Hospital Resources May Be an Important Aspect of Mortality Rate among Critically Ill Patients with COVID-19: The Paradigm of Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Setting
2.2. Data Collection and Definitions
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics and ICU Admission
3.2. Interventions in the ICU
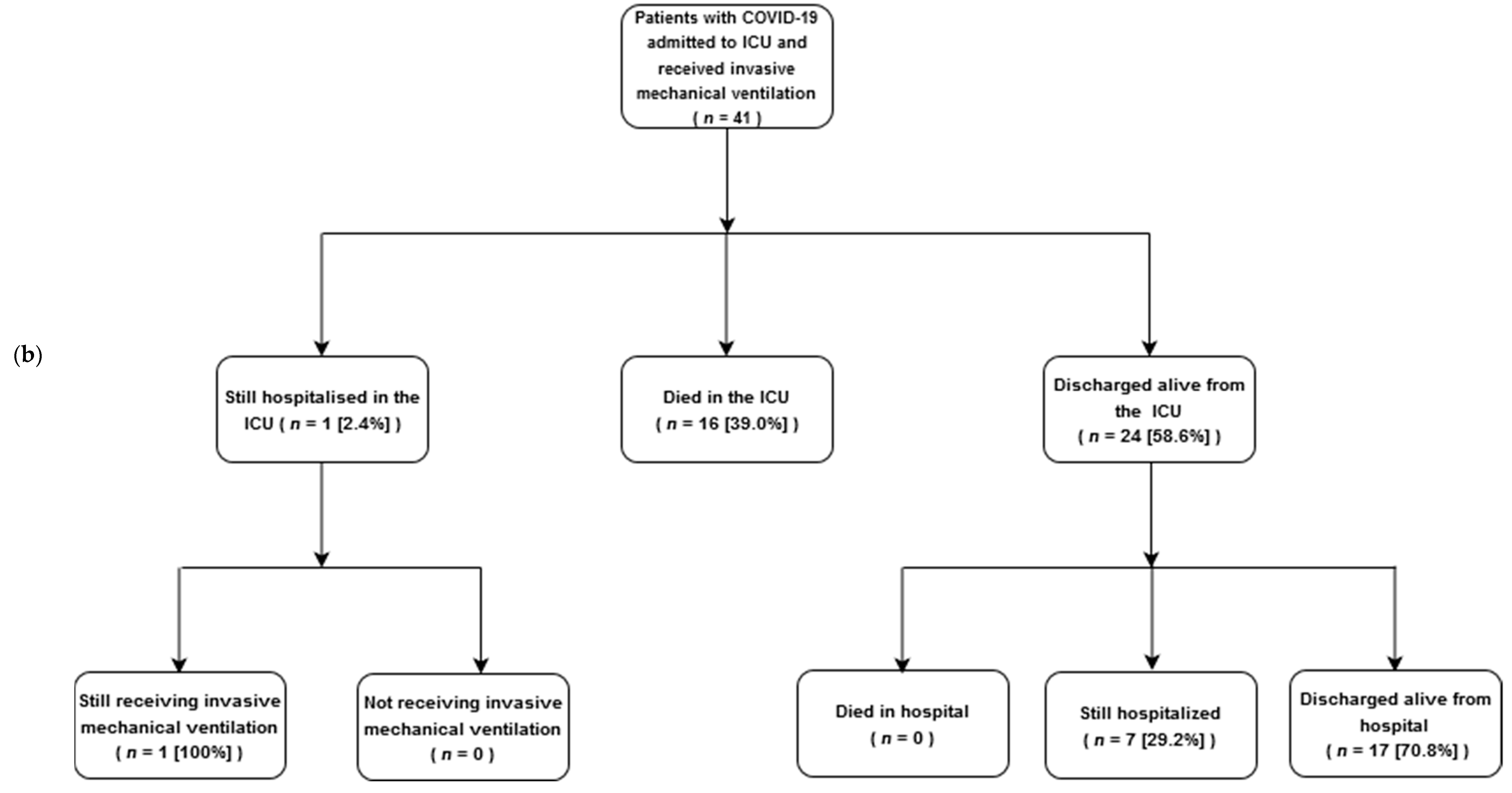
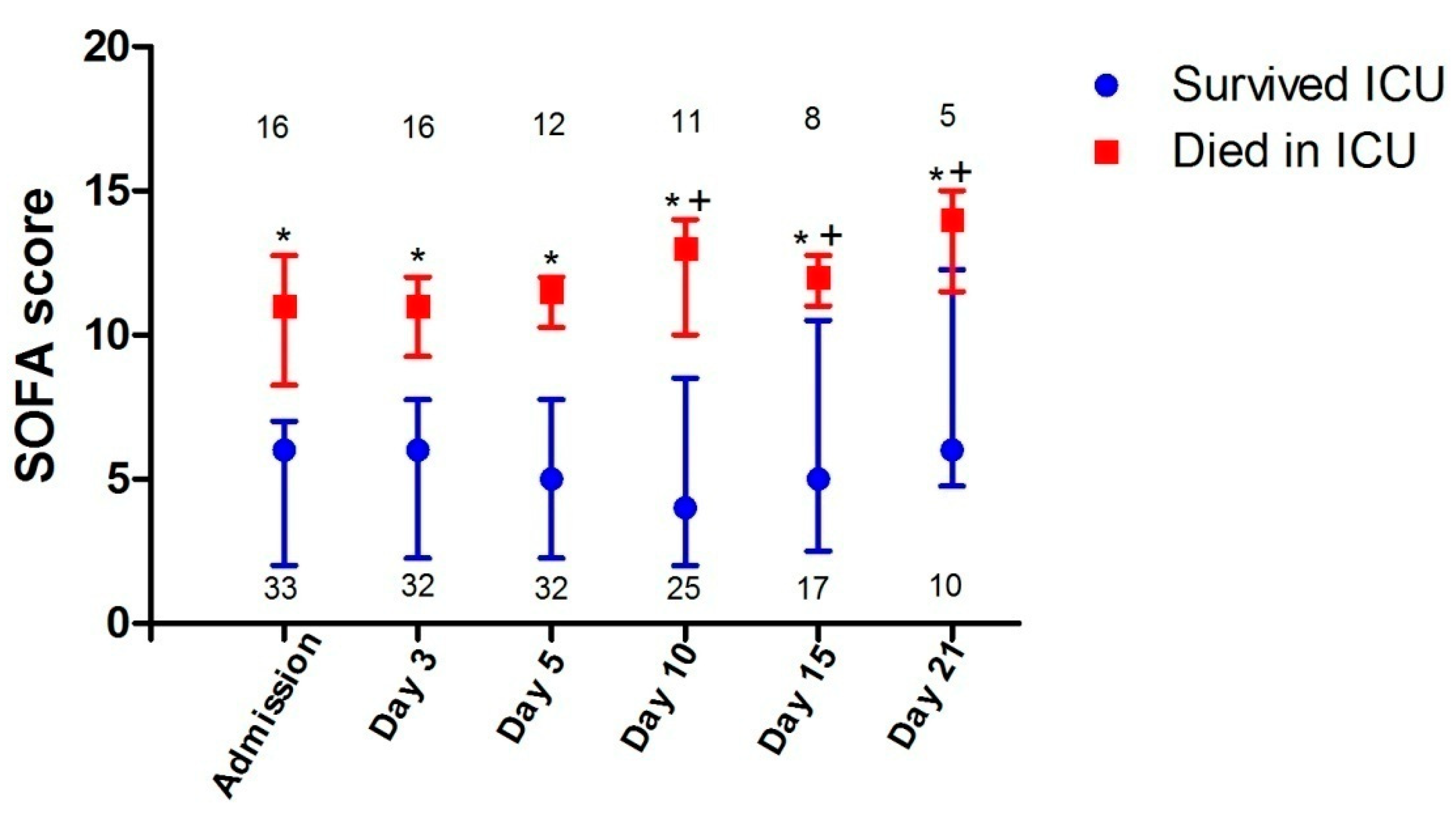
3.3. ICU Outcomes
3.4. The Control Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Declarations
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| ICU | Intensive care unit |
| ARDS | Acute respiratory distress syndrome |
| PEEP | Positive end-expiratory pressure |
| CMV | Conventional mechanical ventilation |
| APACHE | Acute physiology and chronic health evaluation |
| SOFA | Sequential organ failure assessment |
| IQR | Interquartile range |
References
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Karakula-Juchnowicz, H.; Teresinski, G.; Buszewicz, G.; Ciesielka, M.; Sitarz, E. COVID-19: Specific and Non-Specific Clinical Manifestations and Symptoms: The Current State of Knowledge. J. Clin. Med. 2020, 9, 1753. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.; Narasimhan, M.; Crawford, J.; McGinn, T.; Davidson, K. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Arentz, M.; YimE, K.L.; Lokhandwala, S.; Riedo, F.; Chong, M. Characteristics and outcomes of 21 critically ill patients with COVID-19 inWashington State. JAMA 2020, 323, 1612–1614. [Google Scholar] [CrossRef] [PubMed]
- Bhatraju, P.; Ghassemieh, B.; Nichols, M.; Kim, R.; Jerome, K.; Nalla, A. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 1–11. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, X.; Chen, H.; Chen, T.; Su, N.; Huang, F. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am. J. RespirCrit. Care Med. 2020, 201, 1430–1434. [Google Scholar] [CrossRef]
- Auld, S.; Caridi-Scheible, M.; Blum, J.; Robichaux, C.; Kraft, C.; Jacob, J. ICU and Ventilator Mortality among Critically Ill Adults with Coronavirus Disease 2019. Crit. Care Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- National Public Health Organization. Coronavirus Disease (COVID-19). Available online: https://eody.gov.gr/en (accessed on 1 November 2020).
- Moris, D.; Schizas, D. Lockdown during COVID-19: The Greek Success. In Vivo 2020, 34 (Suppl. 3), 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Alhazzani, W.; Møller, M.; Arabi, Y.; Loeb, M.; Gong, M.N.; Fan, E. Surviving sepsis campaign: Guidelines on the management of critically ill adults with Coronovirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887. [Google Scholar] [CrossRef] [PubMed]
- Brower, R.; Matthay, M.; Morris, A.; Schoenfeld, D.; Thompson, T.; Taylor, B. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [PubMed]
- Mentzelopoulos, S.; Malachias, S.; Zintzaras, E.; Kokkoris, S.; Zakynthinos, E.; Makris, D. Intermittent recruitment with high-frequency oscillation/tracheal gas insufflation in acute respiratory distress syndrome. Eur. Respir. J. 2012, 39, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.; Rubenfeld, G.; Thompson, B.; Ferguson, N.; Caldwell, E.; Fan, E.; Camporota, L. ARDS Definition Task Force. Acute respiratory distress syndrome: The Berlin definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Knaus, W.; Draper, E.; Wagner, D.; Zimmerman, J. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; de Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- Murray, J.; Matthay, M.; Luce, J.; Flick, M. An Expanded definition of the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1988, 138, 720–723. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020. [Google Scholar] [CrossRef]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.; Divatia, J. Intensive care management of coronovirus disease 2019 (COVID-19): Challenges and recommendations. Lancet. Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G. Risk factors associated with mortality among patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Laffey, J.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A. LUNG SAFE Investigators; ESICM Trials Group: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Zambon, M.; Vincent, J.L. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 2008, 133, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Cherit, G.; Lapinsky, S.; Macias, A.; Pinto, R.; Espinoza-Perez, L.; de la Torre, A. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 2009, 302, 1880–1887. [Google Scholar] [CrossRef]
- Estenssoro, E.; Rios, F.; Apezteguia, C.; Reina, R.; Neira, J.; Ceraso, D. Pandemic 2009 influenza A in Argentina. Am. J. RespirCrit. Care Med. 2010, 182, 41–48. [Google Scholar] [CrossRef]
- Gattinoni, L.; Coppola, S.; Cressoni, M.; Busana, M.; Rossi, S.; Chiumello, D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 20, 1299–1300. [Google Scholar] [CrossRef]
- Marini, J.; Gattinoni, L. Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329–2330. [Google Scholar] [CrossRef]
- Chiumello, D.; Busana, M.; Coppola, S.; Romitti, F.; Formenti, P.; Bonifazi, M. Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: A matched cohort study. Intensive Care Med. 2020, 1–10. [Google Scholar] [CrossRef]
- Grasselli, G.; Tonetti, T.; Protti, A.; Langer, T.; Girardis, M.; Bellani, G. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020, in press. [Google Scholar] [CrossRef]
- Gupta, S.; Hayek, S.; Wang, W.; Chan, L.; Mathews, K.; Melamed, M. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern. Med. 2020, 180. [Google Scholar] [CrossRef] [PubMed]

| All (n = 50) b | Survived ICU (n = 33) | Died in ICU (n = 16) | p c | |
|---|---|---|---|---|
| Age, years (median (IQR)) | 64 (58–72) | 61 (55–71) | 70 (60–77) | 0.065 |
| ≤64 | 26 (52.0) | 20 (60.6) | 5 (31.3) | 0.054 |
| ≥65 | 24 (48.0) | 13 (39.4) | 11 (68.7) | |
| Gender, male | 38 (76.0) | 25 (75.8) | 12 (75.0) | 0.954 |
| Race | ||||
| Caucasian | 47 (94.0) | 30 (90.9) | 16 (100) | 0.542 |
| Asian | 3 (6.0) | 3 (9.1) | 0 (0) | |
| Comorbidity | ||||
| Hypertension | 14 (28.0) | 9 (27.3) | 4 (25.0) | 0.866 |
| Diabetes mellitus | 9 (18.0) | 6 (18.2) | 3 (18.8) | 0.962 |
| Cardiovascular d | 6 (12.0) | 3 (9.1) | 3 (18.8) | 0.333 |
| Malignancy e | 5 (10.0) | 1 (3.0) | 4 (25.0) | 0.034 |
| Obesity f | 5 (10.0) | 3 (9.1) | 2 (12.5) | 0.712 |
| Chronic lung disease | 4 (8.0) | 2 (6.1) | 2 (12.5) | 0.440 |
| Chronic renal failure | 1 (2.0) | 0 (0) | 1 (6.3) | 0.327 |
| Comorbidities (≥2) | 18 (36.0) | 11 (33.3) | 7 (43.8) | 0.478 |
| Time from symptom onset to ICU admission, days (median (IQR)) | 9 (8–11) | 10 (8–12) | 8 (8–10.5) | 0.352 |
| APACHE II score, median (IQR) | 12 (8–17) | 9 (6–12) | 20 (17–26) | <0.001 |
| SOFA score, median (IQR) | 7 (3–9) | 6 (2–7) | 11 (9–13) | <0.001 |
| Laboratory data, median (IQR) | ||||
| Leukocyte count, ×109/L | 7.6 (5.9–11.2) | 7.3 (5.9–10.8) | 9.5 (6.0–17.3) | 0.386 |
| Lymphocyte count, ×109/L | 0.88 (0.61–1.13) | 0.88 (0.65–1.13) | 0.93(0.48–1.08) | 0.855 |
| Platelet count, ×109/L | 233 (193–318) | 233 (200–322) | 263 (211–329) | 0.553 |
| C-reactive protein, mg/dL g | 14.2 (7.7–24.6) | 13.6 (7.7–20.3) | 21.7 (9.6–31.9) | 0.092 |
| Procalcitonine, ng/mL h | 0.43 (0.12–0.94 | 0.34 (0.15–0.66) | 1.41 (0.11–1.52) | 0.503 |
| Ferritin, μg/L | 835 (353–1882) | 835 (576–1710) | 1509 (479–1740) | 0.345 |
| D-dimer, μg/mL i | 1.23 (0.52–2.46) | 1.30 (0.59–2.43) | 1.17 (0.45–2.17) | 0.987 |
| Lactate dehydrogenase, IU/L | 471 (403–611) | 454 (363–604) | 509 (428–574) | 0.316 |
| Albumin, g/dL | 3.2 (3.0–3.6) | 3.3 (3.1–3.7) | 3.0 (2.8–3.4) | 0.038 |
| Creatinine, mg/dL | 0.9 (0.7–1.1) | 0.8 (0.7–1.0) | 1.1 (0.7–1.8) | 0.123 |
| TroponinΤ, pg/mL j | 15 (10–52) | 12 (8–20) | 31 (14–85) | 0.012 |
| Lactate, mmol/L | 1.3 (1.0–1.8) | 1.0 (0.9–1.3) | 1.8 (1.3–2.1) | <0.001 |
| Bilateral infiltrates on chest x-ray/CT | 49 (98) | 32 (97) | 16 (100) | 1.000 |
| Arterial blood gases, median (IQR) | ||||
| PaO2, mmHg | 100 (83–130) | 101 (86–121) | 99 (75–155) | 0.977 |
| PaO2/FiO2, mmHg | 121 (86–171) | 119 (89–157) | 123 (75–243) | 0.790 |
| PaCO2, mmHg | 40 (33–45) | 36 (29–42) | 44 (42–50) | 0.002 |
| pH | 7.39 (7.32–7.44) | 7.42 (7.35–7.45) | 7.30 (7.25–7.36) | 0.006 |
| Respiratory parameters on day of intubation, median (IQR) k | ||||
| PEEP, cmH2O | 14 (12–16) | 14 (12–17) | 13 (10–16) | 0.436 |
| Plateau pressure, cmH2O | 27 (25–29) | 27 (26–29) | 25 (24–29) | 0.413 |
| Driving pressure, cmH2O | 13 (11–15) | 12 (10–14) | 13 (12–15) | 0.384 |
| Static compliance, mL/cmH2O | 40 (32–50) | 40 (33–50) | 39 (33–42) | 0.683 |
| LIS | 2.7 (2.5–3.2) | 2.7 (2.5–3.2) | 2.8 (2.5–3.5) | 0.730 |
| Noradrenaline | 39 (78) | 21 (64) | 16 (100) | 0.004 |
| All (n = 50) b | Survived ICU (n = 33) | Died in ICU (n = 16) | p c | |
|---|---|---|---|---|
| High-flow nasal cannula | 14 (28) | 13 (39) | 1 (6) | 0.019 |
| Non-invasive mechanical ventilation | 2 (4) | 2 (6) | 0 (0) | 0.551 |
| Invasive mechanical ventilation (IMV) | 41 (82) | 24 (73) | 16 (100) | 0.021 |
| Time to intubation, median (IQR) d | 2 (0–3) | 2 (0–4) | 1 (0–3) | 0.249 |
| IMV days, median (IQR) | 13 (9–33) | 13 (10–32) | 14 (7–27) | 0.847 |
| Neuromuscular blockade | 26 (52) | 14 (42) | 12 (75) | 0.066 |
| Prone position | 6 (12) | 4 (12) | 2 (13) | 0.969 |
| Noradrenaline | 34 (68) | 17 (52) | 16 (100) | <0.001 |
| Noradrenaline days, median (IQR) | 6 (2–13) | 4 (1–8) | 11 (5–15) | 0.009 |
| Renal replacement therapy | 13 (26) | 4 (12) | 8 (50) | 0.003 |
| Renal replacement therapy days | ||||
| median (IQR) | 15 (11–24) | 19 (15–24) | 15 (9–26) | 0.570 |
| Selected inpatient medications | ||||
| Hydroxychloroquine | 44 (88) | 29 (88) | 14 (88) | 0.969 |
| Azithromycin | 38 (76) | 26 (79) | 12 (75) | 0.765 |
| Lopinavir/Ritonavir | 18 (36) | 13 (39) | 5 (31) | 0.811 |
| Anti-Interleukin-6 antibody | 5 (10) | 3 (9) | 2 (13) | 0.893 |
| Remdesivir (or placebo) | 3 (6) | 1 (3) | 1 (6) | 0.813 |
| Glucocorticoids | 5 (10) | 2 (6) | 3 (19) | 0.382 |
| Outcomes | ||||
| Follow-up, days (median (range)) | 50 (29–88) | 50 (29–84) | 50 (30–88) | NA |
| Successful extubation d,e | 17 (41) | 17 (71) f | 0 (0) | NA |
| Time to successful extubation, days | ||||
| (median (IQR)) d,e | 9 (5–14) | 9 (5–14) | NA | |
| Tracheostomy d | 12 (29) | 7 (29) f | 4 (25) | 0.942 |
| Time to tracheostomy, days | ||||
| (median (IQR)) d | 22 (19–25) | 21 (19–26) | 22 (18–26) | 0.968 |
| Mechanical ventilation days, | ||||
| median (IQR) d | 13 (9–33) | 13 (10–32) | 14 (7–27) | 0.847 |
| ICU days, median (IQR) | 14 (9–31) | 15 (10–30) | 14 (7–27) | 0.417 |
| Hospital days, median (IQR) | 21 (10–32) | 24 (15–35) | 14 (7–27) | <0.001 |
| 28-day mortality | 12 (24) | NA | NA | NA |
| 28-day mortality in ventilated d | 12 (29) | NA | NA | NA |
| ARDS with COVID-19 (n = 41) | ARDS without COVID-19 (n = 64) | |
|---|---|---|
| Age, years | 65.7 ± 10.4 | 52.9 ± 17.1 |
| Gender, male | 31 (75.6) | 47 (73.4) |
| Comorbidity | ||
| Hypertension | 12 (29.3) | 19 (29.7) |
| Diabetes mellitus | 7 (17.1) | 7 (10.9) |
| Malignancy | 5 (12.2) | 9 (14.1) |
| Other | 5 (12.2) | 6 (9.4) |
| APACHE II score | 14.6 ± 6.9 | 19.3 ± 7.8 |
| SOFA score | 7.9 ± 3.3 | 12.1 ± 2.6 |
| Hemodynamic variables and support | ||
| MAP, mmHg | 82.2 ± 6.7 | 77.3 ± 11.0 |
| Lactate, mmol/L | 1.7 ± 1.1 | 2.8 ± 2.8 |
| Noradrenaline | 33 (80) | 50 (78) |
| Arterial blood gases | ||
| PaO2, mmHg | 115.8 ± 42.8 | 78.2 ± 12.7 |
| PaO2/FiO2, mmHg | 147.5 ± 74.5 | 106.9 ± 27.7 |
| PaCO2, mmHg | 42.6 ± 10.2 | 47.5 ± 8.0 |
| pH | 7.35 ± 0.09 | 7.30 ± 0.08 |
| Respiratory parameters | ||
| Tidal volume, L | 0.47 ± 0.04 | 0.45 ± 0.06 |
| Ventilation rate, breaths/min | 24.3 ± 3.3 | 27.2 ± 5.3 |
| PEEP, cmH2O | 13.9 ± 3.7 | 13.1 ± 3.0 |
| Plateau pressure, cmH2O | 26.7 ± 3.5 | 29.9 ± 3.0 |
| Driving pressure, cmH2O | 12.8 ± 2.1 | 16.4 ± 2.3 |
| Static compliance, mL/cmH2O | 38.9 ± 9.3 | 28.8 ± 5.3 |
| LIS | 2.9 ± 0.5 | 3.4 ± 0.8 |
| Prone position | 6 (15) | 12 (19) |
| Outcomes | ||
| Follow-up, days (median (range)) | 50 (29–82) | 65 (17–142) |
| ICU days (survivors), median (IQR) | 14 (9–31) | 19 (10–45) |
| Hospital days (survivors), median (IQR) | 20 (8–30) | 31 (12–55) |
| Hospital mortality | 16 (39.0) | 41 (64.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Routsi, C.; Magira, E.; Kokkoris, S.; Siembos, I.; Vrettou, C.; Zervakis, D.; Ischaki, E.; Malahias, S.; Sigala, I.; Asimakos, A.; et al. Hospital Resources May Be an Important Aspect of Mortality Rate among Critically Ill Patients with COVID-19: The Paradigm of Greece. J. Clin. Med. 2020, 9, 3730. https://doi.org/10.3390/jcm9113730
Routsi C, Magira E, Kokkoris S, Siembos I, Vrettou C, Zervakis D, Ischaki E, Malahias S, Sigala I, Asimakos A, et al. Hospital Resources May Be an Important Aspect of Mortality Rate among Critically Ill Patients with COVID-19: The Paradigm of Greece. Journal of Clinical Medicine. 2020; 9(11):3730. https://doi.org/10.3390/jcm9113730
Chicago/Turabian StyleRoutsi, Christina, Eleni Magira, Stelios Kokkoris, Ilias Siembos, Charikleia Vrettou, Dimitris Zervakis, Eleni Ischaki, Sotiris Malahias, Ioanna Sigala, Andreas Asimakos, and et al. 2020. "Hospital Resources May Be an Important Aspect of Mortality Rate among Critically Ill Patients with COVID-19: The Paradigm of Greece" Journal of Clinical Medicine 9, no. 11: 3730. https://doi.org/10.3390/jcm9113730
APA StyleRoutsi, C., Magira, E., Kokkoris, S., Siembos, I., Vrettou, C., Zervakis, D., Ischaki, E., Malahias, S., Sigala, I., Asimakos, A., Daidou, T., Kaltsas, P., Douka, E., Sotiriou, A., Markaki, V., Temberikidis, P., Koroneos, A., Politis, P., Mastora, Z., ... Zakynthinos, S. (2020). Hospital Resources May Be an Important Aspect of Mortality Rate among Critically Ill Patients with COVID-19: The Paradigm of Greece. Journal of Clinical Medicine, 9(11), 3730. https://doi.org/10.3390/jcm9113730






