Decreased Volume of Lateral and Medial Geniculate Nuclei in Patients with LHON Disease—7 Tesla MRI Study
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Optical Coherence Tomography (OCT) Acquisition
2.3. MRI Acquisition
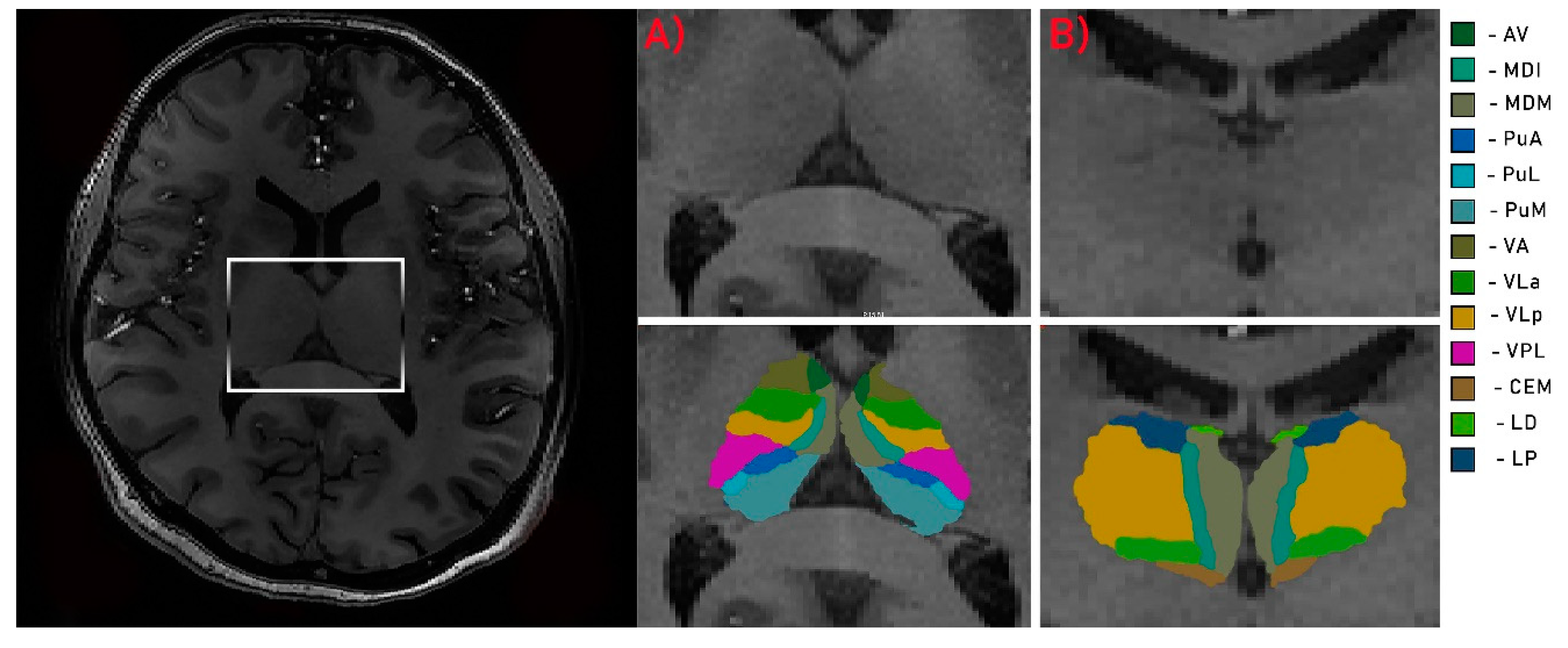
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Participants
3.2. Between-Group Comparisons
3.3. Within-Group Effects
3.4. Volumetric—Clinical Correlations in LHON Sample
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, N.J.; Lott, M.T.; Wallace, D.C. The Clinical Characteristics of Pedigrees of Leber’s Hereditary Optic Neuropathy with the 11778 Mutation. Am. J. Ophthalmol. 1991, 111, 750–762. [Google Scholar] [CrossRef]
- McFarland, R.; Turnbull, D.M. Batteries not included: Diagnosis and management of mitochondrial disease. J. Intern. Med. 2009, 265, 210–228. [Google Scholar] [CrossRef] [PubMed]
- Tońska, K.; Kodroń, A.; Bartnik, E. Genotype-phenotype correlations in Leber hereditary optic neuropathy. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1119–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackey, D.; Howell, N. A variant of Leber hereditary optic neuropathy characterized by recovery of vision and by an unusual mitochondrial genetic etiology. Am. J. Hum. Genet. 1992, 51, 1218–1228. [Google Scholar] [PubMed]
- Carelli, V.; Rugolo, M.; Sgarbi, G.; Ghelli, A.; Zanna, C.; Baracca, A.; Lenaz, G.; Napoli, E.; Martinuzzi, A.; Solaini, G. Bioenergetics shapes cellular death pathways in Leber’s hereditary optic neuropathy: A model of mitochondrial neurodegeneration. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2004, 1658, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Savini, G.; Barboni, P.; Valentino, M.L.; Montagna, P.; Cortelli, P.; De Negri, A.M.; Sadun, F.; Bianchi, S.; Longanesi, L.; Zanini, M. Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber’s hereditary optic neuropathy mutations. Ophthalmology 2005, 112, 127–131. [Google Scholar] [CrossRef]
- Fama, R.; Sullivan, E.V. Thalamic structures and associated cognitive functions: Relations with age and aging. Neurosci. Biobehav. Rev. 2015, 54, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Usrey, W.M.; Alitto, H.J. Visual Functions of the Thalamus. Annu. Rev. Vis. Sci. 2015, 1, 351–371. [Google Scholar] [CrossRef] [Green Version]
- Cudeiro, J.; Sillito, A.M. Looking back: Corticothalamic feedback and early visual processing. Trends Neurosci. 2006, 29, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Covington, B.P.; Al Khalili, Y. Neuroanatomy, Nucleus Lateral Geniculate; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Bizley, J. Audition. In Conn’s Translational Neuroscience; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 579–598. [Google Scholar]
- Diaz, B.; Blank, H.; Von Kriegstein, K. Task-dependent modulation of the visual sensory thalamus assists visual-speech recognition. NeuroImage 2018, 178, 721–734. [Google Scholar] [CrossRef]
- Von Kriegstein, K.; Patterson, R.D.; Griffiths, T.D. Task-Dependent Modulation of Medial Geniculate Body Is Behaviorally Relevant for Speech Recognition. Curr. Biol. 2008, 18, 1855–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deoni, S.C.; Josseau, M.J.; Rutt, B.K.; Peters, T.M. Visualization of thalamic nuclei on high resolution, multi-averaged T1 and T2 maps acquired at 1.5 T. Hum. Brain Mapp. 2005, 25, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Vassal, F.; Coste, J.; Derost, P.; Mendes, V.; Gabrillargues, J.; Nuti, C.; Durif, F.; Lemaire, J.-J. Direct stereotactic targeting of the ventrointermediate nucleus of the thalamus based on anatomic 1.5-T MRI mapping with a white matter attenuated inversion recovery (WAIR) sequence. Brain Stimul. 2012, 5, 625–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudhyadhom, A.; Haq, I.U.; Foote, K.D.; Okun, M.; Bova, F.J. A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: The Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR). NeuroImage 2009, 47, T44–T52. [Google Scholar] [CrossRef]
- Abosch, A.; Yacoub, E.; Ugurbil, K.; Harel, N. An Assessment of Current Brain Targets for Deep Brain Stimulation Surgery with Susceptibility-Weighted Imaging at 7 Tesla. Neurosurgery 2010, 67, 1745–1756. [Google Scholar] [CrossRef] [Green Version]
- Tourdias, T.; Saranathan, M.; Levesque, I.R.; Su, J.; Rutt, B.K. Visualization of intra-thalamic nuclei with optimized white-matter-nulled MPRAGE at 7T. NeuroImage 2014, 84, 534–545. [Google Scholar] [CrossRef] [Green Version]
- García-Gomar, M.G.; Strong, C.; Toschi, N.; Singh, K.; Rosen, B.R.; Wald, L.L.; Bianciardi, M. In vivo Probabilistic Structural Atlas of the Inferior and Superior Colliculi, Medial and Lateral Geniculate Nuclei and Superior Olivary Complex in Humans Based on 7 Tesla MRI. Front. Mol. Neurosci. 2019, 13, 764. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.A.; Knott, M.; Heidemann, R.; Michelson, G.; Kober, T.; Dörfler, A.; Engelhorn, T. Investigation of lateral geniculate nucleus volume and diffusion tensor imaging in patients with normal tension glaucoma using 7 tesla magnetic resonance imaging. PLoS ONE 2018, 13, e0198830. [Google Scholar] [CrossRef]
- Morrissey, S.P.; Borruat, F.X.; Miller, D.H.; Moseley, I.F.; Sweeney, M.G.; Govan, G.G.; Kelly, M.A.; Francis, D.A.; Harding, A.E.; McDonald, W.I. Bilateral simultaneous optic neuropathy in adults: Clinical, imaging, serological, and genetic studies. J. Neurol. Neurosurg. Psychiatry 1995, 58, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Kermode, A.G.; Moseley, I.F.; Kendall, B.E.; Miller, D.H.; MacManus, D.G.; McDonald, W.I. Magnetic resonance imaging in Leber’s optic neuropathy. J. Neurol. Neurosurg. Psychiatry 1989, 52, 671–674. [Google Scholar] [CrossRef]
- Barcella, V.; Rocca, M.A.; Bianchi-Marzoli, S.; Milesi, J.; Melzi, L.; Falini, A.; Pierro, L.; Filippi, M. Evidence for retrochiasmatic tissue loss in Leber’s hereditary optic neuropathy. Hum. Brain Mapp. 2010, 31, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Milesi, J.; Rocca, M.A.; Bianchi-Marzoli, S.; Petrolini, M.; Pagani, E.; Falini, A.; Comi, G.; Filippi, M. Patterns of white matter diffusivity abnormalities in Leber’s hereditary optic neuropathy: A tract-based spatial statistics study. J. Neurol. 2012, 259, 1801–1807. [Google Scholar] [CrossRef]
- Grochowski, C.; Symms, M.R.; Jonak, K.; Krukow, P.; Wood, T.C.; Ljungberg, E.; Enseñat, J.; Nowomiejska, K.; Rejdak, R.; Maciejewski, R.; et al. The Evaluation of Optic Nerves Using 7 Tesla “Silent” Zero Echo Time Imaging in Patients with Leber’s Hereditary Optic Neuropathy with or without Idebenone Treatment. J. Clin. Med. 2020, 9, 1112. [Google Scholar] [CrossRef] [PubMed]
- Madio, D.P.; Lowe, I.J. Ultra-fast imaging using low flip angles and fids. Magn. Reson. Med. 1995. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Why voxel-based morphometry should be used. NeuroImage 2001, 14, 1238–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaretskaya, N.; Fischl, B.; Reuter, M.; Renvall, V.; Polimeni, J.R. Advantages of cortical surface reconstruction using submillimeter 7 T MEMPRAGE. NeuroImage 2018, 165, 11–26. [Google Scholar] [CrossRef]
- Iglesias, J.E.; Insausti, R.; Lerma-Usabiaga, G.; Bocchetta, M.; Van Leemput, K.; Greve, D.N.; Van Der Kouwe, A.; Fischl, B.; Gaudes, C.C.; Paz-Alonso, P.M.; et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. NeuroImage 2018, 183, 314–326. [Google Scholar] [CrossRef]
- Rizzo, G.; Tozer, K.R.; Tonon, C.; Manners, D.; Testa, C.; Malucelli, E.; Valentino, M.L.; La Morgia, C.; Barboni, P.; Randhawa, R.S.; et al. Secondary Post-Geniculate Involvement in Leber’s Hereditary Optic Neuropathy. PLoS ONE 2012, 7, e50230. [Google Scholar] [CrossRef]
- Wei, Q.-P.; Zhou, X.; Yang, L.; Sun, Y.-H.; Zhou, J.; Li, G.; Jiang, R.; Lu, F.; Qu, J.; Guan, M.-X. The coexistence of mitochondrial ND6 T14484C and 12S rRNA A1555G mutations in a Chinese family with Leber’s hereditary optic neuropathy and hearing loss. Biochem. Biophys. Res. Commun. 2007, 357, 910–916. [Google Scholar] [CrossRef]
- Khan, N.A.; Govindaraj, P.; Jyothi, V.; Meena, A.K.; Thangaraj, K. Co-occurrence of m.1555A>G and m.11778G>A mitochondrial DNA mutations in two Indian families with strikingly different clinical penetrance of Leber hereditary optic neuropathy. Mol. Vis. 2013, 19, 1282–1289. [Google Scholar]
- Zhang, A.-M.; Jia, X.; Yao, Y.-G.; Zhang, Q. Co-occurrence of A1555G and G11778A in a Chinese family with high penetrance of Leber’s hereditary optic neuropathy. Biochem. Biophys. Res. Commun. 2008, 376, 221–224. [Google Scholar] [CrossRef]
- Yang, H.; Liu, R.; Wang, C. Searching the co-occurrence of pathogenic mutations for Leber’s hereditary optic neuropathy and hearing loss in more than 26,000 whole mitochondrial genomes. Mitochondrial DNA Part A 2015, 27, 3399–3402. [Google Scholar] [CrossRef]
- Fortuna, F.; Barboni, P.; Liguori, R.; Valentino, M.L.; Savini, G.; Gellera, C.; Mariotti, C.; Rizzo, G.; Tonon, C.; Manners, D.; et al. Visual system involvement in patients with Friedreich’s ataxia. Brain 2008, 132, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, E.; Triolo, G.; Cascavilla, M.L.; La Morgia, C.; Rizzo, G.; Savini, G.; Balducci, N.; Nucci, P.; Giglio, R.; Darvizeh, F.; et al. Changes in Choroidal Thickness follow the RNFL Changes in Leber’s Hereditary Optic Neuropathy. Sci. Rep. 2016, 6, 37332. [Google Scholar] [CrossRef] [Green Version]
- Asanad, S.; Tian, J.J.; Frousiakis, S.; Jiang, J.P.; Kogachi, K.; Felix, C.M.; Fatemeh, D.; Irvine, A.G.; Ter-Zakarian, A.; Falavarjani, K.G.; et al. Optical Coherence Tomography of the Retinal Ganglion Cell Complex in Leber’s Hereditary Optic Neuropathy and Dominant Optic Atrophy. Curr. Eye Res. 2019, 44, 638–644. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Wei, S.; Qiu, H.; Gong, Y.; Li, H.; Dai, Y.; Jiang, Z.; Liu, Z. Characterization of retinal nerve fiber layer thickness changes associated with Leber’s hereditary optic neuropathy by optical coherence tomography. Exp. Ther. Med. 2013, 7, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Fan, K.; Zhang, Y.; Chen, Y.; Tian, Q.; Shi, D. Quantitative assessment of optic nerve in patients with Leber’s hereditary optic neuropathy using reduced field-of-view diffusion tensor imaging. Eur. J. Radiol. 2017, 93, 24–29. [Google Scholar] [CrossRef]
- Hedgesiii, T.R.; Gobuty, M.; Manfready, R.A.; Erlich-Malona, N.; Monaco, C.; Mendoza-Santiesteban, C.E. The Optical Coherence Tomographic Profile of Leber Hereditary Optic Neuropathy. Neuro-Ophthalmol. 2016, 40, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, A.; Hashimoto, Y.; Shinmei, Y.; Nozaki, M.; Ishijima, K.; Tagawa, Y.; Ishida, S. Macular thickness changes in a patient with Leber’s hereditary optic neuropathy. BMC Ophthalmol. 2015, 15, 27. [Google Scholar] [CrossRef] [Green Version]
- Koilkonda, R.D.; Yu, H.; Chou, T.-H.; Feuer, W.J.; Ruggeri, M.; Porciatti, V.; Tse, D.; Hauswirth, W.W.; Chiodo, V.; Boye, S.L.; et al. Safety and effects of the vector for the Leber hereditary optic neuropathy gene therapy clinical trial. JAMA Ophthalmol. 2014, 132, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, Y.; Wang, J.; Tong, Y. Oxidative Stress in Chinese Patients with Leber’s Hereditary Optic Neuropathy. J. Int. Med. Res. 2008, 36, 544–550. [Google Scholar] [CrossRef] [Green Version]






| LHON (n = 15) M (SD) | HC (n = 15) M (SD) | t Value or χ2 | p | |
|---|---|---|---|---|
| Age (years) | 36.21 (14.41) | 32.53 (7.42) | 0.02 | 0.98 |
| Education (years) | 15.33 (1.98) | 16 (1.55) | −1.82 | 0.67 |
| Sex (% male) | 86 | 66 | 1.67 | 0.19 |
| Duration of illness (months) | 132 (144.32) | |||
| Mitochondrial mutation 11778G > A (%) | 100 | |||
| RNFL averaged thickens (left) | 62.153 (2.81) | |||
| RNFL averaged thickens (right) | 62.054 (3.15) |
| Right M (SD) | Left M (SD) | Z | p | |
|---|---|---|---|---|
| Length (cm) | 4.18 (0.32) | 4.23 (0.23) | −0.22 | 0.78 |
| Diameter (mm) 1 | 3.52 (0.56) | 3.75 (0.77) | −0.72 | 0.41 |
| Diameter 2 | 2.28 (0.5) | 2.27 (0.45) | 0.07 | 0.91 |
| Diameter 3 | 3.20 (0.22) | 3.22 (0.29) | −0.12 | 0.86 |
| Surface area (mm2) 1 | 12.93 (3.34) | 13.11 (4.73) | −0.88 | 0.22 |
| Surface area 2 | 3.81 (1.14) | 3.77 (1.31) | 0.22 | 0.61 |
| Surface area 3 | 8.12 (1.22) | 8.15 (1.34) | −0.18 | 0.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonak, K.; Krukow, P.; Jonak, K.E.; Radzikowska, E.; Baj, J.; Niedziałek, A.; Pankowska, A.; Symms, M.; Stępniewski, A.; Podkowiński, A.; et al. Decreased Volume of Lateral and Medial Geniculate Nuclei in Patients with LHON Disease—7 Tesla MRI Study. J. Clin. Med. 2020, 9, 2914. https://doi.org/10.3390/jcm9092914
Jonak K, Krukow P, Jonak KE, Radzikowska E, Baj J, Niedziałek A, Pankowska A, Symms M, Stępniewski A, Podkowiński A, et al. Decreased Volume of Lateral and Medial Geniculate Nuclei in Patients with LHON Disease—7 Tesla MRI Study. Journal of Clinical Medicine. 2020; 9(9):2914. https://doi.org/10.3390/jcm9092914
Chicago/Turabian StyleJonak, Kamil, Paweł Krukow, Katarzyna E. Jonak, Elżbieta Radzikowska, Jacek Baj, Anna Niedziałek, Anna Pankowska, Mark Symms, Andrzej Stępniewski, Arkadiusz Podkowiński, and et al. 2020. "Decreased Volume of Lateral and Medial Geniculate Nuclei in Patients with LHON Disease—7 Tesla MRI Study" Journal of Clinical Medicine 9, no. 9: 2914. https://doi.org/10.3390/jcm9092914
APA StyleJonak, K., Krukow, P., Jonak, K. E., Radzikowska, E., Baj, J., Niedziałek, A., Pankowska, A., Symms, M., Stępniewski, A., Podkowiński, A., Osuchowska, I., & Grochowski, C. (2020). Decreased Volume of Lateral and Medial Geniculate Nuclei in Patients with LHON Disease—7 Tesla MRI Study. Journal of Clinical Medicine, 9(9), 2914. https://doi.org/10.3390/jcm9092914







