Craniofacial Asymmetry from One to Three Years of Age: A Prospective Cohort Study with 3D Imaging
Abstract
1. Introduction
2. Materials and Methods
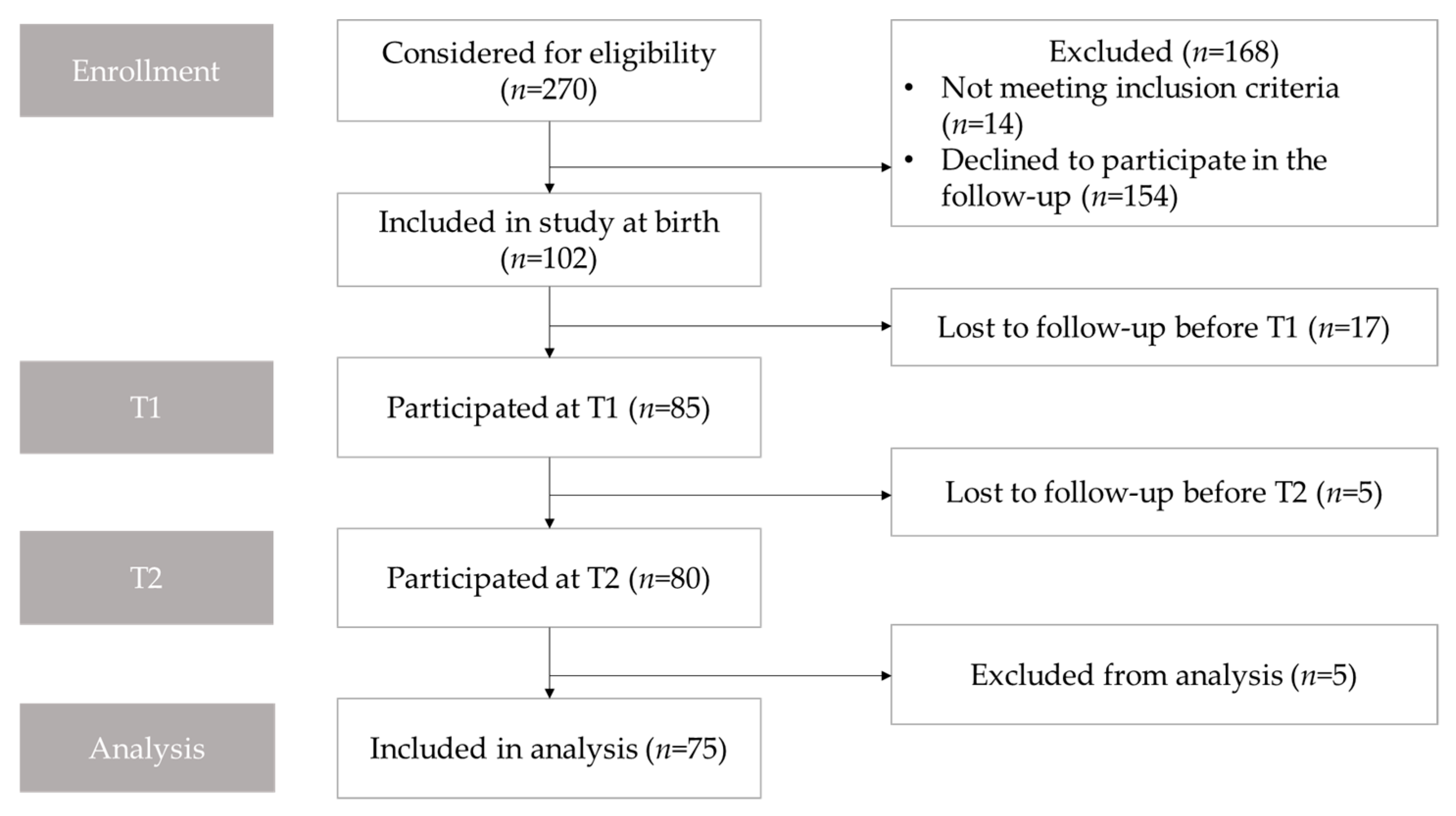
2.1. Study Population and Study Design
2.2. Data Collection
2.3. Image Analysis
2.4. Cranial Asymmetry
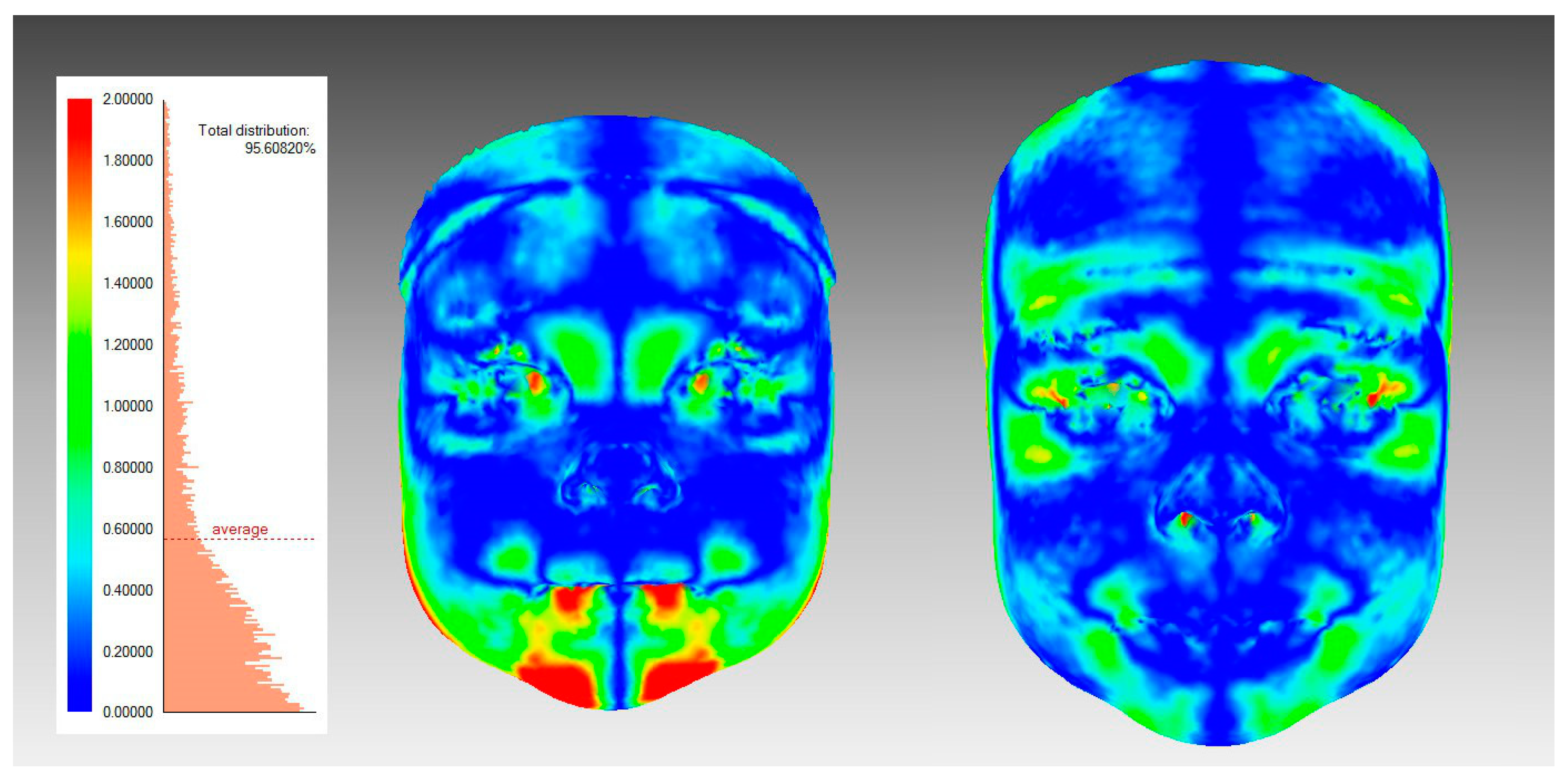
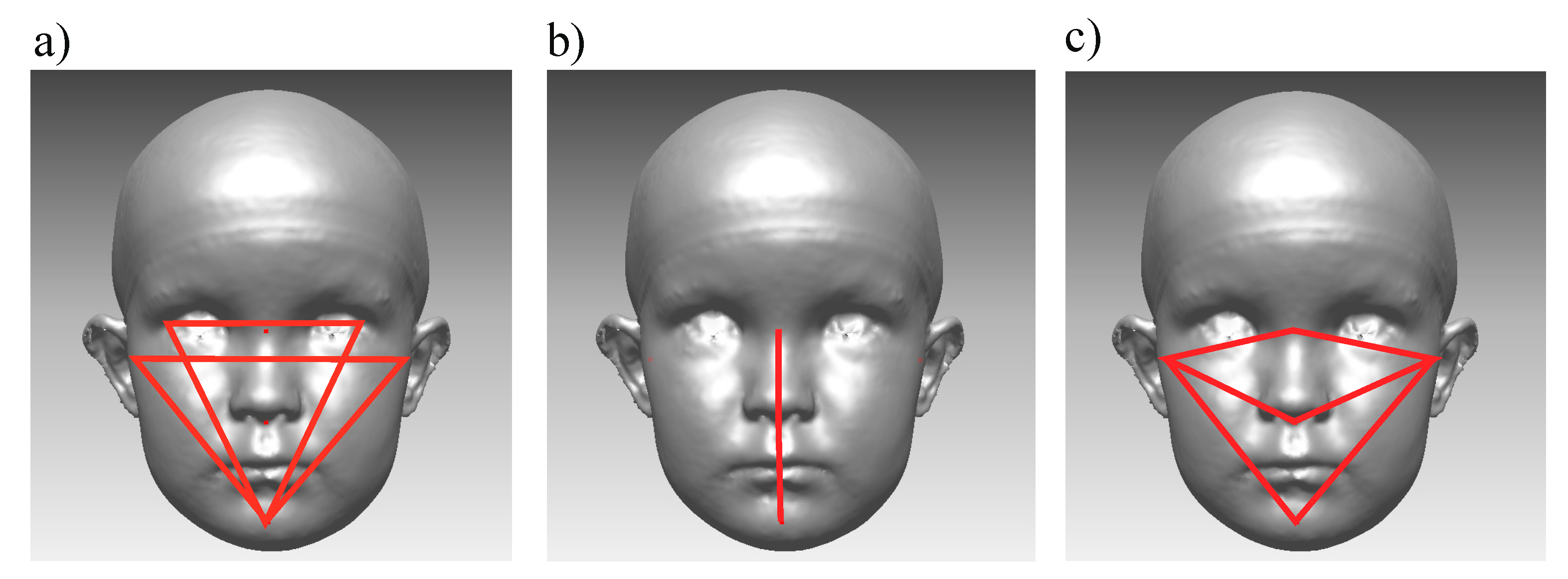
2.5. Surface-Based Facial Symmetry Parameters
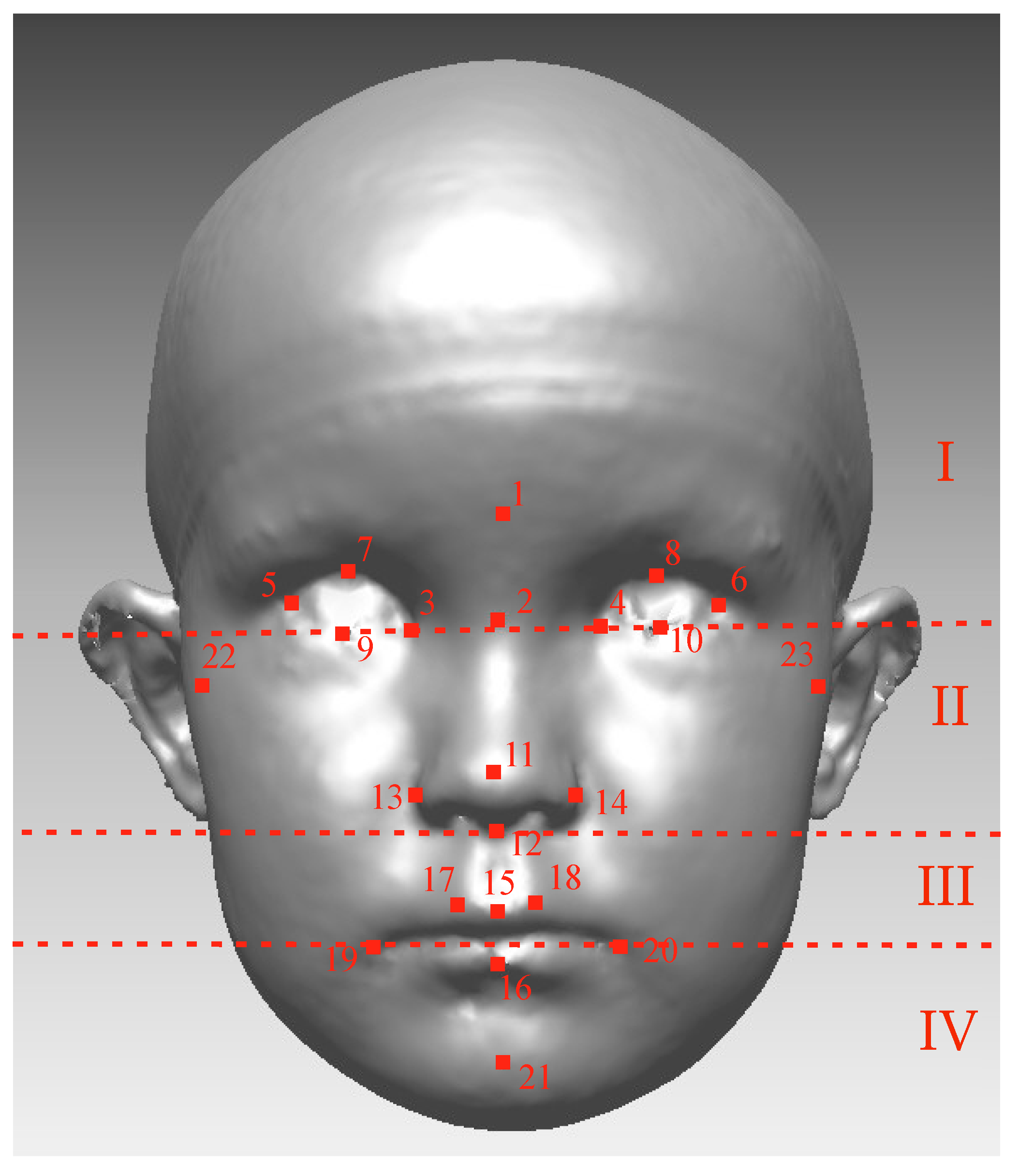
2.6. Landmark-Based Facial Symmetry Parameters
2.7. Subgroups
2.8. Statistical Analysis
3. Results
3.1. The Primary Outcome
3.2. Subgroups
3.3. Deformational Plagiocephaly
3.4. Side of the DP Related to Facial Asymmetry
3.5. Ear-Offset and Facial Asymmetry
3.6. Occlusal Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liukkonen, M.; Sillanmäki, L.; Peltomäki, T. Mandibular asymmetry in healthy children. Acta Odontol. Scand. 2005, 63, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.; Chandhoke, T.; Uribe, F.; Rigali, P.; Nanda, R. Quantification of skeletal asymmetries in normal adolescents: Cone-beam computed tomography analysis. Prog. Orthod. 2014, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Primozic, J.; Perinetti, G.; Zhurov, A.; Richmond, S.; Ovsenik, M. Assessment of facial asymmetry in growing subjects with a three-dimensional laser scanning system. Orthod. Craniofac. Res. 2012, 15, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.; Pirttiniemi, P.; Harila, V.; Heikkinen, T.; Toma, A.M.; Zhurov, A.I.; Richmond, S. Three-dimensional longitudinal assessment of facial symmetry in adolescents. Eur. J. Orthod. 2013, 35, 143–151. [Google Scholar] [CrossRef]
- Cheong, Y.; Lo, L. Facial asymmetry: Etiology, evaluation, and management. Chang Gung Med. J. 2011, 34, 341. [Google Scholar]
- Thiesen, G.; Gribel, B.F.; Freitas, M.P.M. Facial asymmetry: A current review. Dent. Press J. Orthod. 2015, 20, 110–125. [Google Scholar] [CrossRef]
- Primozic, J.; Richmond, S.; Kau, C.H.; Zhurov, A.; Ovsenik, M. Three-dimensional evaluation of early crossbite correction: A longitudinal study. Eur. J. Orthod. 2013, 35, 7–13. [Google Scholar] [CrossRef]
- Pirttiniemi, P. Normal and increased functional asymmetries in the craniofacial area. Acta Odontol. Scand. 1998, 56, 342–345. [Google Scholar] [CrossRef]
- Bialocerkowski, A.E.; Vladusic, S.L.; Wei-Ng, C. Prevalence, risk factors, and natural history of positional plagiocephaly: A systematic review. Dev. Med. Child Neurol. 2008, 50, 577–586. [Google Scholar] [CrossRef]
- Aarnivala, H.; Vuollo, V.; Harila, V.; Heikkinen, T.; Pirttiniemi, P.; Valkama, A. Preventing deformational plagiocephaly through parent guidance: A randomized, controlled trial. Eur. J. Pediatr. 2015, 174, 1197–1208. [Google Scholar] [CrossRef]
- Ballardini, E.; Sisti, M.; Basaglia, N.; Benedetto, M.; Baldan, A.; Borgna-Pignatti, C.; Garani, G. Prevalence and characteristics of positional plagiocephaly in healthy full-term infants at 8–12 weeks of life. Eur. J. Pediatr. 2018, 177, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Smartt, J.; James, M.; Elliott, R.M.; Reid, R.R.; Bartlett, S.P. Analysis of Differences in the Cranial Base and Facial Skeleton of Patients with Lambdoid Synostosis and Deformational Plagiocephaly. Plast. Reconstr. Surg. 2011, 127, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Moon, I.Y.; Lim, S.Y.; Oh, K.S. Analysis of Facial Asymmetry in Deformational Plagiocephaly Using Three-Dimensional Computed Tomographic Review. Arch. Craniofac. Surg. 2014, 15, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Netherway, D.J.; Abbott, A.H.; Gulamhuseinwala, N.; McGlaughlin, K.L.; Anderson, P.J.; Townsend, G.C.; David, D.J. Three-Dimensional Computed Tomography Cephalometry of Plagiocephaly: Asymmetry and Shape Analysis. Cleft Palate-Craniofac. J. 2006, 43, 201–210. [Google Scholar] [CrossRef] [PubMed]
- St. John, D.; Mulliken, J.B.; Kaban, L.B.; Padwa, B.L. Anthropometric analysis of mandibular asymmetry in infants with deformational posterior plagiocephaly. J. Oral Maxillofac. Surg. 2002, 60, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Hwang, J.; Kim, Y.O.; Shim, K.W.; Park, E.K.; Lew, D.H.; Yun, I.S. Three-dimensional analysis of cranial and facial asymmetry after helmet therapy for positional plagiocephaly. Childs Nerv Syst 2015, 31, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Kunz, F.; Schweitzer, T.; Große, S.; Waßmuth, N.; Stellzig-Eisenhauer, A.; Böhm, H.; Meyer-Marcotty, P.; Linz, C. Head orthosis therapy in positional plagiocephaly: Longitudinal 3D-investigation of long-term outcomes, compared with untreated infants and with a control group. Eur. J. Orthod. 2019, 41, 29–37. [Google Scholar] [CrossRef]
- Kluba, S.; Roßkopf, F.; Kraut, W.; Peters, J.; Calgeer, B.; Reinert, S.; Krimmel, M. Malocclusion in the primary dentition in children with and without deformational plagiocephaly. Clin. Oral Invest. 2016, 20, 2395–2401. [Google Scholar] [CrossRef]
- Öwall, L.; Darvann, T.A.; Larsen, P.; Hove, H.D.; Hermann, N.V.; Bøgeskov, L.; Kreiborg, S. Facial Asymmetry in Children with Unicoronal Synostosis who have Undergone Craniofacial Reconstruction in Infancy. Cleft Palate-Craniofac. J. 2016, 53, 385–393. [Google Scholar] [CrossRef]
- Van Vlimmeren, L.A.; Engelbert, R.H.; Pelsma, M.; Groenewoud, H.M.; Boere-Boonekamp, M.M.; Nijhuis-van der Sanden, M.W. The course of skull deformation from birth to 5 years of age: A prospective cohort study. Eur. J. Pediatr. 2017, 176, 11–21. [Google Scholar] [CrossRef]
- Laowansiri, U.; Behrents, R.G.; Araujo, E.; Oliver, D.R.; Buschang, P.H. Maxillary growth and maturation during infancy and early childhood. Angle Orthod. 2013, 83, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Behrents, R.G.; Buschang, P.H. Mandibular growth, remodeling, and maturation during infancy and early childhood. Angle Orthod. 2010, 80, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, S.; Iguchi, Y.; Takada, K. Asymmetry of the Face in Orthodontic Patients. Angle Orthod. 2008, 78, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Melnik, A.K. A cephalometric study of mandibular asymmetry in a longitudinally followed sample of growing children. Am. J. Orthod. Dentofac. Orthop. 1992, 101, 355–366. [Google Scholar] [CrossRef]
- American Academy of Oral and Maxillofacial Radiology. Clinical recommendations regarding use of cone beam computed tomography in orthodontics. Position statement by the American Academy of Oral and Maxillofacial Radiology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 238–257. [Google Scholar] [CrossRef]
- Kau, C.H.; Richmond, S. Three-dimensional analysis of facial morphology surface changes in untreated children from 12 to 14 years of age. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 751–760. [Google Scholar] [CrossRef]
- Kau, C.H.; Richmond, S.; Zhurov, A.I.; Knox, J.; Chestnutt, I.; Hartles, F.; Playle, R. Reliability of measuring facial morphology with a 3-dimensional laser scanning system. Am. J. Orthod. Dentofac. Orthop. 2005, 128, 424–430. [Google Scholar] [CrossRef]
- Aldridge, K.; Boyadjiev, S.A.; Capone, G.T.; DeLeon, V.B.; Richtsmeier, J.T. Precision and error of three-dimensional phenotypic measures acquired from 3dMD photogrammetric images. Am. J. Med. Genet. Part A 2005, 138, 247–253. [Google Scholar] [CrossRef]
- Vuollo, V.; Holmström, L.; Aarnivala, H.; Harila, V.; Heikkinen, T.; Pirttiniemi, P.; Valkama, A.M. Analyzing infant head flatness and asymmetry using kernel density estimation of directional surface data from a craniofacial 3D model. Stat. Med. 2016, 35, 4891–4904. [Google Scholar] [CrossRef]
- Aarnivala, H.E.; Valkama, A.M.; Pirttiniemi, P.M. Cranial shape, size and cervical motion in normal newborns. Early Hum. Dev. 2014, 90, 425–430. [Google Scholar] [CrossRef]
- Aarnivala, H.; Vuollo, V.; Harila, V.; Heikkinen, T.; Pirttiniemi, P.; Holmström, L.; Valkama, A. The course of positional cranial deformation from 3 to 12 months of age and associated risk factors: A follow-up with 3D imaging. Eur. J. Pediatr. 2016, 175, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.D.; Hamilton, M.C. Occlusion in the primary dentition. Study of children at 2 and one-half to 3 years of age. Br. Dent. J. 1969, 126, 76–79. [Google Scholar] [PubMed]
- Björk, A.; Krebs, A.; Solow, B. A Method for Epidemiological Registration of Malocculusion. Acta Odontol. Scand. 1964, 22, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Farkas, L. Anthropometry of the Head and Face; Raven Press: New York, NY, USA, 1994. [Google Scholar]
- Toma, A.; Zhurov, A.; Playle, R.; Ong, E.; Richmond, S. Reproducibility of facial soft tissue landmarks on 3D laser-scanned facial images. Orthod. Craniofac. Res. 2009, 12, 33–42. [Google Scholar] [CrossRef]
- Zhurov, A.; Richmond, S.; Kau, C.H.; Toma, A. Averaging Facial Images. In Three-Dimensional Imaging for Orthodontics and Maxillofacial Surgery; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 126–144. [Google Scholar]
- Aarnivala, H.; Vuollo, V.; Heikkinen, T.; Harila, V.; Holmström, L.; Pirttiniemi, P.; Valkama, A.M. Accuracy of measurements used to quantify cranial asymmetry in deformational plagiocephaly. J. Craniomaxillofac. Surg. 2017, 45, 1349–1356. [Google Scholar] [CrossRef]
- Launonen, A.; Maikku, M.; Vuollo, V.; Pirttiniemi, P.; Valkama, A.M.; Heikkinen, T.; Kau, C.H.; Harila, V. 3D follow-up study of facial asymmetry after developmental dysplasia of the hip. Orthod. Craniofac. Res. 2018, 21, 146–152. [Google Scholar] [CrossRef]
- Kreutz, M.; Fitze, B.; Blecher, C.; Marcello, A.; Simon, R.; Cremer, R.; Zeilhofer, H.; Kunz, C.; Mayr, J. Facial asymmetry correction with moulded helmet therapy in infants with deformational skull base plagiocephaly. J. Cranio-Maxillofac. Surg. 2018, 46, 28–34. [Google Scholar] [CrossRef]
- Pirttiniemi, P.; Kantomaa, T. Relation of glenoid fossa morphology to mandibulofacial asymmetry, studied in dry human Lapp skulls. Acta Odontol. 1992, 50, 235–243. [Google Scholar] [CrossRef]
- Heikkinen, T.; Pirttiniemi, P.; Grön, M.; Alvesalo, L. Primary dentition unilateral crossbite in relation to functional lateralities. J. Dent. Child. 2005, 72, 81–87. [Google Scholar]
- Heikkinen, T.; Rusanen, J.; Harila, V.; Alvesalo, L. Primary tooth wear in functional lateralities. J. Dent. Child. 2009, 76, 5–12. [Google Scholar]
- Heikkinen, T.; Poikela, T.; Grön, M.; Alvesalo, L. Unilateral Angle II in functional lateralities. Eur. J. Orthod. 2004, 26, 93–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ravn, J.J. Occlusion in the primary dentition in 3-year-old children. Scand. J. Dent. Res. 1975, 83, 123. [Google Scholar] [CrossRef] [PubMed]
- Dimberg, L.; Lennartsson, B.; Arnrup, K.; Bondemark, L. Prevalence and change of malocclusions from primary to early permanent dentition:A longitudinal study. Angle Orthod. 2015, 85, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.P.; Teichgraeber, J.F.; Baumgartner, J.E.; Waller, A.L.; English, J.D.; Lasky, R.E.; Miller, C.C.; Gateno, J.; Xia, J.J. Long-Term Treatment Effectiveness of Molding Helmet Therapy in the Correction of Posterior Deformational Plagiocephaly: A Five-Year Follow-Up. Cleft Palate-Craniofac. J. 2008, 45, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Tolleson, S.R.; Kau, C.H.; Lee, R.P.; English, J.D.; Harila, V.; Pirttiniemi, P.; Valkama, M. 3-D analysis of facial asymmetry in children with hip dysplasia. Angle Orthod. 2010, 80, 519–524. [Google Scholar] [CrossRef]
- White, J.E.; Ayoub, A.F.; Hosey, M.; Bock, M.; Bowman, A.; Bowman, J.; Siebert, J.P.; Ray, A. Three-Dimensional Facial Characteristics of Caucasian Infants without Cleft and Correlation with Body Measurements. Cleft Palate-Craniofac. J. 2004, 41, 593–602. [Google Scholar] [CrossRef]
- Djordjevic, J.; Toma, A.M.; Zhurov, A.I.; Richmond, S. Three-dimensional quantification of facial symmetry in adolescents using laser surface scanning. Eur. J. Orthod. 2014, 36, 125–132. [Google Scholar] [CrossRef]
- Vuollo, V.; Sidlauskas, M.; Sidlauskas, A.; Harila, V.; Salomskiene, L.; Zhurov, A.; Holmström, L.; Pirttiniemi, P.; Heikkinen, T. Comparing Facial 3D Analysis With DNA Testing to Determine Zygosities of Twins. Twin Res. Hum. Genet. Off. J. Int. Soc. Twin Stud. 2015, 18, 306–313. [Google Scholar] [CrossRef][Green Version]
- Gwilliam, J.R.; Cunningham, S.J.; Hutton, T. Reproducibility of soft tissue landmarks on three-dimensional facial scans. Eur. J. Orthod. 2006, 28, 408–415. [Google Scholar] [CrossRef]
- Young, N.M.; Sherathiya, K.; Gutierrez, L.; Nguyen, E.; Bekmezian, S.; Huang, J.C.; Hallgrímsson, B.; Lee, J.S.; Marcucio, R.S. Facial surface morphology predicts variation in internal skeletal shape. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 501–508. [Google Scholar] [CrossRef]
- Siqueira de Lima, L.; Brunetto, D.P.; Nojima, M.D.C.G. Evaluation of facial soft tissue thickness in symmetric and asymmetric subjects with the use of cone-beam computed tomography. Am. J. Orthod. Dentofac. Orthop. 2019, 155, 216–223. [Google Scholar] [CrossRef] [PubMed]
| Part of the Face | T1 | T2 | pa | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | IQR | Mean (SD) | Median | IQR | ||
| Average Distance (mm) | |||||||
| Whole face | 0.52 (0.17) | 0.48 | 0.41–0.62 | 0.49 (0.13) | 0.47 | 0.41–0.58 | 0.160 |
| Upper face | 0.45 (0.16) | 0.42 | 0.33–0.55 | 0.41 (0.16) | 0.37 | 0.30–0.50 | 0.024 |
| Upper mid face | 0.48 (0.21) | 0.43 | 0.31–0.55 | 0.46 (0.18) | 0.44 | 0.30–0.58 | 0.312 |
| Lower mid face | 0.56 (0.24) | 0.51 | 0.41–0.68 | 0.58 (0.22) | 0.56 | 0.40–0.75 | 0.478 |
| Lower face | 0.75 (0.40) | 0.65 | 0.48–0.87 | 0.66 (0.36) | 0.55 | 0.36–0.88 | 0.130 |
| Symmetry Percentage (%) | |||||||
| Whole face | 63.3 (10.7) | 63.5 | 56.9–70.4 | 64.4 (10.5) | 65.1 | 58.7–69.9 | 0.444 |
| Upper face | 67.8 (13.6) | 70.0 | 60.4–75.8 | 70.9 (14.2) | 72.1 | 61.4–82.5 | 0.059 |
| Upper mid face | 68 (13.1) | 66.7 | 59.9–77.1 | 67.9 (14.5) | 66.7 | 56.9–81.3 | 0.945 |
| Lower mid face | 59.9 (16.7) | 58.1 | 50.1–70.4 | 56.0 (17.5) | 55.0 | 45.2–70.7 | 0.177 |
| Lower face | 46.3 (18.7) | 48.5 | 32.6–61.4 | 51.2 (23.4) | 52.3 | 29.5–71.1 | 0.131 |
| T1 | T2 | pa | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | IQR | Mean (SD) | Median | IQR | ||
| ExPg difference 3D (°): | 1.45 (1.28) | 1.09 | 0.46–2.09 | 1.13 (0.95) | 0.85 | 0.33–1.57 | 0.095 |
| ExPg difference XY (°): | 1.43 (1.21) | 1.18 | 0.50–1.95 | 1.10 (0.96) | 0.92 | 0.31–1.71 | 0.066 |
| TrPg difference 3D (°): | 1.39 (0.94) | 1.24 | 0.72–2.02 | 1.12 (0.82) | 0.99 | 0.40–1.69 | 0.040 |
| TrPg difference XY (°): | 1.01 (0.92) | 0.86 | 0.39–1.41 | 0.99 (0.82) | 0.87 | 0.28–1.37 | 0.897 |
| Difference of N-Sn-Pg from 180°, XY (°) | 1.38 (1.2) | 1.04 | 0.53–1.85 | 1.37 (1.07) | 1.15 | 0.53–1.95 | 0.948 |
| Absolute value of difference between TrNa Ratio and 1 | 0.019 (0.012) | 0.018 | 0.011–0.027 | 0.017 (0.013) | 0.013 | 0.006–0.024 | 0.173 |
| Absolute value of difference between TrSn Ratio and 1 | 0.019 (0.014) | 0.017 | 0.008–0.029 | 0.020 (0.010) | 0.014 | 0.006–0.023 | 0.032 |
| Absolute value of difference between TrPg Ratio and 1 | 0.017 (0.012) | 0.015 | 0.009–0.026 | 0.010 (0.010) | 0.011 | 0.005–0.019 | 0.010 |
| Absolute value of EO | 1.82 (1.22) | 1.51 | 0.85–2.78 | 1.55 (1.10) | 1.32 | 0.67–2.32 | 0.124 |
| Part of the Face | No History of DP (n = 52) | History of DP (n = 23) | pb | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | IQR | Mean (SD) | Median | IQR | ||
| T1 | |||||||
| Average distance (mm) | |||||||
| Whole face | 0.49 (0.16) | 0.46 | 0.39–0.56 | 0.59 (0.17) | 0.54 | 0.46–0.66 | 0.014 |
| Upper face | 0.43 (0.16) | 0.39 | 0.32–0.48 | 0.51 (0.16) | 0.48 | 0.33–0.59 | 0.022 |
| Upper mid-face | 0.43 (0.16) | 0.41 | 0.31–0.51 | 0.58 (0.26) | 0.53 | 0.43–0.72 | 0.008 |
| Lower mid-face | 0.53 (0.23) | 0.50 | 0.36–0.64 | 0.62 (0.26) | 0.57 | 0.45–0.74 | 0.121 |
| Lower face | 0.74 (0.39) | 0.64 | 0.47–0.86 | 0.78 (0.42) | 0.69 | 0.48–0.99 | 0.890 |
| Symmetry percentage (%) | |||||||
| Whole face | 64.7 (11.4) | 65.6 | 59.1–72.6 | 60.1 (8.5) | 62.6 | 54.8–65.4 | 0.060 |
| Upper face | 69.4 (14.1) | 71.0 | 63.5–77.1 | 64.2 (11.9) | 66.8 | 53.8–74.2 | 0.091 |
| Upper mid-face | 69.9 (13.3) | 68.2 | 61.6–80.6 | 63.8 (11.8) | 64.8 | 53.6–72.5 | 0.049 |
| Lower mid-face | 61.5 (17.7) | 62.3 | 52.1–74.2 | 56.1 (13.7) | 54.3 | 45.4–67.3 | 0.175 |
| Lower face | 46.0 (17.8) | 43.1 | 32.7–61.2 | 47.0 (21.0) | 50.6 | 28.2–63.8 | 0.613 |
| T2 | |||||||
| Average distance (mm) | |||||||
| Whole face | 0.48 (0.13) | 0.45 | 0.41–0.56 | 0.52 (0.14) | 0.51 | 0.40–0.64 | 0.396 |
| Upper face | 0.38 (0.13) | 0.34 | 0.29–0.46 | 0.48 (0.19) | 0.44 | 0.32–0.56 | 0.042 |
| Upper mid-face | 0.43 (0.15) | 0.43 | 0.30–0.53 | 0.52 (0.21) | 0.52 | 0.35–0.68 | 0.093 |
| Lower mid-face | 0.59 (0.24) | 0.51 | 0.38–0.76 | 0.57 (0.17) | 0.61 | 0.45–0.69 | 1.000 |
| Lower face | 0.70 (0.37) | 0.59 | 0.41–0.91 | 0.58 (0.31) | 0.47 | 0.32–0.75 | 0.138 |
| Symmetry percentage (%) | |||||||
| Whole face | 64.7 (10.6) | 65.7 | 58.2–70.0 | 63.9 (10.5) | 64.4 | 59.3–69.9 | 0.890 |
| Upper face | 72.6 (14.3) | 76.4 | 61.7–82.9 | 67.0 (13.7) | 68.7 | 59.6–79.5 | 0.100 |
| Upper mid-face | 68.3 (14.9) | 67.0 | 57.4–81.7 | 67.1 (14.0) | 62.6 | 56.5–77.6 | 0.671 |
| Lower mid-face | 55.1 (18.7) | 54.6 | 40.6–71.1 | 58.1 (14.7) | 55.5 | 47.9–66.3 | 0.550 |
| Lower face | 48.5 (23.1) | 48.8 | 28.5–70.0 | 57.2 (23.3) | 59.8 | 31.4–80.8 | 0.121 |
| Right-Sided DP (n = 15) | Left-Sided DP (n = 8) | Mean Difference | pb | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | IQR | Mean (SD) | Median | IQR | |||
| T1 | ||||||||
| TrNa ratio | 1.012 (0.015) | 1.012 | 1.000–1.026 | 0.981 (0.026) | 0.975 | 0.958–1.010 | 0.031 | 0.011 |
| TrSn ratio | 1.007 (0.016) | 1.009 | 0.998–1.016 | 0.979 (0.027) | 0.973 | 0.957–1.004 | 0.028 | 0.005 |
| TrPg ratio | 1.002 (0.024) | 0.995 | 0.989–1.017 | 0.987 (0.020) | 0.987 | 0.969–1.005 | 0.015 | 0.146 |
| T2 | ||||||||
| TrNa ratio | 1.007 (0.012) | 1.012 | 0.994–1.016 | 0.980 (0.017) | 0.980 | 0.964–0.995 | 0.027 | <0.001 |
| TrSn ratio | 1.000 (0.013) | 1.001 | 0.991–1.014 | 0.977 (0.016) | 0.975 | 0.966–0.989 | 0.023 | 0.001 |
| TrPg ratio | 0.998 (0.013) | 0.998 | 0.986–1.004 | 0.980 (0.008) | 0.981 | 0.973–0.985 | 0.018 | 0.002 |
| Right Tragion Anteriorly | Left Tragion Anteriorly | p | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | IQR | Mean (SD) | Median | IQR | ||
| T1 | T1 (n = 17) | T1 (n = 58) | |||||
| TrNa ratio | 1.017(0.012) | 1.013 | 1.008–1.027 | 0.986(0.019) | 0.983 | 0.974–0.998 | <0.001 |
| TrSN ratio | 1.013(0.012) | 1.013 | 1.003–1.020 | 0.983(0.018) | 0.983 | 0.968–0.996 | <0.001 |
| TrPo ratio | 1.006(0.020) | 1.012 | 0.992–1.015 | 0.987(0.016) | 0.984 | 0.975–0.999 | <0.001 |
| T2 | T2 (n = 21) | T2 (n = 54) | |||||
| TrNa ratio | 1.017(0.019) | 1.016 | 0.999–1.024 | 0.988(0.016) | 0.989 | 0.975–1.000 | <0.001 |
| TrSN ratio | 1.010(0.018) | 1.008 | 0.997–1.019 | 0.985(0.013) | 0.986 | 0.976–0.994 | <0.001 |
| TrPo ratio | 1.003(0.017) | 1.003 | 0.991–1.016 | 0.990(0.012) | 0.990 | 0.981–1.001 | 0.002 |
| History of Deformational Plagiocephaly | ||||
|---|---|---|---|---|
| NO (n = 51) | YES (n = 23) | |||
| N | % | N | % | |
| Asymmetric occlusal trait | ||||
| Deviation of mandibular midline | 8 | 16% | 6 | 26% |
| Asymmetric molar relationship | 4 | 8% | 1 | 4% |
| Crossbite | 1 | 2% | 0 | 0% |
| Total | 11 | 22% | 7 | 30% |
| Sagittal relationship | ||||
| Normal | 35 | 73% | 16 | 73% |
| Distal | 8 | 17% | 4 | 18% |
| Mesial | 1 | 2% | 1 | 4.5% |
| Asymmetric | 4 | 8% | 1 | 4.5% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Launonen, A.M.; Vuollo, V.; Aarnivala, H.; Heikkinen, T.; Pirttiniemi, P.; Valkama, A.M.; Harila, V. Craniofacial Asymmetry from One to Three Years of Age: A Prospective Cohort Study with 3D Imaging. J. Clin. Med. 2020, 9, 70. https://doi.org/10.3390/jcm9010070
Launonen AM, Vuollo V, Aarnivala H, Heikkinen T, Pirttiniemi P, Valkama AM, Harila V. Craniofacial Asymmetry from One to Three Years of Age: A Prospective Cohort Study with 3D Imaging. Journal of Clinical Medicine. 2020; 9(1):70. https://doi.org/10.3390/jcm9010070
Chicago/Turabian StyleLaunonen, Anniina M., Ville Vuollo, Henri Aarnivala, Tuomo Heikkinen, Pertti Pirttiniemi, A. Marita Valkama, and Virpi Harila. 2020. "Craniofacial Asymmetry from One to Three Years of Age: A Prospective Cohort Study with 3D Imaging" Journal of Clinical Medicine 9, no. 1: 70. https://doi.org/10.3390/jcm9010070
APA StyleLaunonen, A. M., Vuollo, V., Aarnivala, H., Heikkinen, T., Pirttiniemi, P., Valkama, A. M., & Harila, V. (2020). Craniofacial Asymmetry from One to Three Years of Age: A Prospective Cohort Study with 3D Imaging. Journal of Clinical Medicine, 9(1), 70. https://doi.org/10.3390/jcm9010070





