Normative Database for All Retinal Layer Thicknesses Using SD-OCT Posterior Pole Algorithm and the Effects of Age, Gender and Axial Lenght
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
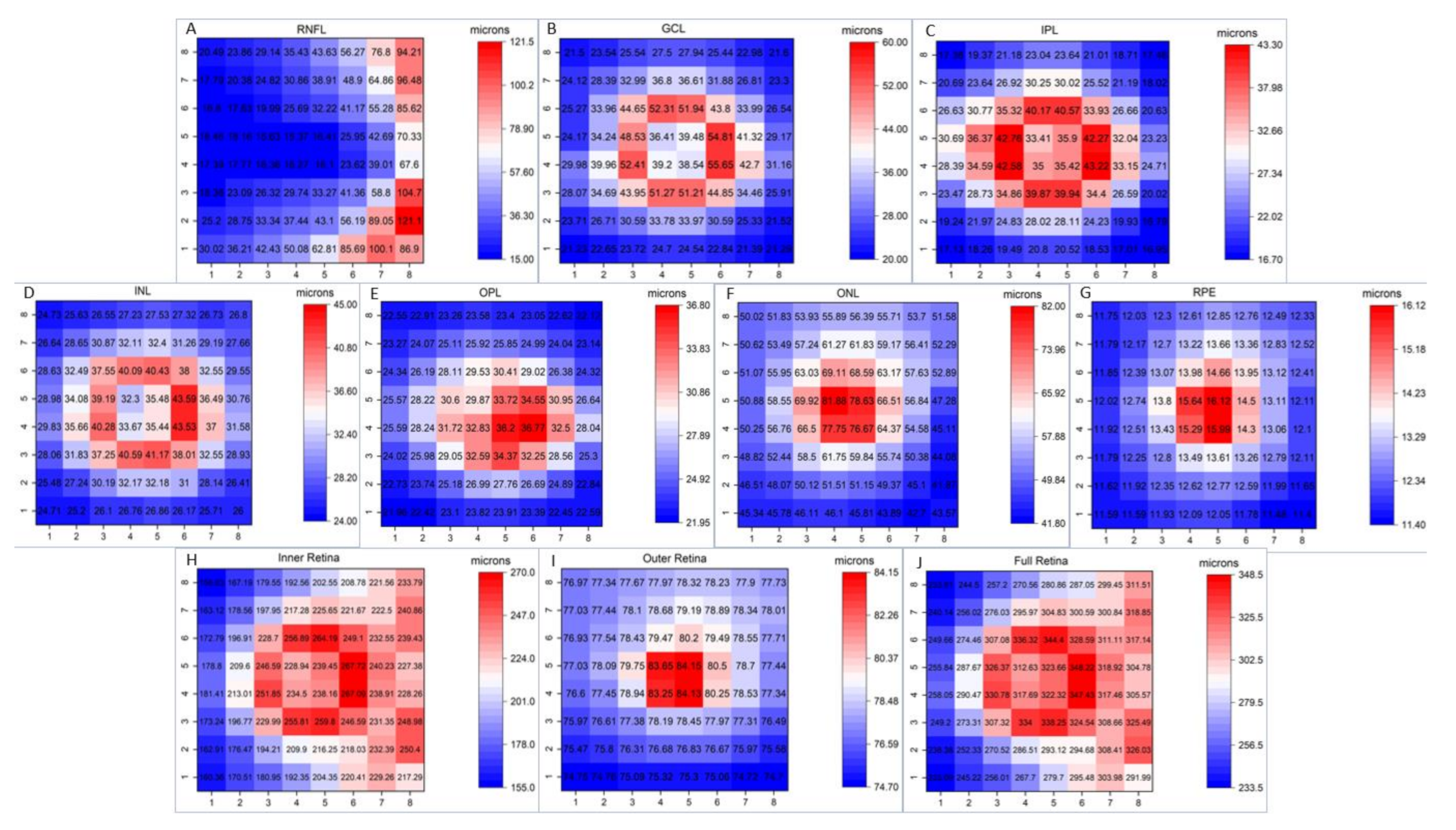
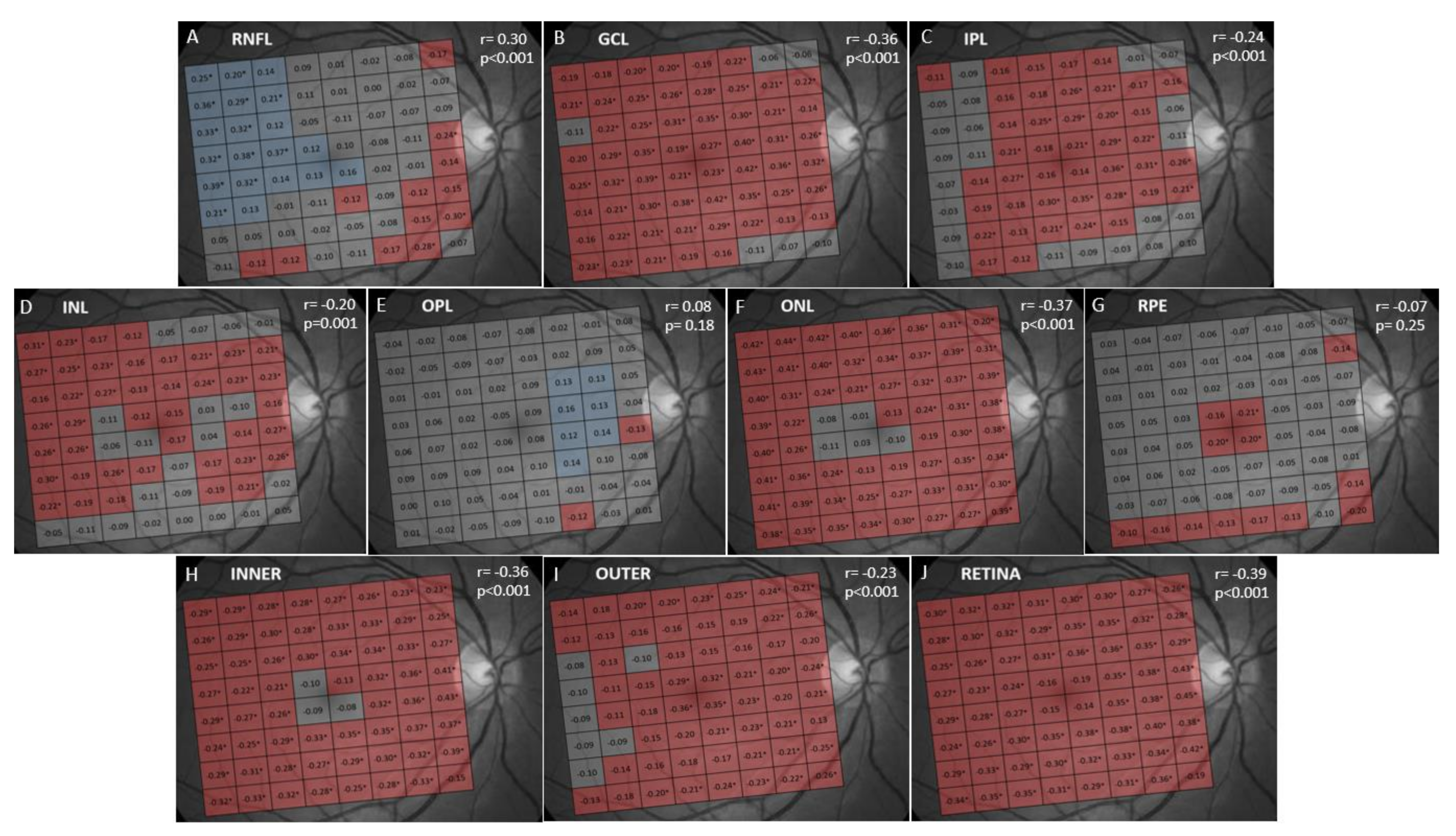
3. Results
4. Discussion
Limitations
5. Conclusions
6. Future Research
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Apple, D.J. Anatomy and Histopathology of the Macular Region. Int. Ophthalmol. Clin. 1981, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, R. Functional anatomy of macula and diagnostic procedures for macular function in clear media. Indian J. Ophthalmol. 1983, 31, 105–108. [Google Scholar] [PubMed]
- Liutkevičienė, R.; Lesauskaitė, V.; Ašmonienė, V.; Gelžinis, A.; Žaliūnienė, D.; Jašinskas, V. Inherited Macular Dystrophies and Differential Diagnostics. Medicina 2012, 48, 485–495. [Google Scholar]
- García-Medina, J.J.; Del-Rio-Vellosillo, M.; Palazón-Cabanes, A. Mapping the thickness changes on retinal layers segmented by spectral-domain optical coherence tomography using the posterior pole program in glaucoma. Arch. Soc. Esp. Oftalmol. 2018, 93, 263–273. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related Macular Degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Bhende, M.; Shetty, S.; Parthasarathy, M.K.; Ramya, S. Optical coherence tomography: A guide to interpretation of common macular diseases. Indian J. Ophthalmol. 2018, 66, 20–35. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Del-Rio-Vellosillo, M.; Palazon-Cabanes, A.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; Villegas-Perez, M.P. Glaucomatous Maculopathy: Thickness Differences on Inner and Outer Macular Layers between Ocular Hypertension and Early Primary Open-Angle Glaucoma Using 8 × 8 Posterior Pole Algorithm of SD-OCT. J. Clin. Med. 2020, 9, 1503. [Google Scholar] [CrossRef]
- Petzold, A.; Balder, L.J.; Calabresi, P.A. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2017, 16, 797–812. [Google Scholar] [CrossRef]
- Chan, V.T.T.; Sun, Z.; Tang, S. Spectral-domain OCT measurements in Alzheimer’s disease: A systematic review and meta-analysis. Ophthalmology 2019, 126, 497–510. [Google Scholar] [CrossRef]
- García-Martin, E.; Larrosa, J.M.; Polo, V. Distribution of retinal layer atrophy in patients with Parkinson disease and association with disease severity and duration. Am. J. Ophthalmol. 2014, 157, 470–478. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Garcia-Piñero, M.; Del-Río-Vellosillo, M. Comparison of Foveal, Macular, and Peripapillary Intraretinal Thicknesses Between Autism Spectrum Disorder and Neurotypical Subjects. Invest Ophthalmol. Vis. Sci. 2017, 58, 5819–5826. [Google Scholar] [CrossRef] [PubMed]
- Almonte, M.T.; Capellàn, P.; Yap, T.E.; Cordeiro, M.F. Retinal correlates of psychiatric disorders. Ther. Adv. Chronic Dis. 2020, 11, 2040622320905215. [Google Scholar] [CrossRef] [PubMed]
- Vajzovic, L.; Hendrickson, A.E.; O’Connerll, R.V. Maturation of the human fovea: Correlation of spectral-domain optical coherence tomography findings with histology. Am. J. Ophthalmol. 2012, 154, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.L.; Wollstein, G.; Ishikawa, H. Optical Coherence Tomography: History, Current Status, and Laboratory Work. Invest Ophthalmol. Vis. Sci. 2011, 52, 2425–2436. [Google Scholar] [CrossRef] [PubMed]
- Mwanza, J.C.; Durbin, M.K.; Budenz, D.L. Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Investig. Ophthalmol Vis. Sci. 2011, 52, 7872–7879. [Google Scholar] [CrossRef] [PubMed]
- Arepalli, S.; Srivastava, S.K.; Hu, M. Assessment of inner and outer retinal layer metrics on the Cirrus HD-OCT Platform in normal eyes. PLoS ONE 2018, 13, 0203324. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.C.; Vianna, J.R.; Sharpe, G.P.; Demirel, S.; Girkin, C.A.; Mardin, C.Y.; Scheuerle, A.F.; Burgoyne, C.F. Differential Effects of Aging in the Macular Retinal Layers, Neuroretinal Rim, and Peripapillary Retinal Nerve Fiber Layer. Ophthalmology 2020, 127, 177–185. [Google Scholar] [CrossRef]
- Nieves-Moreno, M.; Martínez-de-la-Casa, J.M.; Cifuentes-Canorea, P.; Sastre-Ibáñez, M.; Santos-Bueso, E.; Sáenz-Francés, F. Normative database for separate inner retinal layers thickness using spectral domain optical coherence tomography in Caucasian population. PLoS ONE 2017, 12, e0180450. [Google Scholar] [CrossRef]
- Lee, Y.P.; Ju, Y.S.; Choi, D.G. Ganglion cell-inner plexiform layer thickness by swept-source optical coherence tomography in healthy Korean children: Normative data and biometric correlations. Sci. Rep. 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Invernizzi, A.; Pellegrini, M.; Acquistapace, A. Normative Data for Retinal-Layer Thickness Maps Generated by Spectral-Domain OCT in a White Population. Ophthalmol. Retina 2018, 2, 808–815. [Google Scholar] [CrossRef]
- Mauschitz, M.M.; Holz, F.G.; Finger, R.P.; Breteler, M.M.B. Determinants of macular layers and optic disc characteristics on SD-OCT: The Rhineland study. Trans. Vis. Sci Tech. 2019, 8, 34. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, W.B.; Wang, Y.X.; Yan, Y.N.; Yang, J.Y.; Zhou, W.J.; Chan, S.Y.; Xu, L.; Jonas, J.B. Thickness of individual layers at the macula and associated factors: The Beijing Eye Study 2011. BMC Ophthalmol. 2020, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Realini, T.; Zangwill, L.M.; Flanagan, J.G. Normative Databases for Imaging Instrumentation. J. Glaucoma 2015, 24, 480–483. [Google Scholar] [CrossRef]
- Cirrus HD-OCT. User Manual, 2660021162665 Rev. A.; Carl Zeiss Meditec: Jena, Germany, 2016. [Google Scholar]
- Asrani, S.; Rosdahl, J.A.; Allingham, R.R. Novel software strategy for glaucoma diagnosis: Asymmetry analysis of retinal thickness. Arch. Ophthalmol. 2011, 129, 1205–1211. [Google Scholar] [CrossRef]
- Grover, S.; Murthy, R.K.; Brar, V.S.; Chalam, K.V. Normative Data for Macular Thickness by High-Definition Spectral-Domain Optical Coherence Tomography (Spectralis). Am. J. Ophthalmol. 2009, 148, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Choovythayakorn, J.; Watanachai, N.; Chaikitmongkol, V.; Patikulsila, D.; Kunavisarut, P.; Ittipunkul, N. Macular thickness measured by spectral-domain optical coherence tomography in healthy Thai eyes. Jpn. J. Ophthalmol. 2012, 56, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Appukuttan, B.; Giridhar, A.; Gopalakrishnan, M.; Sivaprasad, S. Normative spectral domain optical coherence tomography data on macular and retinal nerve fiber layer thickness in Indians. Indian J. Ophthalmol. 2014, 62, 316–321. [Google Scholar]
- Bafiq, R.; Mathew, R.; Pearce, E.; Abdel-Hey, A.; Richardson, M.; Bailey, T.; Sivaprasad, S. Age, Sex and Ethnic Variations in Inner and Outer Retinal and Choroidal Thickness on Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2015, 160, 1034–1043. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kwak, H.W.; Kim, M.; Kim, Y.G.; Yu, S.Y. New profiles of posterior pole retinal thickness map in healthy Korean eyes measured by spectral-domain optical coherence tomography. Retina 2013, 33, 2139–2148. [Google Scholar] [CrossRef]
- Nieves-Moreno, M.; Martínez-de-la-Casa, J.M.; Morales-Fernández, L.; Sánchez-Jean, R.; Sáenz-Francés, F.; García-Feijoó, J. Impacts of age and sex on retinal layer thicknesses measured by spectral domain optical coherence tomography with Spectralis. PLoS ONE 2018, 13, e0194169. [Google Scholar] [CrossRef]
- Gao, H.; Hollyfield, J.G. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 1992, 33, 1–17. [Google Scholar]
- Harman, A.; Abrahams, B.; Moore, S.; Hoskins, R. Neuronal density in the human retinal ganglion cell layer from 16–77 years. Anat. Rec. 2000, 260, 124–131. [Google Scholar] [CrossRef]
- Ooto, S.; Hangai, M.; Tomidokoro, A.; Saito, H.; Araie, M.; Otani, T.; Kishi, S.; Matsushita, K.; Maeda, N.; Shirakashi, M.; et al. Effects of age, sex, and axial length on the three-dimensional profile of normal macular layer structures. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8769–8779. [Google Scholar] [CrossRef] [PubMed]
- Demirkaya, N.; van Dijk, H.W.; van Schuppen, S.M.; Abràmoff, M.D.; Garvin, M.K.; Sonka, M.; Schlingemann, R.O.; Verbraak, F.D. Effect of age on individual retinal layer thickness in normal eyes as measured with spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4934–4940. [Google Scholar] [CrossRef]
- Won, J.Y.; Kim, S.E.; Park, Y.-H. Effect of age and sex on retinal layer thickness and volume in normal eyes. Medicine 2016, 95, e5441. [Google Scholar] [CrossRef] [PubMed]
- Jorge, L.; Canário, N.; Quental, H.; Bernardes, R.; Castelo-Branco, M. Is the Retina a Mirror of the Aging Brain? Aging of Neural Retina Layers and Primary Visual Cortex Across the Lifespan. Front Aging Neurosci. 2019, 11, 360. [Google Scholar] [CrossRef]
- Neuville, J.M.; Bronson-Castain, K.; Bearse, M.A.; Ng, J.S.; Harrison, W.W.; Schneck, M.E.; Adams, A.J. OCT reveals regional differences in macular thickness with age. Optom Vis. Sci. 2009, 86, E810–E816. [Google Scholar] [CrossRef]
- Arsani, S.; Essaid, L.; Alder, B.; Santiago-Turla, C. Artifacts in Spectral-Domain Optical Coherence Tomography Measurements in Glaucoma. JAMA Ophthalmol. 2014, 132, 396–402. [Google Scholar]
- Altay, L.; Jahn, C.; Arikan Yorgun, M.; Caramoy, A.; Schick, T.; Hoyng, C.B.; den Hollander, A.I.; Fauser, S. Alteration of retinal layers in healthy subjects over 60 years of age until nonagenarians. Clin. Ophthalmol. 2017, 11, 1499–1503. [Google Scholar] [CrossRef]
- Curcio, C.; Messinger, J.D.; Sloan, K.R.; Mitra, A.; McGwin, G.; Spaide, R.F. Human chorioretinal layer thicknesses measured in macula-wide, high-resolution histologic sections. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3943–3954. [Google Scholar] [CrossRef]
- Watzke, R.C.; Soldevilla, J.D.; Trune, D.R. Morphometric analysis of human retinal pigment epithelium: Correlation with age and location. Curr. Eye Res. 1993, 12, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, Y.; Cheng, Y.; Qu, Y. Assessment of the effect of age on macular layer thickness in a healthy Chinese cohort using spectral-domain optical coherence tomography. BMC Ophthalmol. 2018, 18, 169. [Google Scholar] [CrossRef]
- Bonilha, V.L. Age and disease-related structural changes in the retinal pigment epithelium. Clin. Ophthalmol. 2008, 2, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Tham, Y.C.; Tan, B.; Devarajan, K.; Schwarzhans, F.; Gan, A.; Wong, D.; Cheung, C.Y.; Majithia, S.; Thakur, S.; et al. Age-related changes of individual macular retinal layers among Asians. Sci. Rep. 2019, 9, 20352. [Google Scholar] [CrossRef]
- Wong, K.H.; Tham, Y.C.; Al, N.D.Q. Racial differences and determinants of macular thickness profiles in multiethnic Asian population: The Singapore Epidemiology of Eye Diseases Study. Br. J. Ophthalmol. 2019, 103, 894–899. [Google Scholar] [CrossRef]
- Myers, C.E.; Klein, B.E.K.; Meuer, S.M. Retinal thickness measured by spectral-domain optical coherence tomography in eyes without retinal abnormalities: The Beaver Dam Eye Study. Am. J. Ophthalmol. 2015, 159, 445–456. [Google Scholar] [CrossRef] [PubMed]
- von Hanno, T.; Lade, A.C.; Mathiesen, E.B.; Peto, T.; Njølstad, I.; Bertelsen, G. Macular thickness in healthy eyes of adults (N = 4508) and relation to sex, age and refraction: The Tromsø Eye Study (2007–2008). Acta Ophthalmol. 2017, 95, 262–269. [Google Scholar] [CrossRef]
- Wu, P.C.; Chen, Y.J.; Chen, C.H. Assessment of macular retinal thickness and volume in normal eyes and highly myopic eyes with third-generation optical coherence tomography. Eye 2008, 22, 551–555. [Google Scholar] [CrossRef]
- Hirasawa, K.; Shoji, N. Association between ganglion cell complex and axial length. Jpn. J. Ophthalmol. 2013, 57, 429–434. [Google Scholar] [CrossRef]
- Koh, V.T.; Tham, Y.C.; Cheung, C.Y. Determinants of ganglion cell inner plexiform layer thickness measured by high-definition optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2012, 53, 5853–5859. [Google Scholar] [CrossRef]
- Higashide, T.; Ohkubo, S.; Hangai, M. Influence of Clinical Factors and Magnification Correction on Normal Thickness Profiles of Macular Retinal Layers Using Optical Coherence Tomography. PLoS ONE 2016, 11, 014778254. [Google Scholar] [CrossRef]
- Rothman, K.J. No Adjustments Are Needed for Multiple Comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palazon-Cabanes, A.; Palazon-Cabanes, B.; Rubio-Velazquez, E.; Lopez-Bernal, M.D.; Garcia-Medina, J.J.; Villegas-Perez, M.P. Normative Database for All Retinal Layer Thicknesses Using SD-OCT Posterior Pole Algorithm and the Effects of Age, Gender and Axial Lenght. J. Clin. Med. 2020, 9, 3317. https://doi.org/10.3390/jcm9103317
Palazon-Cabanes A, Palazon-Cabanes B, Rubio-Velazquez E, Lopez-Bernal MD, Garcia-Medina JJ, Villegas-Perez MP. Normative Database for All Retinal Layer Thicknesses Using SD-OCT Posterior Pole Algorithm and the Effects of Age, Gender and Axial Lenght. Journal of Clinical Medicine. 2020; 9(10):3317. https://doi.org/10.3390/jcm9103317
Chicago/Turabian StylePalazon-Cabanes, Ana, Begoña Palazon-Cabanes, Elena Rubio-Velazquez, Maria Dolores Lopez-Bernal, Jose Javier Garcia-Medina, and Maria Paz Villegas-Perez. 2020. "Normative Database for All Retinal Layer Thicknesses Using SD-OCT Posterior Pole Algorithm and the Effects of Age, Gender and Axial Lenght" Journal of Clinical Medicine 9, no. 10: 3317. https://doi.org/10.3390/jcm9103317
APA StylePalazon-Cabanes, A., Palazon-Cabanes, B., Rubio-Velazquez, E., Lopez-Bernal, M. D., Garcia-Medina, J. J., & Villegas-Perez, M. P. (2020). Normative Database for All Retinal Layer Thicknesses Using SD-OCT Posterior Pole Algorithm and the Effects of Age, Gender and Axial Lenght. Journal of Clinical Medicine, 9(10), 3317. https://doi.org/10.3390/jcm9103317





