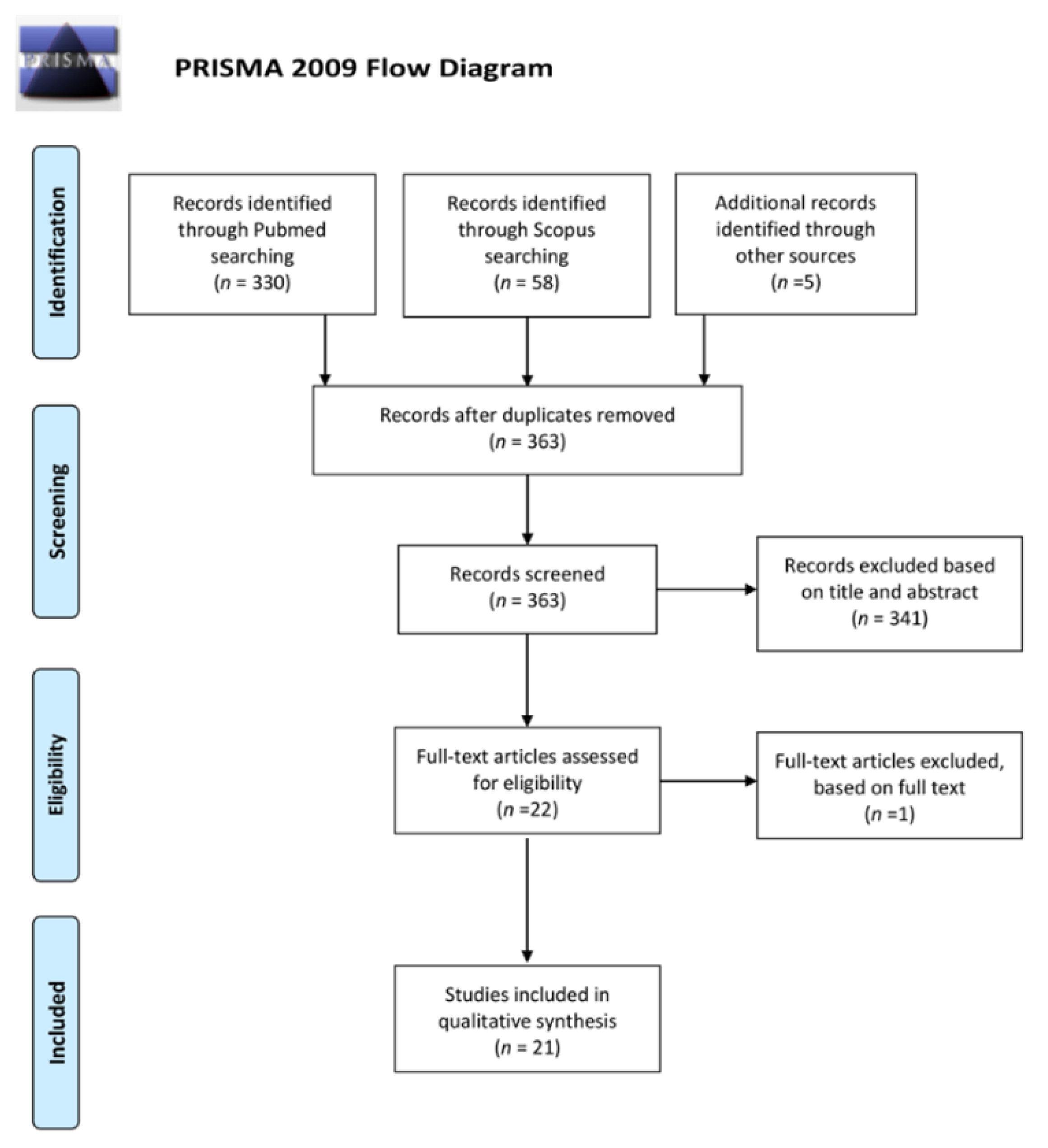
4. Material and Methods
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flaxman, S.R.; Bourne, R.R.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Leasher, J. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Rossetti, L.; Digiuni, M.; Giovanni, M.; Centofanti, M.; Fea, A.M.; Iester, M.; Tanga, L. Blindness and glaucoma: A multicenter data review from 7 academic eye clinics. PLoS ONE 2015, 10, e0136632. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA J. Am. Med. Assoc. 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Hepler, R.S.; Frank, I.R. Marihuana smoking and intraocular pressure. JAMA J. Am. Med. Assoc. 1971, 217, 1392. [Google Scholar] [CrossRef]
- Russo, E.B.; Grotenhermen, F. (Eds.) The Handbook of Cannabis Therapeutics: From Bench to Bedside; Routledge: New York, NY, USA, 2014. [Google Scholar]
- Aghazadeh Tabrizi, M.; Baraldi, P.G.; Borea, P.A.; Varani, K. Medicinal chemistry, pharmacology, and potential therapeutic benefits of cannabinoid CB2 receptor agonists. Chem. Rev. 2016, 116, 519–560. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokin. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Cairns, E.A.; Baldridge, W.H.; Kelly, M.E.M. The endocannabinoid system as a therapeutic target in glaucoma. Neural Plast. 2016. [Google Scholar] [CrossRef]
- Chen, J.; Matias, I.; Dinh, T.; Lu, T.; Venezia, S.; Nieves, A.; Di Marzo, V. Finding of endocannabinoids in human eye tissues: Implications for glaucoma. Biochem. Biophys. Res. Commun. 2005, 330, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Dasilva, M.A.; Grieve, K.L.; Cudeiro, J.; Rivadulla, C. Endocannabinoid CB1 receptors modulate visual output from the thalamus. Psychopharmacology 2012, 219, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Nucci, C.; Bari, M.; Spanò, A.; Corasaniti, M.; Bagetta, G.; Maccarrone, M.; Morrone, L.A. Potential roles of (endo)cannabinoids in the treatment of glaucoma: From intraocular pressure control to neuroprotection. Prog. Brain Res. 2008, 173, 451–464. [Google Scholar] [PubMed]
- Järvinen, T.; Pate, D.W.; Laine, K. Cannabinoids in the treatment of glaucoma. Pharmacol. Ther. 2002, 95, 203–220. [Google Scholar] [CrossRef]
- Katz, J.; Costarides, A.P. Facts vs fiction: The role of cannabinoids in the treatment of glaucoma. Curr. Ophthalmol. Rep. 2019, 7, 177–181. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.Y.; Kim, D.M.; Ko, H.S.; Kim, S.Y.; Yoo, T.; Park, S.S. Investigations on the association between normal tension glaucoma and single nucleotide polymorphisms of the endothelin-1 and endothelin receptor genes. Mol. Vis. 2006, 1, 1016–1021. [Google Scholar]
- Krishnan, G.; Chatterjee, N. Anandamide rescues retinal barrier properties in Müller glia through nitric oxide regulation. Neuroscience 2015, 284, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Lax, P.; Esquiva, G.; Altavilla, C.; Cuenca, N. Neuroprotective effects of the cannabinoid agonist HU210 on retinal degeneration. Exp. Eye Res. 2014, 120, 175–185. [Google Scholar] [CrossRef]
- Marsicano, G.; Goodenough, S.; Monory, K.; Hermann, H.; Eder, M.; Cannich, A.; López-Rodríguez, M.L. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003, 302, 84–88. [Google Scholar] [CrossRef]
- Samy, C.N.; Lui, C.J.; Kaiser, P.K.; Lipton, S.A.; Dreyer, E.B. Toxicity of chronic glutamate administration to the retina. Investig. Ophthalmol. Vis. Sci. 1994, 35, 497–502. [Google Scholar]
- Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Ingster-Moati, I.; Lalanne, L.; Giersch, A.; Laprevote, V. The cannabinoid system and visual processing: A review on experimental findings and clinical presumptions. Eur. Neuropsychopharmacol. 2015, 25, 100–112. [Google Scholar] [CrossRef]
- Flom, M.C.; Adams, A.J.; Jones, R.T. Marijuana smoking and reduced pressure in human eyes: Drug action or epiphenomenon? Investig. Ophthalmol. Vis. Sci. 1975, 14, 52–55. [Google Scholar]
- Panahi, Y.; Manayi, A.; Nikan, M.; Vazirian, M. The arguments for and against cannabinoids application in glaucomatous retinopathy. Biomed. Pharmacother. 2017, 86, 620–627. [Google Scholar] [CrossRef]
- Williamson, E.M.; Evans, F.J. Cannabinoids in clinical practice. Drugs 2000, 60, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.M.; Boubakir, Z.; Ambrose, S.J.; Purves, R.W.; Page, J.E. The hexanoyl-CoA precursor for cannabinoid biosynthesis is formed by an acyl-activating enzyme in Cannabis sativa trichomes. Plant J. 2012, 71, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Yoshikai, K.; Shoyama, Y.; Morimoto, S. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett. 2007, 581, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, J.E.; Ohlsson, A.; Agurell, S.; Hollister, L.; Gillespie, H. Clinical effects and plasma levels of DELTA 9-tetrahydrocannabinol (DELTA 9-THC) in heavy and light users of cannabis. Psychopharmacology 1981, 74, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An overview of some pharmacological aspects. J. Clin. Pharmacol. 2002, 42, 11S–19S. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, C.; De Filippis, D.; Iuvone, T.; Blasio, A.; Steardo, A.; Esposito, G. Cannabidiol in medicine: A review of its therapeutic potential in CNS disorders. Phyther. Res. 2009, 23, 597–602. [Google Scholar] [CrossRef]
- Brenneisen, R.; Egli, A.; Elsohly, M.A.; Henn, V.; Spiess, Y. The effect of orally and rectally administered delta 9-tetrahydrocannabinol on spasticity: A pilot study with 2 patients. Int. J. Clin. Pharm. 1996, 34, 446–452. [Google Scholar]
- Chiang, C.W.; Barnett, G.; Brine, D. Systemic absorption of DELTA9-tetrahydrocannabinol after ophthalmic administration to the rabbit. J. Pharm. Sci. 1983, 72, 136–138. [Google Scholar] [CrossRef]
- Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Giersch, A.; Laprevote, V. The Endocannabinoid System in the Retina: From Physiology to Practical and Therapeutic Applications. Neural Plast. 2016. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ(9)-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharm. 2015, 172, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; Danesh-Meyer, H.V. Cannabinoids and the eye. Surv. Oftalmol. 2020. [Google Scholar] [CrossRef] [PubMed]
- López, E.M.; Tagliaferro, P.; Onaivi, E.S.; López-Costa, J.J. Distribution of CB2 cannabinoid receptor in adult rat retina. Synapse 2011, 65, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Bouskila, J.; Javadi, P.; Elkrief, L.; Casanova, C.; Bouchard, J.F.; Ptito, M. A comparative analysis of the endocannabinoid system in the retina of mice, tree shrews, and monkeys. Neural Plast. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, X.; Wang, L. Presence and regulation of cannabinoid receptors in human retinal pigment epithelial cells. Mol. Vis. 2009, 15, 1243. [Google Scholar] [PubMed]
- Zhan, G.L.; Camras, C.B.; Palmberg, P.F.; Toris, C.B. Effects of marijuana on aqueous humor dynamics in a glaucoma patient. J. Glaucoma 2005, 14, 175–177. [Google Scholar] [CrossRef]
- Romano, M.R. Cannabinoid agonists induce contractile responses through G i/o-dependent activation of phospholipase C in the bovine ciliary muscle. Eur. J. Pharmacol. 2004, 494, 55–62. [Google Scholar]
- Su, E.N.; Kelly, M.E.; Cringle, S.J.; Yu, D.Y. Role of endothelium in abnormal cannabidiol-induced vasoactivity in retinal arterioles. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4029–4037. [Google Scholar] [CrossRef]
- Colasanti, B.K. A comparison of the ocular and central effects of DELTA9-tetrahydrocannabinol and cannabigerol. J. Ocul. Pharmacol. Ther. 1990, 6, 259–269. [Google Scholar] [CrossRef]
- Rösch, S.; Ramer, R.; Brune, K.; Hinz, B. R(+)-methanandamide and other cannabinoids induce the expression of cyclooxygenase-2 and matrix metalloproteinases in human nonpigmented ciliary epithelial cells. J. Pharmacol. Exp. Ther. 2006, 316, 1219–1228. [Google Scholar] [CrossRef]
- Plange, N.; Arend, K.O.; Kaup, M.; Doehmen, B.; Adams, H.; Hendricks, S.; Remky, A. Dronabinol and retinal hemodynamics in humans. Am. J. Ophthalmol. 2007, 143, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Hommer, N.; Kallab, M.; Szegedi, S.; Puchner, S.; Stjepanek, K.; Bauer, M.; Schmidl, D. The effect of orally administered dronabinol on optic nerve head blood flow in healthy subjects—A randomized clinical trial. Clin. Pharmacol. Ther. 2020, 108, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Green, K.; Wynn, H.; Padgett, D. Effects of delta9-tetrahydrocannabinol on ocular blood flow and aqueous humor formation. Exp. Eye Res. 1978, 26, 65–69. [Google Scholar] [CrossRef]
- Yoles, E.; Belkin, M.; Schwartz, M. HU-211, a nonpsychotropic cannabinoid, produces short- and long-term neuroprotection after optic nerve axotomy. J. Neurotrauma 1996, 13, 49–57. [Google Scholar] [CrossRef]
- Zalish, M.; Lavie, V. Dexanabinol (HU-211) has a beneficial effect on axonal sprouting and survival after rat optic nerve crush injury. Vision Res. 2003, 43, 237–242. [Google Scholar] [CrossRef]
- Opere, C.A.; Zheng, W.D.; Zhao, M.; Lee, J.S.; Kulkarni, K.H.; Ohia, S.E. Inhibition of potassium-and ischemia-evoked [3H] D-aspartate release from isolated bovine retina by cannabinoids. Curr. Eye Res. 2006, 31, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Crandall, J.; Matragoon, S.; Khalifa, Y.M.; Borlongan, C.; Tsai, N.T.; Caldwell, R.B.; Liou, G.I. Neuroprotective and intraocular pressure-lowering effects of (-)Delta9-tetrahydrocannabinol in a rat model of glaucoma. Ophthalmic Res. 2007, 39, 69–75. [Google Scholar] [CrossRef]
- Nucci, C.; Gasperi, V.; Tartaglione, R.; Cerulli, A.; Terrinoni, A.; Bari, M.; Bagetta, G. Involvement of the endocannabinoid system in retinal damage after high intraocular pressure-induced ischemia in rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2997–3004. [Google Scholar] [CrossRef]
- Pinar-Sueiro, S.; Zorrilla Hurtado, J.A.; Veiga-Crespo, P.; Sharma, S.C.; Vecino, E. Neuroprotective effects of topical CB1 agonist WIN 55212-2 on retinal ganglion cells after acute rise in intraocular pressure induced ischemia in rat. Exp. Eye Res. 2013, 110, 55–58. [Google Scholar] [CrossRef]
- Liu, H.F.; He, Y.; Jia, J.; Ji, M.L.; Xi, J.W. Clinical study on intravitreal injection of cannabinoid HU-211 for optic nerve damage in glaucoma rats. Int. Eye Sci. 2014, 14, 1584–1586. [Google Scholar]
- Arend, O.; Plange, N.; Sponsel, W.E.; Remky, A. Pathogenetic aspects of the glaucomatous optic neuropathy: Fluorescein angiographic findings in patients with primary open angle glaucoma. Brain Res. Bull. 2004, 62, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Schwitzer, T.; Henrion, M.L.; Sarre, D.; Albuisson, E. Spatial localization of retinal anomalies in regular cannabis users: The relevance of the multifocal electroretinogram. Schizophr. Res. 2020, 219, 56–61. [Google Scholar] [CrossRef]
- Lucas, A.; Thirion, A.; Schwan, R.; Krieg, J.; Angioi-Duprez, K.; Laprevote, V.; Schwitzer, T. Association between increased retinal background noise and co-occurrent regular cannabis and alcohol use. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 335–340. [Google Scholar] [CrossRef]
- Schwitzer, T.; Robert, M.P.; Giersch, A.; Angioi-Duprez, K.; Ingster-Moati, I.; Pon-Monnier, A.; Laprevote, V. Transient retinal dysfunctions after acute cannabis use. Eur. Addict. Res. 2016, 22, 287–291. [Google Scholar] [CrossRef]
- Hepler, R.S.; Petrus, R.J. Experiences with administration of marihuana to glaucoma patients. In The Therapeutic Potential of Marihuana; Cohen, M., Ed.; Springer: New York, NY, USA, 1976; pp. 63–75. [Google Scholar]
- Hepler, R.S.; Frank, I.M.; Petrus, R. Ocular effects of marihuana smoking. In The Pharmacology of Marihuana; Braude, M.C., Szara, S., Eds.; Raven Press: New York, NY, USA, 1976. [Google Scholar]
- Newell, F.W.; Stark, P.; Jay, W.M.; Schanzlin, D.J. Nabilone: A Pressure-reducing synthetic benzopyran in open-angle glaucoma. Ophthalmology 1979, 86, 156–160. [Google Scholar] [CrossRef]
- Tiedeman, J.S.; Shields, M.B.; Weber, P.A.; Crow, J.W.; Cocchetto, D.M.; Harris, W.A.; Howes, J.F. Effect of synthetic cannabinoids on elevated intraocular pressure. Ophthalmology 1981, 88, 270–277. [Google Scholar] [CrossRef]
- Flach, A.J. Delta-9-tetrahydrocannabinol (THC) in the treatment of end-stage open-angle glaucoma. Trans. Am. Ophthalmol. Soc. 2002, 100, 215–222. [Google Scholar] [PubMed]
- Tomida, I.; Azuara-Blanco, A.; House, H.; Flint, M.; Pertwee, R.G.; Robson, P.J. Effect of sublingual application of cannabinoids on intraocular pressure: A pilot study. J. Glaucoma 2006, 15, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, C.; Ortisi, E.; Pulvirenti, L.; Reibaldi, M.; Scollo, D.; Amato, R.; Longo, A. Ocular hypotensive effect of oral palmitoyl-ethanolamide: A clinical trial. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6096–6100. [Google Scholar] [CrossRef]
- Pescosolido, N.; Librando, A.; Puzzono, M.; Nebbioso, M. Palmitoylethanolamide effects on intraocular pressure after Nd: YAG laser iridotomy: An experimental clinical study. J. Ocul. Pharm. 2011, 27, 629–635. [Google Scholar] [CrossRef]
- Strobbe, E.; Cellini, M.; Campos, E.C. Effectiveness of palmitoylethanolamide on endothelial dysfunction in ocular hypertensive patients: A randomized, placebo-controlled cross-over study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.C.; Crawford, W.J.; Alexander, P.C.; Anduze, A.L.; Gelbart, S.S. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology 1980, 87, 222–228. [Google Scholar] [CrossRef]
- Jay, W.M.; Green, K. Multiple-drop study of topically applied 1% delta9-tetrahydrocannabinol in human eyes. Arch. Ophthalmol. 1983, 101, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.C.; Perry, D.D.; Russell, D.N.; Jones, B.F. Topical DELTA 9-Tetrahydrocannabinol and aqueous dynamics in glaucoma. J. Clin. Pharmacol. 1981, 21, 467S–471S. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.C.; Olsen, J.L.; Armstrong, J.R.; McKinnon, S.M. Topical DELTA9-tetrahydrocannabinol in hypertensive glaucomas. J. Pharm. Pharmacol. 1981, 33, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Green, K.; Roth, M. Ocular effects of topical administration of DELTA9-tetrahydrocannabinol in man. Arch. Ophthalmol. 1982, 100, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Porcella, A.; Maxia, C.; Gessa, G.L.; Pani, L. The synthetic cannabinoid WIN55212-2 decreases the intraocular pressure in human glaucoma resistant to conventional therapies. Eur. J. Neurosci. 2001, 13, 409–412. [Google Scholar] [CrossRef]
- Pescosolido, N.; Stefanucci, A.; Librando, A.; Pezzino, S.; Rusciano, D. Evaluation of cannabinoid eye drops on five patients with intractable hypertensive open angle glaucoma. Opth. Clin. Ther. 2018, 2, 1–4. [Google Scholar]
- West, M.E.; Homi, J. Cannabis as a medicine. Br. J. Anaesth. 1996, 76, 167. [Google Scholar] [CrossRef]
- Purnell, W.D.; Gregg, J.M. Delta (9)-tetrahydrocannabinol, euphoria and intraocular pressure in man. Ann. Ophthalmol. 1975, 7, 921–923. [Google Scholar]
- Cooler, P.; Gregg, J.M. Effect of delta-9-tetrahydrocannabinol on intraocular pressure in humans. South Med. J. 1977, 70, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Rapino, C.; Tortolani, D.; Scipioni, L.; Maccarrone, M. Neuroprotection by (endo)Cannabinoids in glaucoma and retinal neurodegenerative diseases. Curr. Neuropharmacol. 2017, 16, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Belyea, D.A.; Alhabshan, R.; Del Rio-Gonzalez, A.M.; Chadha, N.; Lamba, T.; Golshani, C.; Dan, J.A. Marijuana use among patients with glaucoma in a city with legalized medical marijuana use. JAMA Ophthalmol. 2016, 134, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, E.J.; Higginbotham, L.A. Shaping patients’ perspective of medical marijuana for glaucoma treatment. JAMA Ophthalmol. 2016, 134, 265–266. [Google Scholar] [CrossRef]
- Green, K. Marijuana smoking vs cannabinoids for glaucoma therapy. Arch. Ophthalmol. 1998, 116, 1433–1437. [Google Scholar] [CrossRef]
- Keppel Hesselink, J.M.; Costagliola, C.; Fakhry, J.; Kopsky, D.J. Palmitoylethanolamide, a natural retinoprotectant: Its putative relevance for the treatment of glaucoma and diabetic retinopathy. J. Ophthalmol. 2015. [Google Scholar] [CrossRef]
- Pinar-Sueiro, S.; Rodríguez-Puertas, R.; Vecino, E. Cannabinoid applications in glaucoma. Arch. Soc. Esp. Oftalmol. 2011, 86, 16–23. [Google Scholar] [CrossRef]
- Kaufman, P.L. Marijuana and glaucoma. Arch. Ophthalmol. 1998, 16, 1512–1513. [Google Scholar] [CrossRef]
- Peña, J.; Jiménez, C.; Schmidt, J. Do cannabinoids play a role in the control of glaucoma? Medwave 2018, 18. [Google Scholar] [CrossRef]
- Pujari, R.; Jampel, H.D. Treating Glaucoma with medical marijuana: Peering through the smoke. Ophthalmol. Glaucoma 2019, 2, 201–203. [Google Scholar] [CrossRef]
- Sun, X.; Xu, C.S.; Chadha, N.; Chen, A.; Liu, J. Marijuana for glaucoma: A recipe for disaster or treatment? Yale J. Biol. Med. 2015, 88, 265. [Google Scholar] [PubMed]
- De Aquino, J.P.; Sherif, M.; Radhakrishnan, R.; Cahill, J.D.; Ranganathan, M.; D’Souza, D.C. The psychiatric consequences of cannabinoids. Clin. Ther. 2018, 40, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.J.; Pope, H.G.; Hudson, J.I.; Yurgelun-Todd, D. Attributes of long-term heavy cannabis users: A case-control study. Psychol. Med. 2003, 33, 1415. [Google Scholar] [CrossRef] [PubMed]
- Karila, L.; Roux, P.; Rolland, B.; Benyamina, A.; Reynaud, M.; Aubin, H.J.; Lançon, C. Acute and long-term effects of cannabis use: A Review. Curr. Pharm. Des. 2014, 20, 4112–4118. [Google Scholar] [CrossRef]
- Panlilio, L.V.; Goldberg, S.R.; Justinova, Z. Cannabinoid abuse and addiction: Clinical and preclinical findings. Clin. Pharmacol. Ther. 2015, 97, 616–627. [Google Scholar] [CrossRef]
- Huestegge, L.; Radach, R.; Kunert, H.J.; Heller, D. Visual search in long-term cannabis users with early age of onset. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Laffi, G.L.; Safran, A.B. Persistent visual changes following hashish consumption. Br. J. Ophthalmol. 1993, 77, 601. [Google Scholar] [CrossRef][Green Version]
- Levi, L.; Miller, N.R. Visual illusions associated with previous drug abuse. J. Clin. Neuroophthalmol. 1990, 10, 103–110. [Google Scholar]
- Novack, G.D. Cannabinoids for treatment of glaucoma. Curr. Opin. Ophthalmol. 2016, 27, 146–150. [Google Scholar] [CrossRef]
- Cvenkel, B.; Kolko, M. Current medical therapy and future trends in the management of glaucoma treatment. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Howick, J.; Glasziou, P.; Aronson, J.K. Evidence-based mechanistic reasoning. J. R. Soc. Med. 2010, 103, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Keaney, M.; Lorimer, A.R. Auditing the implementation of SIGN (Scottish Intercollegiate Guidelines Network) clinical guidelines. Int. J. Health Care Qual. Assur. 1999, 12, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]


| Authors | Study Population | Intervention | Results |
|---|---|---|---|
| Plange et al., 2007 [42] | 8 healthy subjects | Single dose of 7.5 mg oral Dronabinol (Delta-9-THC) | Significant decrease in artero-venous retinal passage time |
| Hommer et al., 2020 [43] | 24 healthy subjects | 5 mg oral Dronabinol (Delta-9-THC) | Increased optic nerve head blood flow |
| Green et al., 1978 [44] | Rabbits | Intravenous THC | Increased ocular blood flow (choroidal, iris and ciliary processes flow) |
| Yoles et al., 1996 [45] | Rats | Intraperitoneal Dexanabinol (HU-211) or vehicle alone | Reduction in electrophysiological and metabolic deficits after optic nerve injury in HU-211 group |
| Zalish and Lavie, 2003 [46] | 18 rats | Intraperitoneal HU-211 or vehicle alone | Observation of unmyelinated and thinly myelinated axons 30 days after optic nerve injury in the treated group compared with controls |
| Opere et al., 2006 [47] | Isolated bovine retina | Anandamide, ACEA, Methanandamide, WIN55212-2 (superfusion method) | Inhibition of K+-induced aspartate retinal release induced by ischemia |
| Crandall et al., 2007 [48] | 14 rats | 20-week treatment of intraperitoneal THC or ethanol solution vehicle once weekly | Reduction in GCL loss after episcleral vessel cauterization induced glaucoma in the treated group |
| Nucci et al., 2007 [49] | Rats | Single systemic administration of URB597 or intravitreal methanandamide alone or in combination with SR141716 or capsazepine | URB597 and methanandamide alone induced a reduction in GCL loss after acute rise IOP-induced ischemia |
| Pinar-Sueiro et al., 2013 [50] | 27 rats | Topical administration of 1% WIN55212-2 or WIN55212-2 1% and AM251 1%, or vehicle alone for two days | Reduction in GCL loss after acute rise IOP-induced ischemia in the WIN55212-2 1% group |
| Liu et al., 2014 [51] | 18 rats | Four-week treatment with intravitreal HU-211 1 mg or saline solution | Lower reduction in RGC density in the HU-211-group after episcleral vessel cauterization induced glaucoma |
| Authors | Study Population | Intervention | Results |
|---|---|---|---|
| Hepler et al., 1976 [56] | 120 subjects affected by POAG | single dose of 5 mg delta-9-THC | 14% and 15% IOP lowering after 30 and 180 min from administration |
| single dose of 10 mg delta-9-THC | 23% and 18% IOP lowering after 30 and 180 min from administration | ||
| single dose of 20 mg delta-9-THC | 24% and 23% IOP lowering after 30 and 180 min from administration | ||
| Hepler et al., 1976 [57] | 40 healthy subjects | single dose of 5 mg synthetic delta-9-THC | 10% IOP lowering at 30 min after administration |
| single dose of 10 mg synthetic delta-9-THC | 10% IOP lowering at 30 min after administration | ||
| single dose of 20 mg synthetic delta-9-THC | 16% IOP lowering at 30 min after administration | ||
| Newell et al., 1979 [58] | 18 subjects affected by POAG or ocular hypertension | single dose of Nabilone 0.5 mg | 27.9% IOP lowering between two to four hours after administration |
| Tiedeman et al., 1981 [59] | 15 subjects affected by ocular hypertension | 5 mg BW29Y (5 subjects) | no significant effects on IOP |
| 10 mg BW29Y (10 subjects) | no significant effects on IOP | ||
| 22 subjects affected by ocular hypertension | 4 mg BW146 (9 subjects) | 23% IOP lowering | |
| 8 mg BW146 (10 subjects) | 25% IOP lowering | ||
| 12 mg BW146 (3 subjects) | 42% IOP lowering | ||
| Flach AJ 2002 [60] | 9 patients affected by POAG on maximally tolerated medical therapy | 10 mg to 80 mg oral delta-9-THC daily (in addition to regular glaucoma therapy). Treatment duration between 3 and 36 weeks. | initial IOP lowering limited by tolerance development (mean IOP reduction not disclosed) |
| Tomida et al., 2006 [61] | 6 subjects affected by POAG or ocular hypertension | single dose of 5 mg delta-9-THC | 14% and 5.3% IOP lowering at 2 and 4 h from administration |
| single dose of 20 mg cannabidiol | no significant effects on IOP | ||
| single dose of 40 mg cannabidiol | no significant effects on IOP | ||
| Plange et al., 2007 [42] | 8 healthy subjects | single dose of 7.5 mg Dronabinol | 10% IOP lowering two hours after administration |
| Gagliano et al., 2011 [62] | 42 subjects affected by POAG or ocular hypertension | 300 mg palmitoyl-ethanolamide twice a day for two months (in addition to timolol 0.5%) | 16.2% IOP lowering after two months of treatment |
| Pescosolido et al., 2011 [63] | 15 subjects undergoing prophylactic iridotomy | 300 mg palmitoyl-ethanolamide twice a day for 15 days before iridotomy | 24%, 28% and 31% IOP lowering at 15, 30 and 120 min after iridotomy (vs. placebo) |
| Strobbe et al., 2013 [64] | 40 subjects affected by ocular hypertension | 300 mg palmitoyl-ethanolamide twice a day for 3 months | 3.7% IOP lowering |
| Authors | Study Population | Intervention | Results |
|---|---|---|---|
| Hepler et al., 1971 [4] | 11 healthy subjects | 18 mg delta-9-THC | 24% IOP lowering (limited data available) |
| Flom et al., 1975 [20] | 15 healthy subjects | 12 mg delta-9-THC | 13% IOP lowering after 80 min from administration |
| Hepler et al., 1976 [56] | 136 subjects affected by POAG administered with 1, 2, or 4% THC cigarette | 20 mg delta-9-THC (2 g marijuana cigarette with 1% delta-9-THC) | 29% and 22% IOP lowering after 30 and 180 min from administration |
| 40 mg delta-9-THC (2 g marijuana cigarette with 2% delta-9-THC) | 25% and 17% IOP lowering after 30 and 180 min from administration | ||
| 80 mg delta-9-THC (2 g marijuana cigarette with 4% delta-9-THC) | 34% and 22% IOP lowering after 30 and 180 min from administration | ||
| Hepler et al., 1976 [57] | 40 healthy subjects | 1% natural delta-9-THC cigarette | 30% IOP lowering 30 min after administration |
| 2% natural delta-9-THC cigarette | 25% IOP lowering 30 min after administration | ||
| 4% natural delta-9-THC cigarette | 34% IOP lowering min after administration | ||
| 1% synthetic delta-9-THC cigarette | 15% IOP lowering 30 min after administration | ||
| 2% synthetic delta-9-THC cigarette | 23% IOP lowering 30 min after administration | ||
| 4% synthetic delta-9-THC cigarette | 24% IOP lowering 30 min after administration | ||
| Merritt et al., 1980 [65] | 18 glaucoma patients (12 affected by POAG, 6 affected by secondary glaucoma) | 18 mg delta-9-THC (0.9 g marijuana cigarette with 2% delta-9-THC) | 14.5% and 23.4% IOP lowering after 30 and 90 min from administration |
| Authors | Study Population | Intervention | Results |
|---|---|---|---|
| Merritt et al., 1981 [67] | 6 hypertensive glaucoma patients | single 0.05% delta-9-THC eye drop | No significant IOP lowering |
| single 0.1% delta-9-THC eye drop | No significant IOP lowering | ||
| Merritt et al., 1981 [68] | 8 hypertensive glaucoma patients | single 0.01% delta-9-THC eye drop | No significant IOP lowering |
| single 0.05% delta-9-THC eye drop | No significant IOP lowering | ||
| single 0.1% delta-9-THC eye drop | No significant IOP lowering | ||
| Green et al., 1982 [69] | 16 healthy subjects | single administration of 1% delta-9-THC eye drop | No significant IOP lowering |
| Jay et al., 1983 [70] | 28 healthy subjects | 1% delta-9-THC eye drop four times a day for a week | No significant IOP lowering |
| Porcella et al., 2001 [71] | 8 glaucoma patients (4 POAG, 2 malformative glaucoma, 1 pigmentary glaucoma, 1 angle closure glaucoma) | 25 µg of WIN55212-2, two drops | 15 ± 0.5% IOP lowering after 30 min from administration |
| 50 µg of WIN55212-2, two drops | 23 ± 0.9% IOP lowering after 30 min from administration | ||
| Pescosolido et al., 2018 [72] | 5 glaucoma patients (4 POAG and 1 post-uveitic glaucoma) | Bediol (containing 3–6 mg/mL of delta-9-THC and 4–8 mg/mL of cannabidiol) twice a day for 30 days and then switched to topical Bedrocan (containing 18–23 mg/mL of delta-9-THC and 1.2–18 mg/mL of cannabidiol) twice a day for 30 days | No significant IOP lowering regardless of employed compound. |
| Authors | Patient Population | Intervention | Results |
|---|---|---|---|
| Purnell et al., 1975 [74] | 2 healthy subjects | Single dose of 6.7 mg THC in subject 1 | 62% IOP lowering 90 min after administration |
| Single dose of 3.0 mg THC in subject 2 | 42% IOP lowering 30 min after administration | ||
| Cooler et al., 1976 [75] | 10 healthy subjects | 0.022 mg/Kg delta-9-THC | 29% IOP lowering |
| 0.044 mg/Kg delta-9-THC | 37% IOP lowering |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passani, A.; Posarelli, C.; Sframeli, A.T.; Perciballi, L.; Pellegrini, M.; Guidi, G.; Figus, M. Cannabinoids in Glaucoma Patients: The Never-Ending Story. J. Clin. Med. 2020, 9, 3978. https://doi.org/10.3390/jcm9123978
Passani A, Posarelli C, Sframeli AT, Perciballi L, Pellegrini M, Guidi G, Figus M. Cannabinoids in Glaucoma Patients: The Never-Ending Story. Journal of Clinical Medicine. 2020; 9(12):3978. https://doi.org/10.3390/jcm9123978
Chicago/Turabian StylePassani, Andrea, Chiara Posarelli, Angela Tindara Sframeli, Laura Perciballi, Marco Pellegrini, Gianluca Guidi, and Michele Figus. 2020. "Cannabinoids in Glaucoma Patients: The Never-Ending Story" Journal of Clinical Medicine 9, no. 12: 3978. https://doi.org/10.3390/jcm9123978
APA StylePassani, A., Posarelli, C., Sframeli, A. T., Perciballi, L., Pellegrini, M., Guidi, G., & Figus, M. (2020). Cannabinoids in Glaucoma Patients: The Never-Ending Story. Journal of Clinical Medicine, 9(12), 3978. https://doi.org/10.3390/jcm9123978





