ACE2 Interaction Networks in COVID-19: A Physiological Framework for Prediction of Outcome in Patients with Cardiovascular Risk Factors
Abstract
1. Introduction
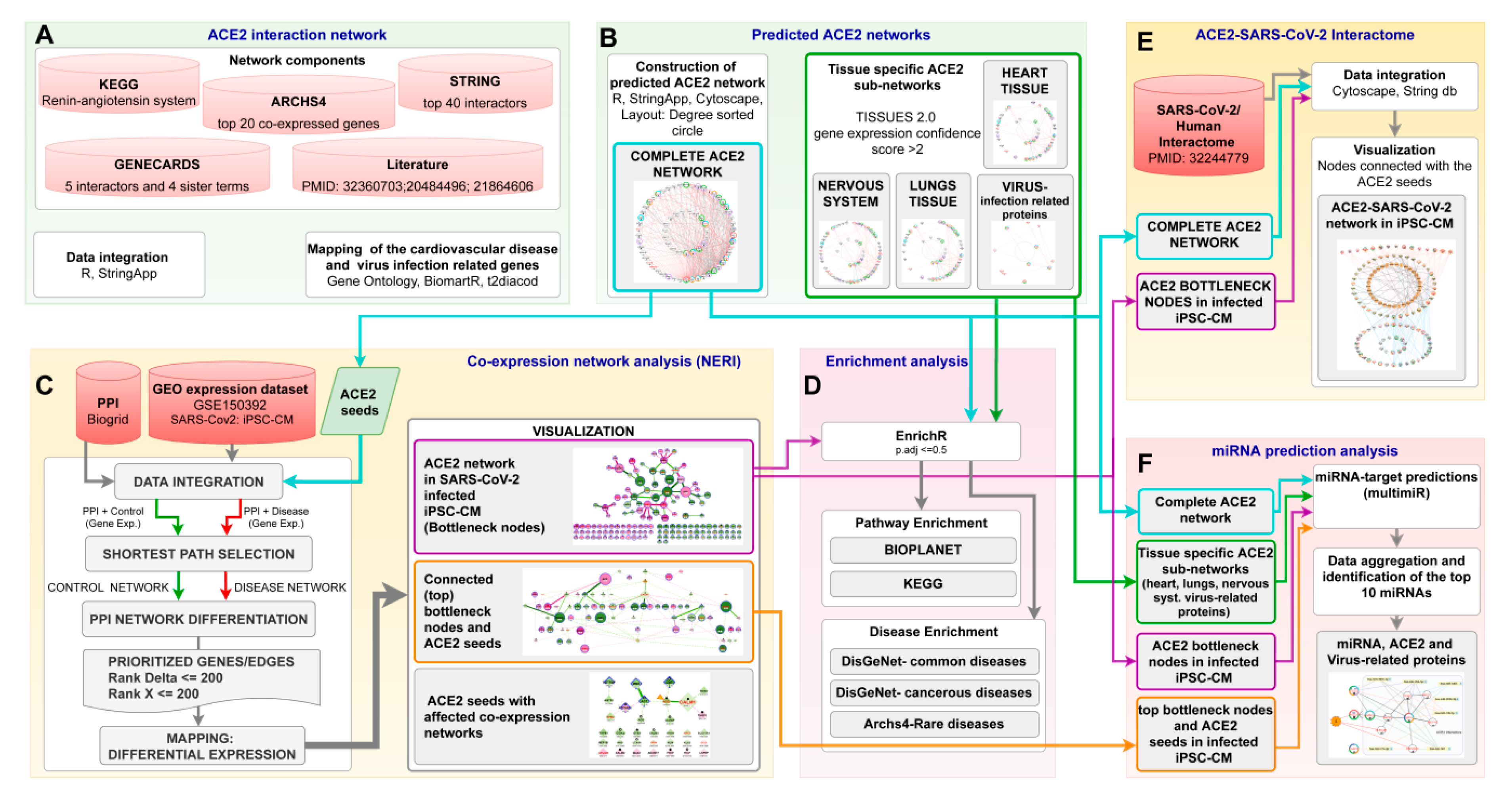
2. Materials and Methods
2.1. Data Collection
2.2. Tissue Expression Analysis
2.3. Interaction Network Analysis
2.3.1. ACE2 Interactome
2.3.2. NERI Method
2.4. Extraction of Disease-Relevant Ontological Terms
2.5. Enrichment Analysis
2.6. miRNA Predictions
3. Results
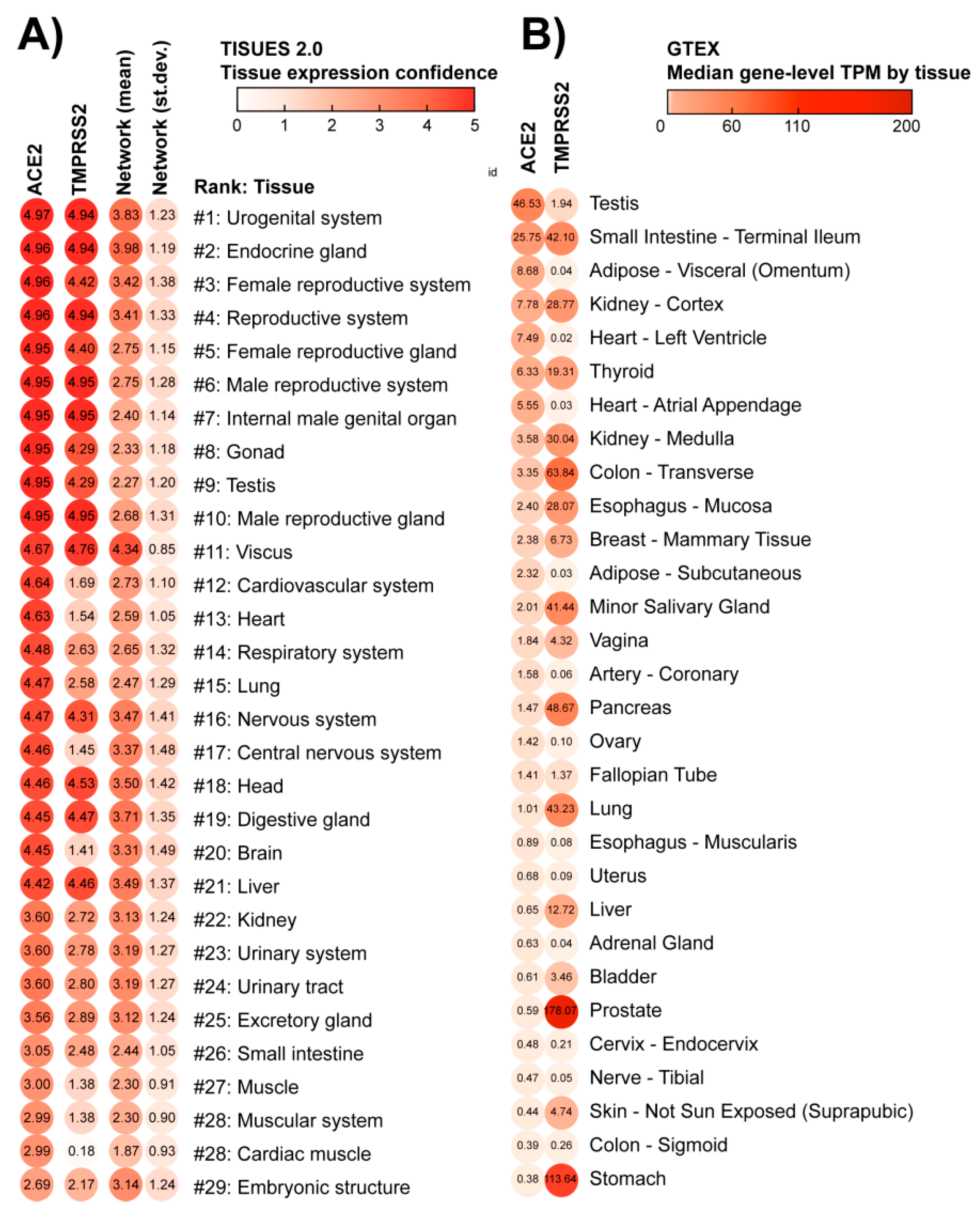
3.1. ACE2 Tissue-Specific Expression
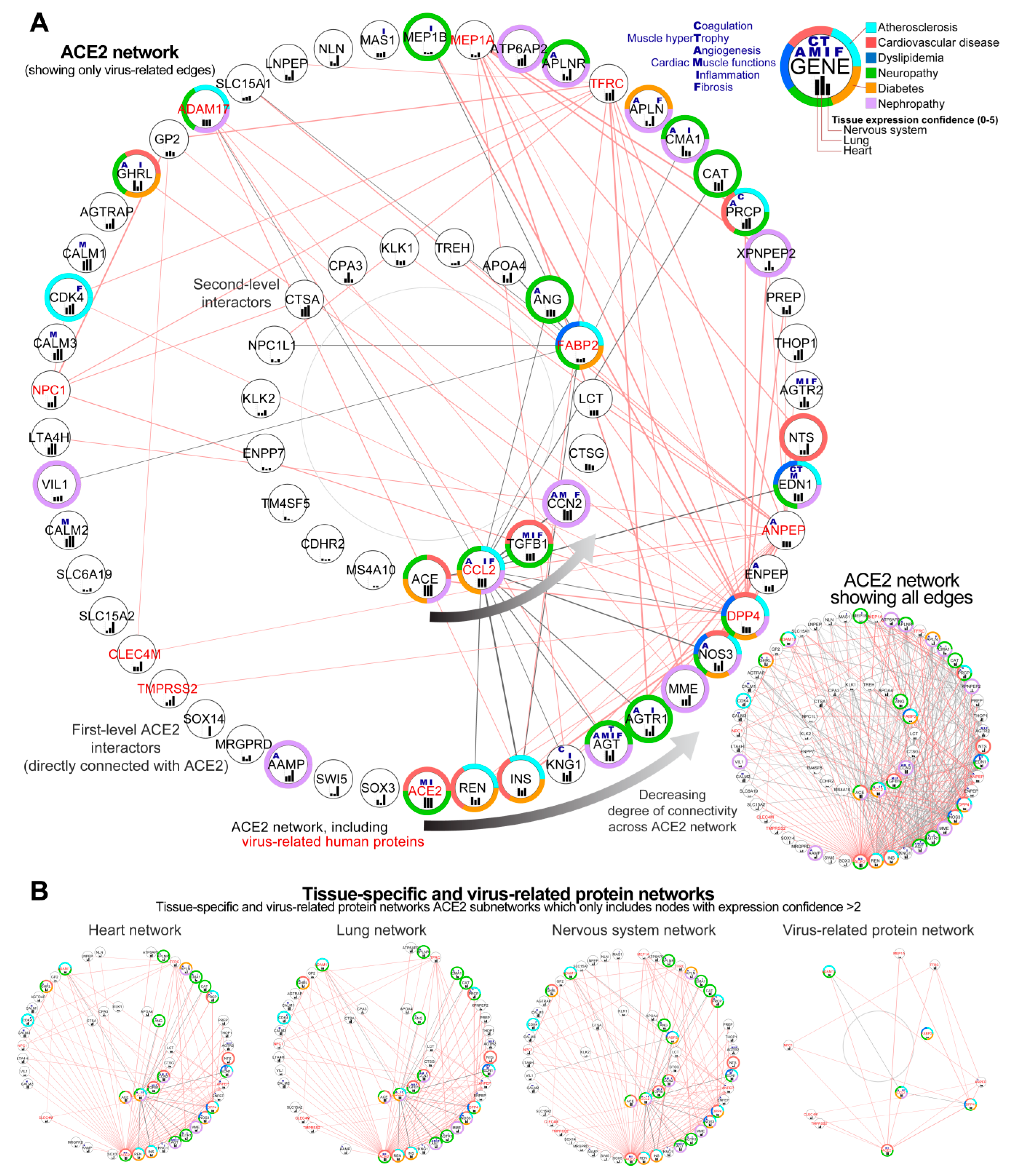
3.2. Construction of the Complete ACE2 Network
3.2.1. Identification of Genes Showing the Highest Connectivity within the Complete ACE2 Network
3.2.2. Identification of the Virus Infection-Related Proteins within the Complete ACE2 Network
3.2.3. Sub-Setting of the Tissues-Specific ACE-2 Related Networks
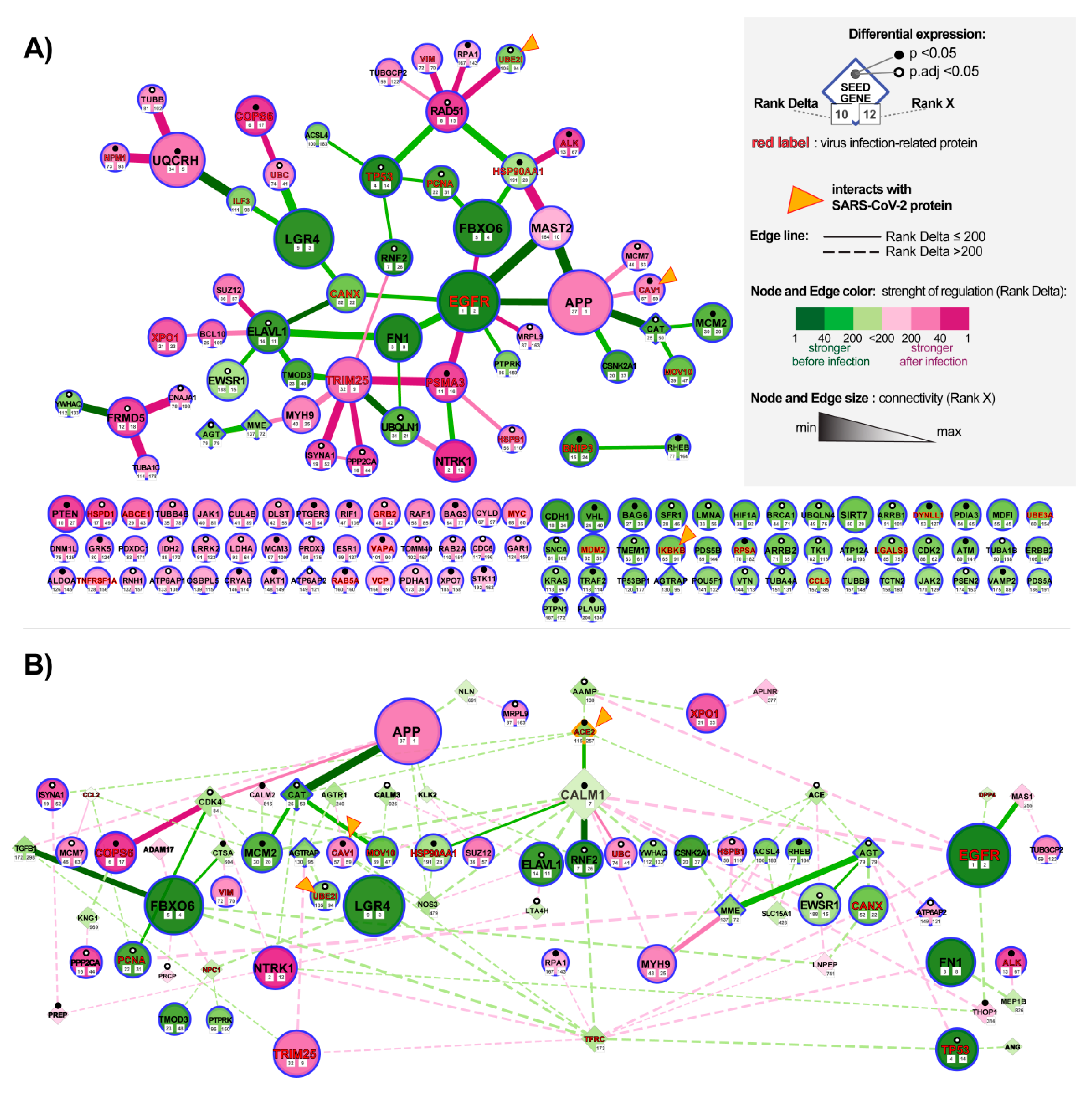
3.3. Analysis of Changes in ACE2 Co-Expression Network in Infected Cardiomyocytes
3.3.1. Identification of the ACE-2 Related Hub Genes Related to Corrupted Co-expression Networks
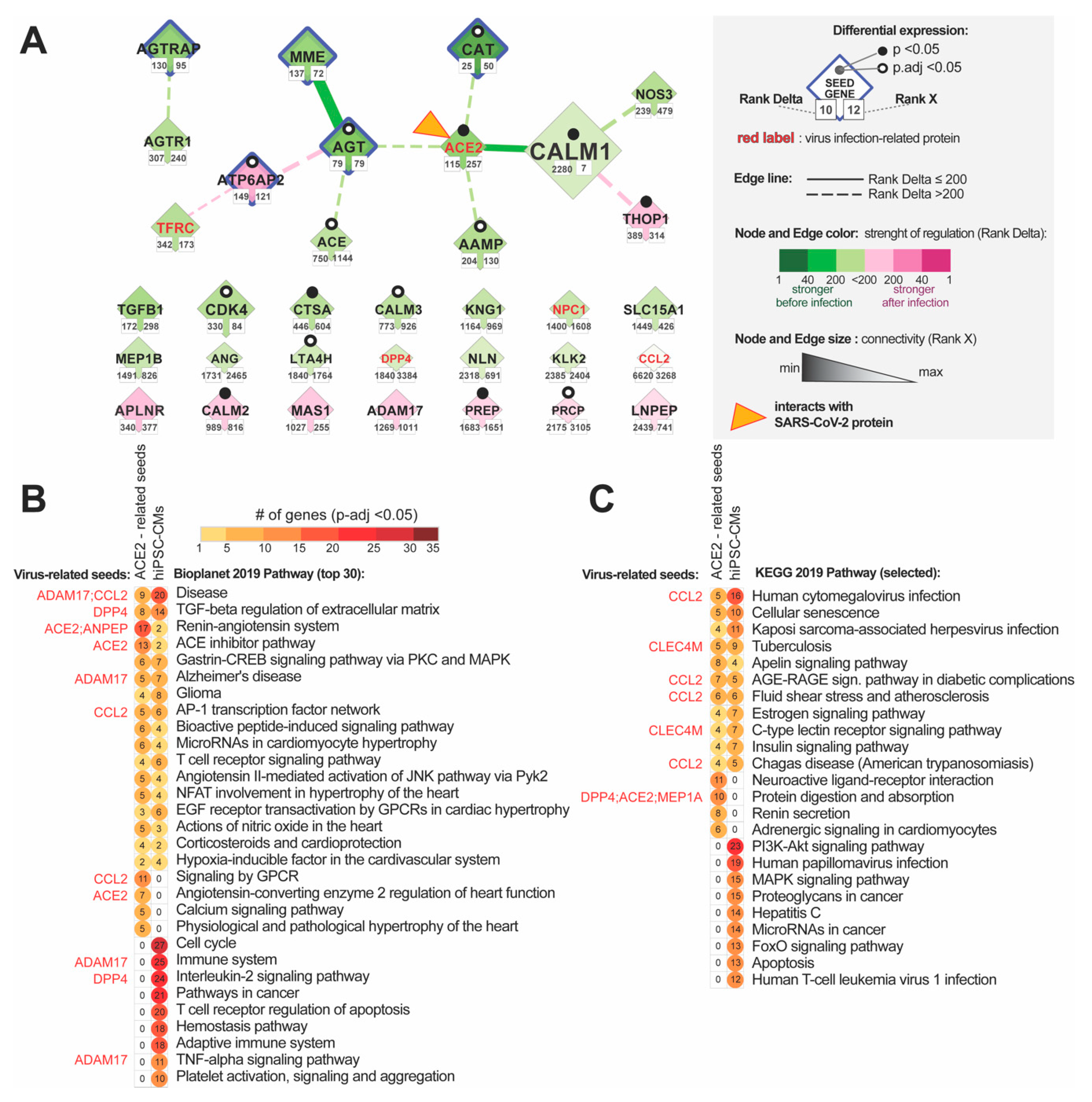
3.3.2. Identification of the Most Altered Connections between ACE2-Related Hub Genes
3.3.3. Identification of the Most Altered Connections between ACE2-Related Seed Genes and Hub Genes
3.3.4. Identification of the Most Altered Connections between ACE2-Related Seed Genes
3.4. Enrichment Analysis of the Signalling within ACE2-Tissue-Specific Network
3.5. Enrichment Analysis of the Disease Terms Associated with ACE2 Tissue-Specific Network
3.5.1. Analysis of the ACE2-Related Common Diseases
3.5.2. Analysis of ACE2-Related Cancerous Diseases
3.5.3. Analysis of ACE2-Related Rare Diseases
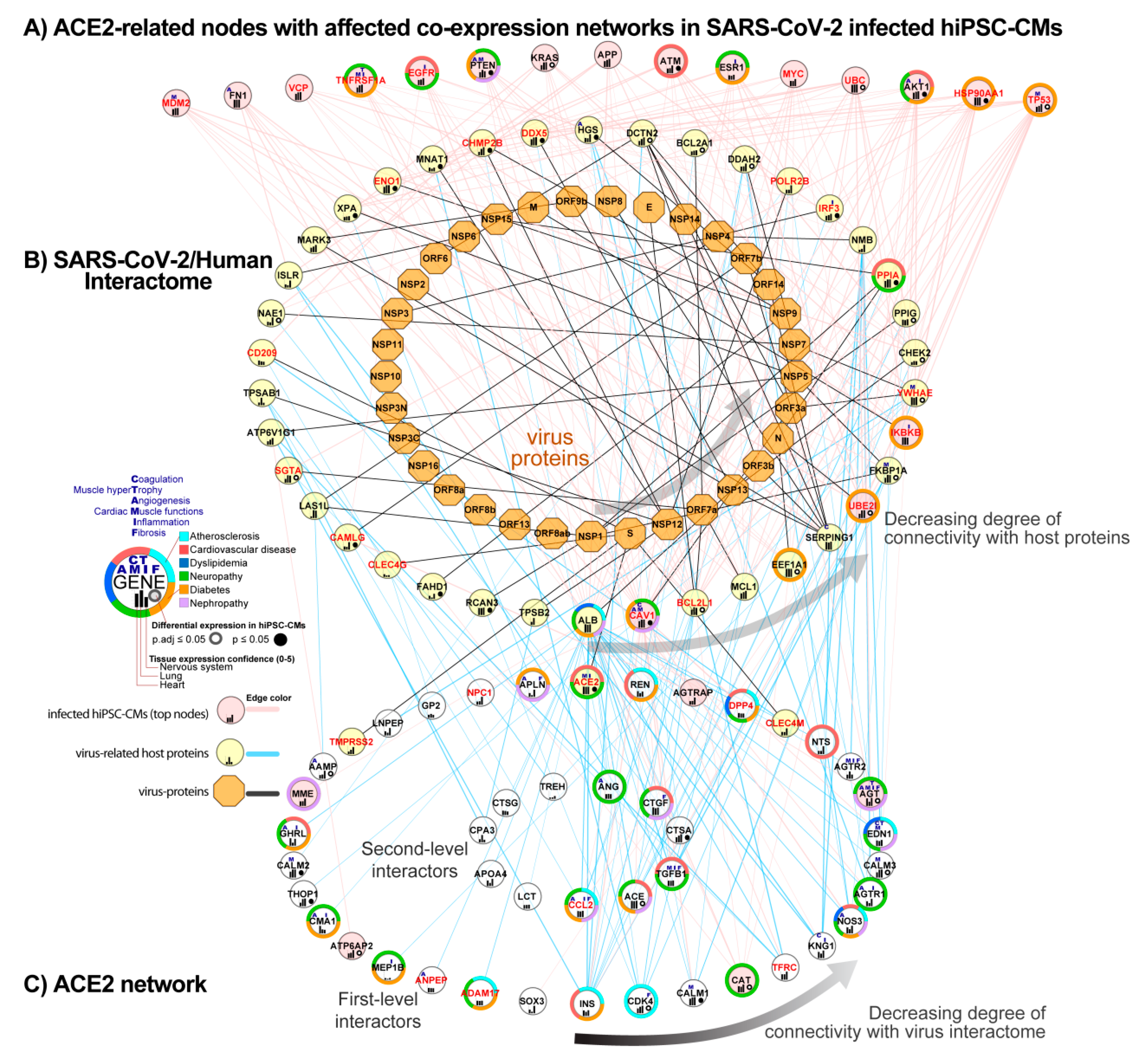
3.6. Integration of ACE2 Network with SARS-CoV-2/Human Interactome
3.6.1. Identification of ACE2-Related Genes Interacting with the Virus Proteins
3.6.2. Identification of ACE2-Related Genes Interacting with the Host Proteins
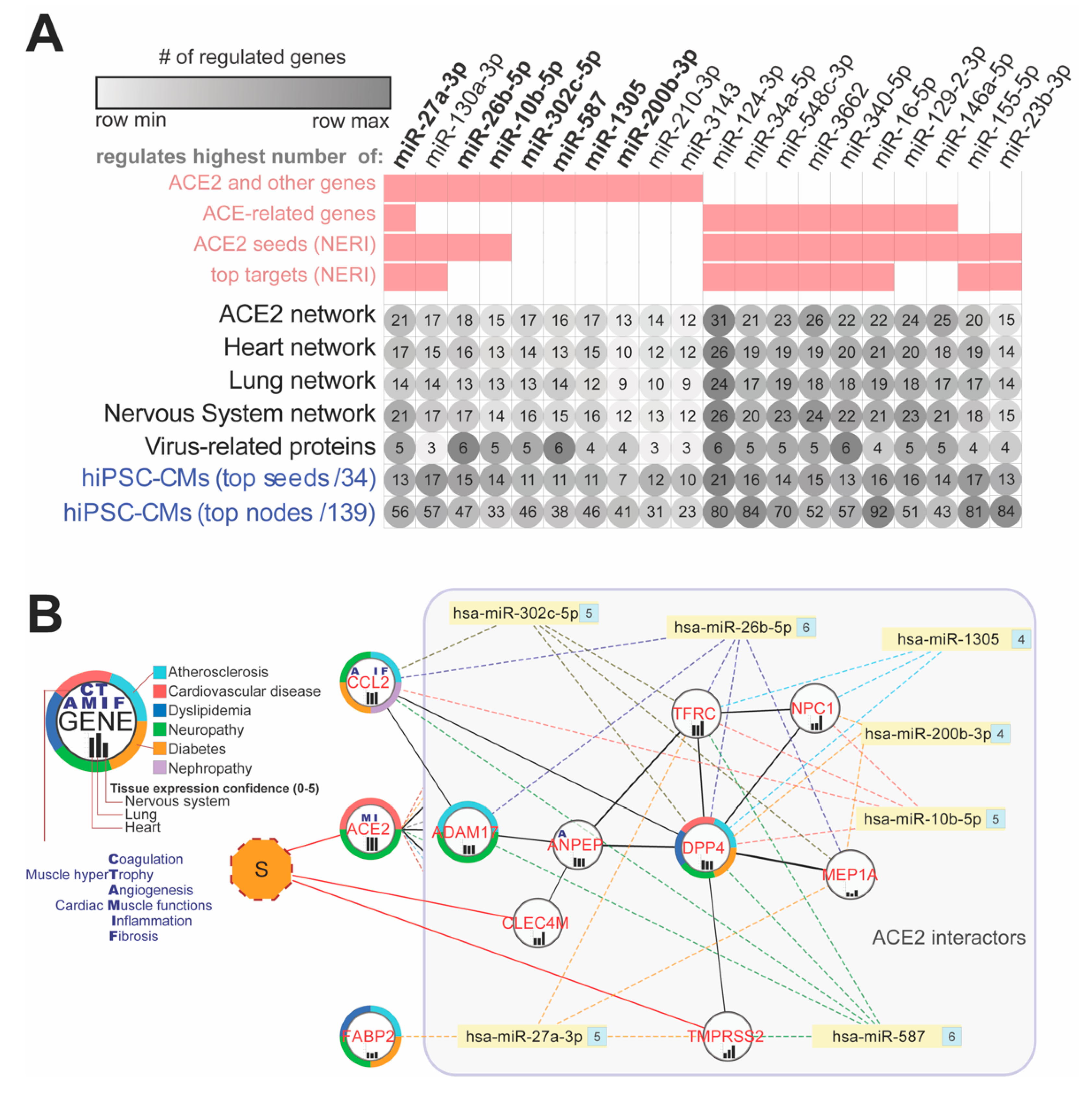
3.7. ACE2 Network-Related miRNA Predictions
4. Discussion
4.1. The High Expression of ACE2 in the Cardiovascular System Explains Why SARS-CoV-2 Infection May Target the Heart
4.2. The High Expression of ACE2 in the Reproductive System and Endocrine Glands and Enrichment of Cancerous Diseases Related to Those Organs Suggest Wider Implications of SARS-CoV-2
4.3. ACE, REN, INS, KNG1, and AGT Play A Role in Cardiovascular Risk Factors Related to SARS-CoV-2 Binding to the ACE2 Receptor
4.4. Analysis of Infected hiPSC-CMS Using the NERI Algorithm Revealed Affected Co-expression Networks of EGFR, APP, and CALM1 Implicating Their Role in Cardiac and Thromboembolic Complications
4.5. Alterations in the ACE2 Interaction Network Can Aggravate Major Comorbidities in COVID19 through Related Signalling Pathways
4.6. Renin-Angiotensin Pathway, AGE-RAGE, and Apelin Signalling as Fundamental Mediators of the Blood Pressure Dysregulation Mediated through ACE2 in COVID-19
4.7. The Role of Virus-Infection Related Proteins from ACE2 Network in COVID-19 Adverse Outcomes
4.8. miRNAs as Promising Antiviral Modulators of the ACE2 Network and a Potential Biomarker of HF Associated with COVID-19
4.8.1. miR-1305 and miR-587: TGF-β Signaling Pathway Regulators in HF Progression
4.8.2. miR-26b-5p: Anti-fibrotic Agent and AGTR1-Dependent Hypertension Modulator
4.8.3. miR-302c-5p: Potential Antiviral Therapeutic and Biomarker of HF
4.8.4. miR-27a-3p: A Potential Biomarker of Acute HF and NF-κB Signaling Regulator
4.8.5. Hsa-miR-16-5p: Modulates Inflammatory Signaling and Cytokines including IL-1β, IL-6, and TNF-α, NF-κB mTOR-Related Pathways
4.8.6. Hsa-miR-124-3p: Has a Potentially Aggravating Role in Cardiovascular Consequences of COVID19
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-Converting Enzyme 2 |
| ACE | Angiotensin-Converting Enzyme 1 |
| ARDS | Acute respiratory distress syndrome |
| COVID-19 | Coronavirus disease 2019 |
| CVD | Cardiovascular disease |
| DEG | Differentially expressed genes |
| DM | Diabetes mellitus |
| eNOS | Endothelial nitric oxide synthase |
| GEO | Gene Expression Omnibus |
| GO | Gene Ontology |
| GPCRs | G-protein-coupled receptors |
| GTEx | Genotype-Tissue Expression |
| HF | Heart failure |
| HK | High molecular weight kininogen |
| hiPSC-CMs | Human-induced pluripotent stem cell-derived cardiomyocytes |
| INS | Insulin |
| KKS | Kallikrein-Kinin system |
| KNG1 | Kininogen 1 |
| MERS | Middle-East respiratory syndrome coronavirus |
| MI | Myocardial infarction |
| miRNAs, miR | MicroRNAs |
| NERI | NEtwork by Relative Importance |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| PPI | Protein–protein interaction |
| RAS | Renin–angiotensin system |
| REN | Renin |
| ROS | Reactive oxygen species |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| TMPRSS2 | Transmembrane protease serine 2 |
| Gene/protein | |
| ACE2 | Angiotensin-Converting Enzyme 2 |
| ADAM17 | ADAM metallopeptidase domain 17 |
| AGT | Angiotensinogen |
| AGTR1 | Angiotensin II Receptor Type 1 |
| ALB | Albumin |
| APP | Amyloid Beta Precursor Protein |
| ANPEP | Alanyl Aminopeptidase |
| APN/CD13 | Aminopeptidase N/CD13 |
| ATP6AP2 | ATPase H+ Transporting Accessory Protein 2 |
| CALM1 | Calmodulin 1 |
| CAT | Catalase |
| CAV1 | Caveolin-1 |
| CCL2 | C-C Motif Chemokine Ligand 2 |
| CCN2 | Cellular Communication Network Factor 2 |
| CDHR2 | Cadherin Related Family Member 2 |
| CDK4 | Cyclin-Dependent Kinase 4 |
| CLEC4M | C-Type Lectin Domain Family 4 Member M |
| CTSA | Cathepsin A |
| CTSG | Cathepsin G |
| DPP4 | Dipeptidyl Peptidase 4 |
| EGFR | Epidermal Growth Factor Receptor |
| ENPEP | Glutamyl Aminopeptidase |
| EV71 | Enterovirus 71 |
| EWSR1 | EWS RNA Binding Protein 1 (EWSR1) |
| FABP2 | Fatty acid-binding protein 2 |
| FN1 | Fibronectin 1 |
| FoxO2 | Forkhead box O-2 |
| HSP90AA1 | Heat Shock Protein 90 Alpha Family Class A Member 1 |
| INS | Insulin |
| KNG1 | Kininogen 1 |
| KPNA2 | Karyopherin Subunit Alpha 2 |
| LNPEP | Leucyl And Cystinyl Aminopeptidase |
| LTA4H | Leukotriene A4 hydrolase |
| MAPK | Mitogen-activated protein kinase |
| MAST2 | Microtubule Associated Serine/Threonine Kinase 2 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MEP1A | Meprin A subunit alpha |
| MME | Membrane metallo-endopeptidase |
| MS4A10 | Membrane Spanning 4-Domains A10 |
| NFKB1 | Nuclear Factor Kappa B Subunit 1 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NPC1 | Niemann-Pick disease, type C1 |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PDGFR-b | Platelet-derived growth factor receptor-beta |
| PPARγ | Peroxisome proliferator-activated receptor-gamma |
| PRCP | Prolyl-carboxypeptidase |
| TFRC | Transferrin Receptor |
| TGFB1 | Transforming Growth Factor Beta 1 |
| THOP1 | Thimet Oligo-peptidase 1 |
| TMPRSS2 | Transmembrane protease, serine 2 |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| Signaling pathways | |
| AGE-RAGE | Advanced glycation end products-Receptor for Advanced glycation end products |
| ERK1/2/AP-1 | Extracellular signal-regulated kinases 1/2/AP-1 |
| PI3K/AKT/Nrf2 | Phosphatidylinositol 3′-kinase/AKT/NF-E2-related factor 2 |
| ERK1/2/AP-1 | Extracellular signal-regulated kinases 1/2/AP-1 |
| PI3K/AKT/Nrf2 | Phosphatidylinositol 3′-kinase/AKT/NF-E2-related factor 2 |
| RAS | Renin-Angiotensin System |
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.M.; Hedley, A.J.; Ho, L.-M.; Chau, P.; Wong, I.O.; Thach, T.Q.; Ghani, A.C.; Donnelly, C.A.; Fraser, C.; Riley, S.; et al. The Epidemiology of Severe Acute Respiratory Syndrome in the 2003 Hong Kong Epidemic: An Analysis of All 1755 Patients. Ann. Intern. Med. 2004, 141, 662–673. [Google Scholar] [CrossRef]
- Assiri, A.; Al-Tawfiq, J.A.; Al-Rabeeah, A.A.; Al-Rabiah, F.A.; Al-Hajjar, S.; Al-Barrak, A.; Flemban, H.; Al-Nassir, W.N.; Balkhy, H.H.; Al-Hakeem, R.F.; et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect. Dis. 2013, 13, 752–761. [Google Scholar] [CrossRef]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System. JAMA Cardiol. 2020, 5, 831. [Google Scholar] [CrossRef]
- Wang, K.; Gheblawi, M.; Oudit, G.Y. Angiotensin Converting Enzyme 2. Circulation 2020, 142, 426–428. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Amraei, R.; Rahimi, N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 2020, 9, 1652. [Google Scholar] [CrossRef]
- Patel, V.B.; Zhong, J.-C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/Angiotensin 1–7 Axis of the Renin–Angiotensin System in Heart Failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Krüger, N.; Müller, M.A.; Drosten, C.; Pöhlmann, S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Sharma, A.; Garcia, G.; Wang, Y.; Plummer, J.T.; Morizono, K.; Arumugaswami, V.; Svendsen, C.N. Human iPSC-Derived Cardiomyocytes Are Susceptible to SARS-CoV-2 Infection. Cell Rep. Med. 2020, 1, 100052. [Google Scholar] [CrossRef] [PubMed]
- Bojkova, D.; Wagner, J.; Shumliakivska, M.; Aslan, G.; Saleem, U.; Hansen, A.; Luxan, G.; Guenther, S.; Pham, M.D.; Krishnan, J.; et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. bioRxiv 2020. [Google Scholar] [CrossRef]
- Bulfamante, G.; Perrucci, G.L.; Falleni, M.; Sommariva, E.; Tosi, D.; Martinelli, C.; Songia, P.; Poggio, P.; Carugo, S.; Pompilio, G. Evidence of SARS-CoV-2 transcriptional activity in cardiomyocytes of COVID-19 patients without clinical signs of cardiac involvement. medRxiv 2020. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Kassiri, Z.; Jiang, C.; Liu, P.P.; Poutanen, S.M.; Penninger, J.M.; Butany, J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009, 39, 618–625. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802. [Google Scholar] [CrossRef]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Kanehisa, M. The KEGG Database. In “In Silico” Simulation of Biological Processes; John Wiley & Sons: Hoboken, NJ, USA, 2002; pp. 91–103. [Google Scholar]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-Y.; Chang, S.C.; Wu, H.-Y.; Yu, T.-C.; Wei, W.-C.; Lin, S.; Chien, C.-L.; Chang, M.-F. Upregulation of the Chemokine (C-C Motif) Ligand 2 via a Severe Acute Respiratory Syndrome Coronavirus Spike-ACE2 Signaling Pathway. J. Virol. 2010, 84, 7703–7712. [Google Scholar] [CrossRef] [PubMed]
- Kuan, T.-C.; Yang, T.-H.; Wen, C.-H.; Chen, M.-Y.; Lee, I.L.; Lin, C.-S. Identifying the regulatory element for human angiotensin-converting enzyme 2 (ACE2) expression in human cardiofibroblasts. Peptides 2011, 32, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Wicik, Z.; Neto, L.H.J.; Guzman, L.E.; Pavão, R.; Takayama, L.; Caparbo, V.; Lopes, N.; Pereira, A.C.; Pereira, R.M. The crosstalk between bone metabolism, lncRNAs, microRNAs and mRNAs in coronary artery calcification. Genomics 2020. [Google Scholar] [CrossRef] [PubMed]
- Palasca, O.; Santos, A.; Stolte, C.; Gorodkin, J.; Jensen, L.J. TISSUES 2.0: An integrative web resource on mammalian tissue expression. Database 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.N.; Martins, D.C., Jr.; Ab Pereira, C.; Hashimoto, R.F.; Brentani, H.B. NERI: Network-medicine based integrative approach for disease gene prioritization by relative importance. BMC Bioinform. 2015, 16, S9. [Google Scholar] [CrossRef] [PubMed]
- Eyileten, C.; Wicik, Z.; De Rosa, S.; Mirowska-Guzel, D.; Soplinska, A.; Indolfi, C.; Kurkowska-Jastrzębska, I.; Członkowska, A.; Postula, M. MicroRNAs as Diagnostic and Prognostic Biomarkers in Ischemic Stroke—A Comprehensive Review and Bioinformatic Analysis. Cells 2018, 7, 249. [Google Scholar] [CrossRef]
- Rani, J.; Mittal, I.; Pramanik, A.; Singh, N.; Dube, N.; Sharma, S.; Puniya, B.L.; Raghunandanan, M.V.; Mobeen, A.; Ramchandran, S. T2DiACoD: A Gene Atlas of Type 2 Diabetes Mellitus Associated Complex Disorders. Sci. Rep. 2017, 7, 6892. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’Ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Kechris, K.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The multiMiR R package and database: Integration of microRNA–target interactions along with their disease and drug associations. Nucleic Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef] [PubMed]
- Pordzik, J.; Jakubik, D.; Jarosz-Popek, J.; Wicik, Z.; Eyileten, C.; De Rosa, S.; Indolfi, C.; Siller-Matula, J.M.; Czajka, P.; Postula, M. Significance of circulating microRNAs in diabetes mellitus type 2 and platelet reactivity: Bioinformatic analysis and review. Cardiovasc. Diabetol. 2019, 18, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; Wicik, Z.; De Rosa, S.; Eyileten, C.; Jakubik, D.; Spaccarotella, C.; Mongiardo, A.; Postula, M.; Indolfi, C. MicroRNAs fingerprint of bicuspid aortic valve. J. Mol. Cell. Cardiol. 2019, 134, 98–106. [Google Scholar] [CrossRef]
- Barabási, A.-L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry. 2020, 10, 875–882. [Google Scholar] [CrossRef]
- Feltrin, A.S.; Tahira, A.C.; Simões, S.N.; Brentani, H.; Martins, D.C., Jr. Assessment of complementarity of WGCNA and NERI results for identification of modules associated to schizophrenia spectrum disorders. PLoS ONE 2019, 14, e0210431. [Google Scholar] [CrossRef]
- Guzzi, P.H.; Mercatelli, D.; Ceraolo, C.; Giorgi, F.M. Master Regulator Analysis of the SARS-CoV-2/Human Interactome. J. Clin. Med. 2020, 9, 982. [Google Scholar] [CrossRef]
- Garvin, M.R.; Alvarez, C.; Miller, J.I.; Prates, E.T.; Walker, A.M.; Amos, B.K.; Mast, A.E.; Justice, A.; Aronow, B.; Jacobson, D.A. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. eLife 2020, 9, 9. [Google Scholar] [CrossRef]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. Covid-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef]
- Yan, J.; Risacher, S.L.; Shen, L.; Saykin, A.J. Network approaches to systems biology analysis of complex disease: Integrative methods for multi-omics data. Brief. Bioinform. 2017, 19, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-A.; Wuchty, S.; Przytycka, T.M. Identifying Causal Genes and Dysregulated Pathways in Complex Diseases. PLoS Comput. Biol. 2011, 7, e1001095. [Google Scholar] [CrossRef] [PubMed]
- Suratanee, A.; Plaimas, K. Network-based association analysis to infer new disease-gene relationships using large-scale protein interactions. PLoS ONE 2018, 13, e0199435. [Google Scholar] [CrossRef] [PubMed]
- Sadegh, S.; Matschinske, J.; Blumenthal, D.B.; Galindez, G.; Kacprowski, T.; List, M.; NasiriGerdeh, R.; Oubounyt, M.; Pichlmair, A.; Rose, T.D.; et al. Exploring the SARS-CoV-2 virus-host-drug interactome for drug repurposing. Nat. Commun. 2020, 11, 3518. [Google Scholar] [CrossRef] [PubMed]
- Maroli, N.; Bhasuran, B.; Natarajan, J.; Kolandaivel, P. The potential role of procyanidin as a therapeutic agent against SARS-CoV-2: A text mining, molecular docking and molecular dynamics simulation approach. J. Biomol. Struct. Dyn. 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Muthuramalingam, P.; Jeyasri, R.; Valliammai, A.; Selvaraj, A.; Karthika, C.; Gowrishankar, S.; Pandian, S.K.; Ramesh, M.; Chen, J.-T. Global multi-omics and systems pharmacological strategy unravel the multi-targeted therapeutic potential of natural bioactive molecules against COVID-19: An in silico approach. Genomics 2020, 112, 4486–4504. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Tang, W.; Zhang, L.; Chen, W.; Yan, Z.; Yuan, P.; Yang, M.; Kong, S.; Yan, L.; et al. Single-cell transcriptome analysis of the novel coronavirus (SARS-CoV-2) associated gene ACE2 expression in normal and non-obstructive azoospermia (NOA) human male testes. Sci. China Life Sci. 2020, 63, 1006–1015. [Google Scholar] [CrossRef]
- Ding, Y.; He, L.; Zhang, Q.; Huang, Z.; Che, X.; Hou, J.; Wang, H.; Shen, H.; Qiu, L.; Li, Z.; et al. Organ distribution of severe acute respiratory syndrome(SARS) associated coronavirus(SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J. Pathol. 2004, 203, 622–630. [Google Scholar] [CrossRef]
- Reis, F.M.; Bouissou, D.R.; Pereira, V.M.; Camargos, A.F.; Dos Reis, A.M.; Santos, R.A. Angiotensin-(1-7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil. Steril. 2011, 95, 176–181. [Google Scholar] [CrossRef]
- Somasundaram, N.P.; Ranathunga, I.; Ratnasamy, V.; Wijewickrama, P.S.A.; Dissanayake, H.A.; Yogendranathan, N.; Gamage, K.K.K.; De Silva, N.L.; Sumanatilleke, M.; Katulanda, P.; et al. The Impact of SARS-Cov-2 Virus Infection on the Endocrine System. J. Endocr. Soc. 2020, 4. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xie, W.; Li, D.; Shi, L.; Mao, Y.; Xiong, Y.; Zhang, Y.; Zhang, M. Effect of SARS-CoV-2 infection upon male gonadal function: A single center-based study. medRxiv 2020. [Google Scholar] [CrossRef]
- Fan, C.; Li, K.; Ding, Y.; Lu, W.L.; Wang, J. ACE2 Expression in Kidney and Testis May Cause Kidney and Testis Damage After 2019-nCoV Infection. Urology 2020. [Google Scholar] [CrossRef]
- Kaplan, S.A. Re: Urinary Frequency as a Possibly Overlooked Symptom in COVID-19 Patients: Does SARS–CoV-2 Cause Viral Cystitis? J. Urol. 2020, 204, 1071–1072. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, A.; Li, H.; Zheng, K.; Zhuang, Z.; Chen, Z.; Shi, Y.; Zhang, Z.; Chen, S.-B.; Liu, X.; et al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg. Microbes Infect. 2020, 9, 991–993. [Google Scholar] [CrossRef]
- Mak, T.W.; Hauck, L.; Grothe, D.; Billia, F. p53 regulates the cardiac transcriptome. Proc. Natl. Acad. Sci. USA 2017, 114, 2331–2336. [Google Scholar] [CrossRef]
- Rhaleb, N.-E.; Yang, X.-P.; Carretero, O.A. The Kallikrein-Kinin System as a Regulator of Cardiovascular and Renal Function. Compr. Physiol. 2011, 1, 971–993. [Google Scholar] [CrossRef]
- Isordia-Salas, I.; Pixley, R.A.; Sainz, I.M.; Martínez-Murillo, C.; Colman, R.W. The role of plasma high molecular weight kininogen in experimental intestinal and systemic inflammation. Arch. Med. Res. 2004, 35, 369–377. [Google Scholar] [CrossRef]
- Mattsson, E.; Herwald, H.; Cramer, H.; Persson, K.; Sjöbring, U.; Björck, L. Staphylococcus aureus Induces Release of Bradykinin in Human Plasma. Infect. Immun. 2001, 69, 3877–3882. [Google Scholar] [CrossRef]
- Schreier, B.; Rabe, S.; Schneider, B.; Bretschneider, M.; Rupp, S.; Ruhs, S.; Neumann, J.; Rueckschloss, U.; Sibilia, M.; Gotthardt, M.; et al. Loss of Epidermal Growth Factor Receptor in Vascular Smooth Muscle Cells and Cardiomyocytes Causes Arterial Hypotension and Cardiac Hypertrophy. Hypertension 2013, 61, 333–340. [Google Scholar] [CrossRef]
- Mitchell, H.D.; Eisfeld, A.J.; Stratton, K.G.; Heller, N.C.; Bramer, L.M.; Wen, J.; McDermott, J.E.; Gralinski, L.E.; Sims, A.C.; Le, M.Q.; et al. The Role of EGFR in Influenza Pathogenicity: Multiple Network-Based Approaches to Identify a Key Regulator of Non-lethal Infections. Front. Cell Dev. Biol. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Eimer, W.A.; Kumar, D.K.V.; Shanmugam, N.K.N.; Rodriguez, A.S.; Mitchell, T.; Washicosky, K.J.; György, B.; Breakefield, X.O.; Tanzi, R.E.; Moir, R.D. Alzheimer’s Disease-Associated β-Amyloid Is Rapidly Seeded by Herpesviridae to Protect against Brain Infection. Neuron 2018, 99, 56–63.e3. [Google Scholar] [CrossRef] [PubMed]
- Inyushin, M.; Zayas-Santiago, A.; Rojas, L.; Kucheryavykh, Y.; Kucheryavykh, L. Platelet-generated amyloid beta peptides in Alzheimer’s disease and glaucoma. Histol. Histopathol. 2019, 34, 843–856. [Google Scholar] [PubMed]
- Kucheryavykh, L.Y.; Kucheryavykh, Y.V.; Washington, A.V.; Inyushin, M. Amyloid Beta Peptide Is Released during Thrombosis in the Skin. Int. J. Mol. Sci. 2018, 19, 1705. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Basak, T.; Nayak, M.K.; Bhardwaj, G.; Mukherjee, A.; Bhowmick, R.; Sengupta, S.; Chakrabarti, O.; Chatterjee, N.S.; Chawla-Sarkar, M. Identification of Cellular Calcium Binding Protein Calmodulin as a Regulator of Rotavirus A Infection during Comparative Proteomic Study. PLoS ONE 2013, 8, e56655. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.W.; Clarke, N.E.; Hooper, N.M.; Turner, A.J. Calmodulin interacts with angiotensin-converting enzyme-2 (ACE2) and inhibits shedding of its ectodomain. FEBS Lett. 2008, 582, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Cat, A.N.D.; Rios, F.J.; Touyz, R.M. Angiotensin II and Vascular Injury. Curr. Hypertens. Rep. 2014, 16, 1–11. [Google Scholar] [CrossRef]
- Crackower, M.A.; Sarao, R.; Oudit, G.Y.; Yagil, C.; Kozieradzki, I.; Scanga, S.E.; Oliveira-Dos-Santos, A.J.; Da Costa, J.; Zhang, L.; Pei, Y.; et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nat. Cell Biol. 2002, 417, 822–828. [Google Scholar] [CrossRef]
- Zou, M.-X.; Liu, H.-Y.; Haraguchi, Y.; Soda, Y.; Tatemoto, K.; Hoshino, H. Apelin peptides block the entry of human immunodeficiency virus (HIV). FEBS Lett. 2000, 473, 15–18. [Google Scholar] [CrossRef]
- Pagliaro, P.; Penna, C. ACE/ACE2 Ratio: A Key Also in 2019 Coronavirus Disease (Covid-19)? Front. Med. 2020, 7, 335. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.; Liu, K.; Guo, F.; Zhang, Y.; Zhang, G.; Jiang, C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008, 18, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, A.; Owczarek, K.; Milewska, A.; Baster, Z.; Rajfur, Z.; Mitchell, J.A.; Pyrć, K. Canine respiratory coronavirus employs caveolin-1-mediated pathway for internalization to HRT-18G cells. Vet. Res. 2018, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wyler, E.; Mösbauer, K.; Franke, V.; Diag, A.; Gottula, L.T.; Arsie, R.; Klironomos, F.; Koppstein, D.; Ayoub, S.; Buccitelli, C.; et al. Bulk and single-cell gene expression profiling of SARS-CoV-2 infected human cell lines identifies molecular targets for therapeutic intervention. bioRxiv 2020. [Google Scholar] [CrossRef]
- Foy, B.H.; Carlson, J.C.T.; Reinertsen, E.; Valls, R.P.I.; Lopez, R.P.; Palanques-Tost, E.; Mow, C.; Westover, M.B.; Aguirre, A.D.; Higgins, J.M. Association of Red Blood Cell Distribution Width With Mortality Risk in Hospitalized Adults With SARS-CoV-2 Infection. JAMA Netw. Open 2020, 3, e2022058. [Google Scholar] [CrossRef]
- Qin, M.; Cao, Z.; Wen, J.; Yu, Q.; Liu, C.; Wang, F.; Zhang, J.; Yang, F.; Li, Y.; Fishbein, G.; et al. An Antioxidant Enzyme Therapeutic for COVID-19. Adv. Mater. 2020, 2004901. [Google Scholar] [CrossRef]
- Valiente-Alandi, I.; Potter, S.J.; Salvador, A.M.; Schafer, A.E.; Schips, T.; Carrillo-Salinas, F.; Gibson, A.M.; Nieman, M.L.; Perkins, C.; Sargent, M.A.; et al. Inhibiting Fibronectin Attenuates Fibrosis and Improves Cardiac Function in a Model of Heart Failure. Circulation 2018, 138, 1236–1252. [Google Scholar] [CrossRef]
- Torre, D.; Pugliese, A.; Ferrario, G.; Marietti, G.; Forno, B.; Zeroli, C. Interaction of human plasma fibronectin with viral proteins of human immunodeficiency virus. FEMS Immunol. Med. Microbiol. 1994, 8, 127–131. [Google Scholar] [CrossRef]
- Barchetta, I.; Cavallo, M.G.; Baroni, M.G. COVID-19 and diabetes: Is this association driven by the DPP4 receptor? Potential clinical and therapeutic implications. Diabetes Res. Clin. Pract. 2020, 163, 108165. [Google Scholar] [CrossRef]
- Li, K.; Wohlford-Lenane, C.L.; Channappanavar, R.; Park, J.-E.; Earnest, J.T.; Bair, T.B.; Bates, A.M.; Brogden, K.A.; Flaherty, H.A.; Gallagher, T.; et al. Mouse-adapted MERS coronavirus causes lethal lung disease in human DPP4 knockin mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3119–E3128. [Google Scholar] [CrossRef]
- Fan, C.; Wu, X.; Liu, Q.; Li, Q.; Liu, S.; Lyu, J.; Yang, Y.; Cao, Y.; Huang, W.; Liang, C.; et al. A Human DPP4-Knockin Mouse’s Susceptibility to Infection by Authentic and Pseudotyped MERS-CoV. Viruses 2018, 10, 448. [Google Scholar] [CrossRef]
- Iacobellis, G. COVID-19 and diabetes: Can DPP4 inhibition play a role? Diabetes Res. Clin. Pract. 2020, 162, 108125. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.E.; Sung, S.-S.J.; Fu, S.M. Significant Involvement of CCL2 (MCP-1) in Inflammatory Disorders of the Lung. Microcirculation 2003, 10, 273–288. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ding, Y.; Zhang, Q.; Che, X.; He, Y.; Shen, H.; Wang, H.; Li, Z.; Zhao, L.; JianzhaoGeng, C.L.; et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+cells in SARS patients: Relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006, 210, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Chen, S.; Yang, C.; Shi, B.; Zhou, L.; Zhao, J. Long noncoding RNA DANCR regulates miR-1305-Smad 4 axis to promote chondrogenic differentiation of human synovium-derived mesenchymal stem cells. Biosci. Rep. 2017, 37, 37. [Google Scholar] [CrossRef]
- Su, Y.; Feng, W.; Shi, J.; Chen, L.; Huang, J.; Lin, T. circRIP2 accelerates bladder cancer progression via miR-1305/Tgf-β2/smad3 pathway. Mol. Cancer 2020, 19. [Google Scholar] [CrossRef]
- Yang, X.; Letterio, J.J.; Lechleider, R.J.; Chen, L.; Hayman, R.; Gu, H.; Roberts, A.B.; Deng, C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 1999, 18, 1280–1291. [Google Scholar] [CrossRef]
- Mirzaei, H.; Faghihloo, E. Viruses as key modulators of the TGF-β pathway; a double-edged sword involved in cancer. Rev. Med. Virol. 2018, 28, e1967. [Google Scholar] [CrossRef]
- Taimor, G.; Heger, J. The complex pattern of SMAD signaling in the cardiovascular system? Cardiovasc. Res. 2006, 69, 15–25. [Google Scholar] [CrossRef]
- Qiu, H.; Zhang, Y.; Zhao, Q.; Jiang, H.; Yan, J.; Liu, Y. Platelet miR-587 may be Used as a Potential Biomarker for Diagnosis of Patients with Acute Coronary Syndrome. Clin. Lab. 2020, 66, 66. [Google Scholar] [CrossRef]
- Tang, C.-M.; Zhang, M.; Huang, L.; Hu, Z.-Q.; Zhu, J.-N.; Xiao, Z.; Zhang, Z.; Lin, Q.-X.; Zheng, X.-L.; Yang, M.; et al. CircRNA_000203 enhances the expression of fibrosis-associated genes by derepressing targets of miR-26b-5p, Col1a2 and CTGF, in cardiac fibroblasts. Sci. Rep. 2017, 7, 40342. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, L.; Peng, N.; Yu, H.; Li, M.; Cao, Z.; Lin, Y.; Wang, X.; Li, Q.; Wang, J.; et al. MicroRNA-302a suppresses influenza A virus–stimulated interferon regulatory factor-5 expression and cytokine storm induction. J. Biol. Chem. 2017, 292, 21291–21303. [Google Scholar] [CrossRef] [PubMed]
- Hamada-Tsutsumi, S.; Naito, Y.; Sato, S.; Takaoka, A.; Kawashima, K.; Isogawa, M.; Ochiya, T.; Tanaka, Y. The antiviral effects of human microRNA miR-302c-3p against hepatitis B virus infection. Aliment. Pharmacol. Ther. 2019, 49, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Yang, X.; Zhu, C.; Zhou, L.; Yu, H.; Li, M.; Lin, Y.; Wang, X.; Li, Q.; She, Y.; et al. MicroRNA-302 Cluster Downregulates Enterovirus 71–Induced Innate Immune Response by Targeting KPNA2. J. Immunol. 2018, 201, 145–156. [Google Scholar] [CrossRef]
- Li, G.; Song, Y.; Li, Y.-D.; Jie, L.-J.; Wu, W.-Y.; Li, J.-Z.; Zhang, Q.; Wang, Y. Circulating miRNA-302 family members as potential biomarkers for the diagnosis of acute heart failure. Biomark. Med. 2018, 12, 871–880. [Google Scholar] [CrossRef]
- Braga, L.; Ali, H.; Secco, I.; Giacca, M. Non-coding RNA therapeutics for cardiac regeneration. Cardiovasc. Res. 2020. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Xu, Q.; Zheng, G.; Qiu, G.; Ge, M.; Shu, Q.; Xu, J. Mesenchymal Stem Cell–Derived Extracellular Vesicles Alleviate Acute Lung Injury Via Transfer of miR-27a-3p. Crit. Care Med. 2020, 1. [Google Scholar] [CrossRef]
- Zhao, X.-R.; Zhang, Z.; Gao, M.; Li, L.; Sun, P.-Y.; Xu, L.-N.; Qi, Y.; Yin, L.-H.; Peng, J.-Y. MicroRNA-27a-3p aggravates renal ischemia/reperfusion injury by promoting oxidative stress via targeting growth factor receptor-bound protein 2. Pharmacol. Res. 2020, 155, 104718. [Google Scholar] [CrossRef]
- Lv, X.; Yan, J.; Jiang, J.; Zhou, X.; Lu, Y.; Jiang, H. MicroRNA-27a-3p suppression of peroxisome proliferator-activated receptor-γ contributes to cognitive impairments resulting from sevoflurane treatment. J. Neurochem. 2017, 143, 306–319. [Google Scholar] [CrossRef]
- Vegter, E.L.; Ovchinnikova, E.S.; Van Veldhuisen, D.J.; Jaarsma, T.; Berezikov, E.; Van Der Meer, P.; Voors, A.A. Low circulating microRNA levels in heart failure patients are associated with atherosclerotic disease and cardiovascular-related rehospitalizations. Clin. Res. Cardiol. 2017, 106, 598–609. [Google Scholar] [CrossRef][Green Version]
- Ovchinnikova, E.S.; Schmitter, D.; Vegter, E.L.; Ter Maaten, J.M.; Valente, M.A.; Liu, L.C.; Van Der Harst, P.; Pinto, Y.M.; De Boer, R.A.; Meyer, S.; et al. Signature of circulating microRNAs in patients with acute heart failure. Eur. J. Heart Fail. 2015, 18, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Takizawa, S.; Ohgaku, Y.; Asami, T.; Furuya, K.; Yamamoto, K.; Takahashi, F.; Hamajima, C.; Inaba, C.; Endo, K.; et al. MicroRNA 16-5p is upregulated in calorie-restricted mice and modulates inflammatory cytokines of macrophages. Gene 2020, 725, 144191. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.-A.; Liu, L.; Jiang, Y.; Jan, J.; Gaddipati, S.; Suvas, S.; Steinle, J.J. miR-15a/16 reduces retinal leukostasis through decreased pro-inflammatory signaling. J. Neuroinflamm. 2016, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mariconti, M.; Vola, A.; Manciulli, T.; Genco, F.; Lissandrin, R.; Meroni, V.; Rosenzvit, M.; Tamarozzi, F.; Brunetti, E. Correction to: Role of microRNAs in host defense against Echinococcus granulosus infection: A preliminary assessment. Immunol. Res. 2018, 67, 98. [Google Scholar] [CrossRef]
- Biswas, S.; Haleyurgirisetty, M.; Lee, S.; Hewlett, I.; Devadas, K. Development and validation of plasma miRNA biomarker signature panel for the detection of early HIV-1 infection. EBioMedicine 2019, 43, 307–316. [Google Scholar] [CrossRef]
- Ketprasit, N.; Cheng, I.S.; Deutsch, F.; Tran, N.; Imwong, M.; Combes, V.; Palasuwan, D. The characterization of extracellular vesicles-derived microRNAs in Thai malaria patients. Malar. J. 2020, 19, 1–14. [Google Scholar] [CrossRef]
- Jia, H.; He, C.-H.; Wang, Z.-Y.; Xu, Y.-F.; Yin, G.-Q.; Mao, L.-J.; Liu, C.-W.; Deng, L. MicroRNA expression profile in exosome discriminates extremely severe infections from mild infections for hand, foot and mouth disease. BMC Infect. Dis. 2014, 14, 506. [Google Scholar] [CrossRef]
- Marquez-Pedroza, J.; Cárdenas-Bedoya, J.; Morán-Moguel, M.C.; Escoto-Delgadillo, M.; Torres-Mendoza, B.M.; Pérez-Ríos, A.M.; González-Enriquez, G.V.; Vázquez-Valls, E. Plasma microRNA expression levels in HIV-1-positive patients receiving antiretroviral therapy. Biosci. Rep. 2020, 40, 40. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, M.; Chen, C.; Gong, W.; Yin, Z.; Li, H.; Fan, J.; Zhang, X.A.; Wang, D.W.; Zuo, H. MiR-124 aggravates failing hearts by suppressing CD151-facilitated angiogenesis in heart. Oncotarget 2018, 9, 14382–14396. [Google Scholar] [CrossRef]
- Dang, J.W.; Tiwari, S.K.; Qin, Y.; Rana, T.M. Genome-wide Integrative Analysis of Zika-Virus-Infected Neuronal Stem Cells Reveals Roles for MicroRNAs in Cell Cycle and Stemness. Cell Rep. 2019, 27, 3618–3628.e5. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wicik, Z.; Eyileten, C.; Jakubik, D.; Simões, S.N.; Martins, D.C., Jr.; Pavão, R.; Siller-Matula, J.M.; Postula, M. ACE2 Interaction Networks in COVID-19: A Physiological Framework for Prediction of Outcome in Patients with Cardiovascular Risk Factors. J. Clin. Med. 2020, 9, 3743. https://doi.org/10.3390/jcm9113743
Wicik Z, Eyileten C, Jakubik D, Simões SN, Martins DC Jr., Pavão R, Siller-Matula JM, Postula M. ACE2 Interaction Networks in COVID-19: A Physiological Framework for Prediction of Outcome in Patients with Cardiovascular Risk Factors. Journal of Clinical Medicine. 2020; 9(11):3743. https://doi.org/10.3390/jcm9113743
Chicago/Turabian StyleWicik, Zofia, Ceren Eyileten, Daniel Jakubik, Sérgio N. Simões, David C. Martins, Jr., Rodrigo Pavão, Jolanta M. Siller-Matula, and Marek Postula. 2020. "ACE2 Interaction Networks in COVID-19: A Physiological Framework for Prediction of Outcome in Patients with Cardiovascular Risk Factors" Journal of Clinical Medicine 9, no. 11: 3743. https://doi.org/10.3390/jcm9113743
APA StyleWicik, Z., Eyileten, C., Jakubik, D., Simões, S. N., Martins, D. C., Jr., Pavão, R., Siller-Matula, J. M., & Postula, M. (2020). ACE2 Interaction Networks in COVID-19: A Physiological Framework for Prediction of Outcome in Patients with Cardiovascular Risk Factors. Journal of Clinical Medicine, 9(11), 3743. https://doi.org/10.3390/jcm9113743




